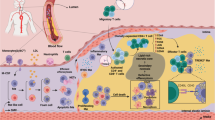
Abstract
Currently, atherosclerosis, characterized by the dysfunction of lipid metabolism and chronic inflammation in the intimal space of the vessel, is considered to be a metabolic disease. As the most abundant innate immune cells in the body, macrophages play a key role in the onset, progression, or regression of atherosclerosis. For example, macrophages exhibit several polarization states in response to microenvironmental stimuli; an increasing proportion of macrophages, polarized toward M2, can suppress inflammation, scavenge cell debris and apoptotic cells, and contribute to tissue repair and fibrosis. Additionally, specific exosomes, generated by macrophages containing certain miRNAs and effective efferocytosis of macrophages, are crucial for atherosclerosis. Therefore, macrophages have emerged as a novel potential target for anti-atherosclerosis therapy. This article reviews the role of macrophages in atherosclerosis from different aspects: origin, phenotype, exosomes, and efferocytosis, and discusses new approaches for the treatment of atherosclerosis.



Similar content being viewed by others
Data Availability
Not applicable
References
Fang F, Xiao C, Li C, Liu X, Li S. Tuning macrophages for atherosclerosis treatment. Regen Biomater. 2023;10:rbac103. https://doi.org/10.1093/rb/rbac103
Sun X, Lyu L, Zhong X, Ni Z, Xu Q. Application of genetic cell-lineage tracing technology to study cardiovascular diseases. J Mol Cell Cardiol. 2021;156:57–68. https://doi.org/10.1016/j.yjmcc.2021.03.006
Mass E, Nimmerjahn F, Kierdorf K, Schlitzer A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat Rev Immunol. 2023; https://doi.org/10.1038/s41577-023-00848-y
Tomas L, Prica F, Schulz C. Trafficking of mononuclear phagocytes in healthy arteries and atherosclerosis. Front Immunol. 2021;12:718432. https://doi.org/10.3389/fimmu.2021.718432
Zhang L. Contribution of resident and recruited macrophages in vascular physiology and pathology. Curr Opin Hematol. 2018;25(3):196–203. https://doi.org/10.1097/MOH.0000000000000421
Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–72. https://doi.org/10.1038/nm.3258
Weinberger T, Esfandyari D, Messerer D, et al. Ontogeny of arterial macrophages defines their functions in homeostasis and inflammation. Nat Commun. 2020;11(1):4549. https://doi.org/10.1038/s41467-020-18287-x
Susser LI, Rayner KJ. Through the layers: how macrophages drive atherosclerosis across the vessel wall. J Clin Invest. 2022;132(9) https://doi.org/10.1172/JCI157011
Tong Y, Cai L, Yang S, et al. The research progress of vascular macrophages and atherosclerosis. Oxidative Med Cell Longev. 2020;2020:7308736. https://doi.org/10.1155/2020/7308736
Wu J, He S, Song Z, et al. Macrophage polarization states in atherosclerosis. Front Immunol. 2023;14:1185587. https://doi.org/10.3389/fimmu.2023.1185587
Zhang J, Ma CR, Hua YQ, et al. Contradictory regulation of macrophages on atherosclerosis based on polarization, death and autophagy. Life Sci. 2021;276:118957. https://doi.org/10.1016/j.lfs.2020.118957
Eshghjoo S, Kim DM, Jayaraman A, Sun Y, Alaniz RC. Macrophage polarization in atherosclerosis. Genes (Basel). 2022;13(5) https://doi.org/10.3390/genes13050756
Lee J, Choi J-H. Deciphering macrophage phenotypes upon lipid uptake and atherosclerosis. Immune Netw. 2020;20(3):e22. https://doi.org/10.4110/in.2020.20.e22
Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res. 2020;126(6):789–806. https://doi.org/10.1161/CIRCRESAHA.119.312321
Rendra E, Riabov V, Mossel DM, et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242–53. https://doi.org/10.1016/j.imbio.2018.11.010
Bi C, Fu Y, Li B. Brain-derived neurotrophic factor alleviates diabetes mellitus-accelerated atherosclerosis by promoting M2 polarization of macrophages through repressing the STAT3 pathway. Cell Signal. 2020;70:109569. https://doi.org/10.1016/j.cellsig.2020.109569
de-Brito NM, Duncan-Moretti J, da-Costa HC, et al. Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim Biophys Acta Mol. Cell Res. 2020;1867(2):118604. https://doi.org/10.1016/j.bbamcr.2019.118604
Kotwal GJ, Chien S. Macrophage differentiation in normal and accelerated wound healing. Results Probl Cell Differ. 2017;62:353–64. https://doi.org/10.1007/978-3-319-54090-0_14
Xu H, Jiang J, Chen W, Li W, Chen Z. Vascular macrophages in atherosclerosis. J Immunol Res. 2019;2019:4354786. https://doi.org/10.1155/2019/4354786
Theofilis P, Oikonomou E, Tsioufis K, Tousoulis D. The role of macrophages in atherosclerosis: pathophysiologic mechanisms and treatment considerations. Int J Mol Sci. 2023;24(11) https://doi.org/10.3390/ijms24119568
Liberale L, Dallegri F, Montecucco F, Carbone F. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb Haemost. 2017;117(1) https://doi.org/10.1160/TH16-08-0593
Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107(6):737–46. https://doi.org/10.1161/CIRCRESAHA.109.215715
Boyle JJ. Heme and haemoglobin direct macrophage Mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr Opin Lipidol. 2012;23(5):453–61. https://doi.org/10.1097/MOL.0b013e328356b145
Barrett TJ. Macrophages in atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2020;40(1):20–33. https://doi.org/10.1161/ATVBAHA.119.312802
Skuratovskaia D, Vulf M, Khaziakhmatova O, et al. Tissue-specific role of macrophages in noninfectious inflammatory disorders. Biomedicines. 2020;8(10) https://doi.org/10.3390/biomedicines8100400
Xie Y, Chen H, Qu P, et al. Novel insight on the role of macrophages in atherosclerosis: focus on polarization, apoptosis and efferocytosis. Int Immunopharmacol. 2022;113(Pt A):109260. https://doi.org/10.1016/j.intimp.2022.109260
Jinnouchi H, Guo L, Sakamoto A, et al. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell Mol Life Sci. 2020;77(10):1919–32. https://doi.org/10.1007/s00018-019-03371-3
Lin P, Ji H-H, Li Y-J, Guo S-D. Macrophage plasticity and atherosclerosis therapy. Front Mol Biosci. 2021;8:679797. https://doi.org/10.3389/fmolb.2021.679797
Qi JR, Zhao DR, Zhao L, Luo F, Yang M. MiR-520a-3p inhibited macrophage polarization and promoted the development of atherosclerosis via targeting UVRAG in apolipoprotein E knockout mice. Front Mol Biosci. 2020;7:621324. https://doi.org/10.3389/fmolb.2020.621324
Ouimet M, Ediriweera HN, Gundra UM, et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125(12):4334–48. https://doi.org/10.1172/JCI81676
Guo Q, Zhu X, Wei R, et al. miR-130b-3p regulates M1 macrophage polarization via targeting IRF1. J Cell Physiol. 2021;236(3):2008–22. https://doi.org/10.1002/jcp.29987
Zhao X, Di Q, Liu H, et al. MEF2C promotes M1 macrophage polarization and Th1 responses. Cell Mol Immunol. 2022;19(4):540–53. https://doi.org/10.1038/s41423-022-00841-w
Meng M, Cao Y, Zhang Y, et al. HnRNPA2B1 aggravates inflammation by promoting M1 macrophage polarization. Nutrients. 2023;15(7) https://doi.org/10.3390/nu15071555
Li J, Yang S, Han Z, et al. Akt2 inhibitor promotes M2 macrophage polarization in rats with periapical inflammation by reducing miR-155-5p expression. Nan Fang Yi Ke Da Xue Xue Bao. 2023;43(4):568–76. https://doi.org/10.12122/j.issn.1673-4254.2023.04.09
Bai L, Li Z, Li Q, et al. Mediator 1 is atherosclerosis protective by regulating macrophage polarization. Arterioscler Thromb Vasc Biol. 2017;37(8):1470–81. https://doi.org/10.1161/ATVBAHA.117.309672
Song F, Li J-Z, Wu Y, et al. Ubiquitinated ligation protein NEDD4L participates in MiR-30a-5p attenuated atherosclerosis by regulating macrophage polarization and lipid metabolism. Mol Ther Nucleic Acids. 2021;26:1303–17. https://doi.org/10.1016/j.omtn.2021.10.030
Lin E-S, Hsu Y-A, Chang C-Y, et al. Ablation of galectin-12 inhibits atherosclerosis through enhancement of M2 macrophage polarization. Int J Mol Sci. 2020;21(15) https://doi.org/10.3390/ijms21155511
Nagenborg J, Goossens P, Biessen EAL, Donners MMPC. Heterogeneity of atherosclerotic plaque macrophage origin, phenotype and functions: implications for treatment. Eur J Pharmacol. 2017;816:14–24. https://doi.org/10.1016/j.ejphar.2017.10.005
Park S-J, Lee K-P, Kang S, et al. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell Signal. 2014;26(10):2249–58. https://doi.org/10.1016/j.cellsig.2014.07.009
Zhang X, Qin Y, Wan X, et al. Rosuvastatin exerts anti-atherosclerotic effects by improving macrophage-related foam cell formation and polarization conversion via mediating autophagic activities. J Transl Med. 2021;19(1):62. https://doi.org/10.1186/s12967-021-02727-3
Zhang X, Xiao S, Li Q. Pravastatin polarizes the phenotype of macrophages toward M2 and elevates serum cholesterol levels in apolipoprotein E knockout mice. J Int Med Res. 2018;46(8):3365–73. https://doi.org/10.1177/0300060518787671
Ma Y, Zhang Y, Qiu C, et al. Rivaroxaban suppresses atherosclerosis by inhibiting FXa-induced macrophage M1 polarization-mediated phenotypic conversion of vascular smooth muscle cells. Front Cardiovasc Med. 2021;8:739212. https://doi.org/10.3389/fcvm.2021.739212
Brenner C, Franz WM, Kühlenthal S, et al. DPP-4 inhibition ameliorates atherosclerosis by priming monocytes into M2 macrophages. Int J Cardiol. 2015;199:163–9. https://doi.org/10.1016/j.ijcard.2015.07.044
Li Z, Ma D, Wang Y, et al. Herb pair attenuates atherosclerosis in ApoE-/- mice by regulating the M1/M2 and Th1/Th2 immune balance and activating the STAT6 signaling pathway. Evid Based Complementary Altern Med. 2022;2022:7421265. https://doi.org/10.1155/2022/7421265
Xie Y, Tian L, Fang Z, et al. Bushen Kangshuai tablet inhibits progression of atherosclerosis by intervening in macrophage autophagy and polarization. J Tradit Chin Med = Chung I Tsa Chih Ying Wen Pan. 2020;40(1):28–37.
Song M-Y, Cho H, Lee S, Lee KH, Kim W. Attenuates atherosclerosis by regulating cholesterol metabolism and inducing M2 macrophage polarization. Life. 2022;12(2) https://doi.org/10.3390/life12020197
Wang X, Du H, Li X. Artesunate attenuates atherosclerosis by inhibiting macrophage M1-like polarization and improving metabolism. Int Immunopharmacol. 2022;102:108413. https://doi.org/10.1016/j.intimp.2021.108413
Li J, Lei H-t, Cao L, et al. Crocin alleviates coronary atherosclerosis via inhibiting lipid synthesis and inducing M2 macrophage polarization. Int Immunopharmacol. 2018;55:120–7. https://doi.org/10.1016/j.intimp.2017.11.037
Jin Z, Li J, Pi J, et al. Geniposide alleviates atherosclerosis by regulating macrophage polarization via the FOS/MAPK signaling pathway. Biomed Pharmacother. 2020;125:110015. https://doi.org/10.1016/j.biopha.2020.110015
Luo Y, Lu S, Gao Y, et al. Araloside C attenuates atherosclerosis by modulating macrophage polarization via Sirt1-mediated autophagy. Aging. 2020;12(2):1704–24. https://doi.org/10.18632/aging.102708
Zhang Y, Shi X, Han J, et al. Convallatoxin promotes M2 macrophage polarization to attenuate atherosclerosis through PPARγ-Integrin αβ signaling pathway. Drug Des Devel Ther. 2021;15:803–12. https://doi.org/10.2147/DDDT.S288728
Wang F, Li M, Zhang A, et al. PCSK9 modulates macrophage polarization-mediated ventricular remodeling after myocardial infarction. J Immunol Res. 2022;2022:7685796. https://doi.org/10.1155/2022/7685796
Lin B, Yang J, Song Y, Dang G, Feng J. Exosomes and atherogenesis. Front Cardiovasc Med. 2021;8:738031. https://doi.org/10.3389/fcvm.2021.738031
Yao J, Cai L, Chen Y, et al. Exosomes: mediators regulating the phenotypic transition of vascular smooth muscle cells in atherosclerosis. Cell Commun Signal. 2022;20(1):153. https://doi.org/10.1186/s12964-022-00949-6
Anakor E, Le Gall L, Dumonceaux J, Duddy WJ, Duguez S. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11) https://doi.org/10.3390/cells10112930
Yang K, Xiao Q, Niu M, Pan X, Zhu X. Exosomes in atherosclerosis: convergence on macrophages. Int J Biol Sci. 2022;18(8):3266–81. https://doi.org/10.7150/ijbs.71862
Shan X, Zhang C, Mai C, et al. The biogenesis, biological functions, and applications of macrophage-derived exosomes. Front Mol Biosci. 2021;8:715461. https://doi.org/10.3389/fmolb.2021.715461
Bouchareychas L, Duong P, Covarrubias S, et al. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via microRNA cargo. Cell Rep. 2020;32(2):107881. https://doi.org/10.1016/j.celrep.2020.107881
Cheng X, Zhou H, Zhou Y, Song C. M2 Macrophage-derived exosomes inhibit apoptosis of HUVEC cell through regulating miR-221-3p expression. Biomed Res Int. 2022;2022:1609244. https://doi.org/10.1155/2022/1609244
Liu Y, Zhang WL, Gu JJ, et al. Exosome-mediated miR-106a-3p derived from ox-LDL exposed macrophages accelerated cell proliferation and repressed cell apoptosis of human vascular smooth muscle cells. Eur Rev Med Pharmacol Sci. 2020;24(12):7039–50. https://doi.org/10.26355/eurrev_202006_21697
Ren L, Chen S, Yao D, Yan H. OxLDL-stimulated macrophage exosomes promote proatherogenic vascular smooth muscle cell viability and invasion via delivering miR-186-5p then inactivating SHIP2 mediated PI3K/AKT/mTOR pathway. Mol Immunol. 2022;146:27–37. https://doi.org/10.1016/j.molimm.2022.02.018
Chen F, Li J, She J, Chen T, Yuan Z. Exosomal microRNA-16-5p from macrophage exacerbates atherosclerosis via modulating mothers against decapentaplegic homolog 7. Microvasc Res. 2022;142:104368. https://doi.org/10.1016/j.mvr.2022.104368
Liu P, Wang S, Wang G, et al. Macrophage-derived exosomal miR-4532 promotes endothelial cells injury by targeting SP1 and NF-κB P65 signalling activation. J Cell Mol Med. 2022;26(20):5165–80. https://doi.org/10.1111/jcmm.17541
Zhu J, Liu B, Wang Z, et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9(23):6901–19. https://doi.org/10.7150/thno.37357
Huang C, Huang Y, Zhou Y, et al. Exosomes derived from oxidized LDL-stimulated macrophages attenuate the growth and tube formation of endothelial cells. Mol Med Rep. 2018;17(3):4605–10. https://doi.org/10.3892/mmr.2018.8380
Wei L-H, Chao N-X, Gao S, et al. Homocysteine induces vascular inflammatory response via SMAD7 hypermethylation in human umbilical vein smooth muscle cells. Microvasc Res. 2018:120. https://doi.org/10.1016/j.mvr.2018.05.003
Wei L, Zhao S, Wang G, et al. SMAD7 methylation as a novel marker in atherosclerosis. Biochem Biophys Res Commun. 2018;496(2):700–5. https://doi.org/10.1016/j.bbrc.2018.01.121
Nanda V, Downing KP, Ye J, et al. CDKN2B regulates TGFβ signaling and smooth muscle cell investment of hypoxic neovessels. Circ Res. 2016;118(2):230–40. https://doi.org/10.1161/CIRCRESAHA.115.307906
Chen W, Schilperoort M, Cao Y, et al. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19(4):228–49. https://doi.org/10.1038/s41569-021-00629-x
Gangadaran P, Madhyastha H, Madhyastha R, et al. The emerging role of exosomes in innate immunity, diagnosis and therapy. Front Immunol. 2022;13:1085057. https://doi.org/10.3389/fimmu.2022.1085057
Wu M, Ouyang Y, Wang Z, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci USA. 2017;114(40):10584–9. https://doi.org/10.1073/pnas.1709210114
Sen S, Xavier J, Kumar N, Ahmad MZ, Ranjan OP. Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech. 2023;13(3):101. https://doi.org/10.1007/s13205-023-03521-2
Xi X-M, Xia S-J, Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76(2):61–7. https://doi.org/10.1691/ph.2021.0128
Wang C, Li Z, Liu Y, Yuan L. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11(8):3996–4010. https://doi.org/10.7150/thno.56035
Wu G, Zhang J, Zhao Q, et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem, Int Ed Engl. 2020;59(10):4068–74. https://doi.org/10.1002/anie.201913700
Zhao Y, Zheng Y, Zhu Y, et al. Docetaxel-loaded M1 macrophage-derived exosomes for a safe and efficient chemoimmunotherapy of breast cancer. J Nanobiotechnology. 2022;20(1):359. https://doi.org/10.1186/s12951-022-01526-2
Zhao C, Song W, Ma J, Wang N. Macrophage-derived hybrid exosome-mimic nanovesicles loaded with black phosphorus for multimodal rheumatoid arthritis therapy. Biomater Sci. 2022;10(23):6731–9. https://doi.org/10.1039/d2bm01274j
Zheng X, Sun K, Liu Y, et al. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J Control Release. 2022;353:675–84. https://doi.org/10.1016/j.jconrel.2022.12.026
Li H, Feng Y, Zheng X, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30. https://doi.org/10.1016/j.jconrel.2021.11.019
Gao Z-S, Zhang C-J, Xia N, et al. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–23. https://doi.org/10.1016/j.actbio.2021.03.018
Reiss AB, Ahmed S, Johnson M, Saeedullah U, De Leon J. Exosomes in cardiovascular disease: from mechanism to therapeutic target. Metabolites. 2023;13(4) https://doi.org/10.3390/metabo13040479
Fu W, Li T, Chen H, Zhu S, Zhou C. Research progress in exosome-based nanoscale drug carriers in tumor therapies. Front Oncol. 2022;12:919279. https://doi.org/10.3389/fonc.2022.919279
Hu R, Dai C, Dong C, et al. Living macrophage-delivered tetrapod PdH nanoenzyme for targeted atherosclerosis management by ROS scavenging, hydrogen anti-inflammation, and autophagy activation. ACS Nano. 2022;16(10):15959–76. https://doi.org/10.1021/acsnano.2c03422
Yousefpour P, Chilkoti A. Co-opting biology to deliver drugs. Biotechnol Bioeng. 2014;111(9):1699–716. https://doi.org/10.1002/bit.25307
Fliervoet LAL, Mastrobattista E. Drug delivery with living cells. Adv Drug Deliv Rev. 2016;106(Pt A):63–72. https://doi.org/10.1016/j.addr.2016.04.021
Gao C, Huang Q, Liu C, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1):2622. https://doi.org/10.1038/s41467-020-16439-7
Peng R, Ji H, Jin L, et al. Macrophage-based therapies for atherosclerosis management. J Immunol Res. 2020;2020:8131754. https://doi.org/10.1155/2020/8131754
Zhang R, Wu S, Ding Q, et al. Recent advances in cell membrane-camouflaged nanoparticles for inflammation therapy. Drug Deliv. 2021;28(1):1109–19. https://doi.org/10.1080/10717544.2021.1934188
Wu Y, Wan S, Yang S, et al. Macrophage cell membrane-based nanoparticles: a new promising biomimetic platform for targeted delivery and treatment. J Nanobiotechnology. 2022;20(1):542. https://doi.org/10.1186/s12951-022-01746-6
Li Y, Che J, Chang L, et al. CD47- and Integrin α4/β1-comodified-macrophage-membrane-coated nanoparticles enable delivery of colchicine to atherosclerotic plaque. Adv Healthc Mater. 2022;11(4):e2101788. https://doi.org/10.1002/adhm.202101788
Wang Y, Zhang K, Li T, et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11(1):164–80. https://doi.org/10.7150/thno.47841
Sha X, Dai Y, Chong L, et al. Pro-efferocytic macrophage membrane biomimetic nanoparticles for the synergistic treatment of atherosclerosis via competition effect. J Nanobiotechnology. 2022;20(1):506. https://doi.org/10.1186/s12951-022-01720-2
Zhao J, Ling L, Zhu W, et al. M1/M2 re-polarization of kaempferol biomimetic NPs in anti-inflammatory therapy of atherosclerosis. J Control Release. 2023;353:1068–83. https://doi.org/10.1016/j.jconrel.2022.12.041
Bianconi E, Piovesan A, Facchin F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40(6):463–71. https://doi.org/10.3109/03014460.2013.807878
Kinchen JM, Ravichandran KS. Phagocytic signaling: you can touch, but you can’t eat. Curr Biol. 2008;18(12):R521–R4. https://doi.org/10.1016/j.cub.2008.04.058
Tajbakhsh A, Farahani N, Gheibihayat SM, et al. Autoantigen-specific immune tolerance in pathological and physiological cell death: nanotechnology comes into view. Int Immunopharmacol. 2021;90:107177. https://doi.org/10.1016/j.intimp.2020.107177
Morioka S, Maueröder C, Ravichandran KS. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity. 2019;50(5):1149–62. https://doi.org/10.1016/j.immuni.2019.04.018
Mehrotra P, Ravichandran KS. Drugging the efferocytosis process: concepts and opportunities. Nat Rev Drug Discov. 2022;21(8):601–20. https://doi.org/10.1038/s41573-022-00470-y
Baraniecki Ł, Tokarz-Deptuła B, Syrenicz A, Deptuła W. Macrophage efferocytosis in atherosclerosis. Scand J Immunol. 2022:e13251. https://doi.org/10.1111/sji.13251
Vorselen D. Dynamics of phagocytosis mediated by phosphatidylserine. Biochem Soc Trans. 2022;50(5):1281–91. https://doi.org/10.1042/BST20211254
Wang L, Li H, Tang Y, Yao P. Potential mechanisms and effects of efferocytosis in atherosclerosis. Front Endocrinol. 2020;11:585285. https://doi.org/10.3389/fendo.2020.585285
Kojima Y, Weissman IL, Leeper NJ. The role of efferocytosis in atherosclerosis. Circulation. 2017;135(5):476–89. https://doi.org/10.1161/CIRCULATIONAHA.116.025684
Thorp EB. Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis: Int J Program Cell Death. 2010;15(9):1124–36. https://doi.org/10.1007/s10495-010-0516-6
Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125(4):1620–36. https://doi.org/10.1172/JCI75417
Yurdagul A. Metabolic consequences of efferocytosis and its impact on atherosclerosis. Immunometabolism. 2021;3(2) https://doi.org/10.20900/immunometab20210017
Schrijvers DM, De Meyer GRY, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(6):1256–61.
Yurdagul A, Doran AC, Cai B, Fredman G, Tabas IA. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med. 2017;4:86. https://doi.org/10.3389/fcvm.2017.00086
Dhawan UK, Singhal A, Subramanian M. Dead cell and debris clearance in the atherosclerotic plaque: mechanisms and therapeutic opportunities to promote inflammation resolution. Pharmacol Res. 2021;170:105699. https://doi.org/10.1016/j.phrs.2021.105699
Kasikara C, Doran AC, Cai B, Tabas I. The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128(7):2713–23. https://doi.org/10.1172/JCI97950
Wang Y, Subramanian M, Yurdagul A, et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. 2017;171(2) https://doi.org/10.1016/j.cell.2017.08.041
Yin C, Vrieze AM, Rosoga M, et al. Efferocytic defects in early atherosclerosis are driven by GATA2 overexpression in macrophages. Front Immunol. 2020;11:594136. https://doi.org/10.3389/fimmu.2020.594136
Wei Y, Zhu M, Corbalán-Campos J, et al. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):796–803. https://doi.org/10.1161/ATVBAHA.114.304723
Singh B, Li K, Cui K, et al. Defective efferocytosis of vascular cells in heart disease. Front Cardiovasc Med. 2022;9:1031293. https://doi.org/10.3389/fcvm.2022.1031293
Kiss RS, Elliott MR, Ma Z, Marcel YL, Ravichandran KS. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr Biol. 2006;16(22):2252–8.
Chen W, Li L, Wang J, et al. The ABCA1-efferocytosis axis: a new strategy to protect against atherosclerosis. Clinica Chimica Acta; International Journal of. Clin Chem. 2021;518:1–8. https://doi.org/10.1016/j.cca.2021.02.025
Tao H, Yancey PG, Babaev VR, et al. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J Lipid Res. 2015;56(8):1449–60. https://doi.org/10.1194/jlr.M056689
Doran AC, Ozcan L, Cai B, et al. CAMKIIγ suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J Clin Invest. 2017;127(11):4075–89. https://doi.org/10.1172/JCI94735
Wiersma VR, Michalak M, Abdullah TM, Bremer E, Eggleton P. Mechanisms of translocation of ER chaperones to the cell surface and immunomodulatory roles in cancer and autoimmunity. Front Oncol. 2015;5:7. https://doi.org/10.3389/fonc.2015.00007
Kojima Y, Volkmer J-P, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536(7614):86–90.
Chen L, Zhou Z, Hu C, et al. Platelet membrane-coated nanocarriers targeting plaques to deliver anti-CD47 antibody for atherosclerotic therapy. Research (Wash D C). 2022;2022:9845459. https://doi.org/10.34133/2022/9845459
Singla B, Lin H-P, Ahn W, et al. Loss of myeloid cell-specific SIRPα, but not CD47, attenuates inflammation and suppresses atherosclerosis. Cardiovasc Res. 2022;118(15):3097–111. https://doi.org/10.1093/cvr/cvab369
Gerlach BD, Marinello M, Heinz J, et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27(2):525–39. https://doi.org/10.1038/s41418-019-0370-1
Karunakaran D, Geoffrion M, Wei L, et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv. 2016;2(7):e1600224. https://doi.org/10.1126/sciadv.1600224
Flores AM, Hosseini-Nassab N, Jarr K-U, et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat Nanotechnol. 2020;15(2):154–61. https://doi.org/10.1038/s41565-019-0619-3
Tajbakhsh A, Bianconi V, Pirro M, et al. Efferocytosis and atherosclerosis: regulation of phagocyte function by microRNAs. Trends Endocrinol Metab. 2019;30(9):672–83. https://doi.org/10.1016/j.tem.2019.07.006
Yurdagul A, Subramanian M, Wang X, et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 2020;31(3) https://doi.org/10.1016/j.cmet.2020.01.001
Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. https://doi.org/10.1038/ncomms12859
Fernanández-Ruiz I. Atherosclerosis: mitochondrial fission is crucial for efferocytosis. Nat Rev Cardiol. 2017;14(12):696. https://doi.org/10.1038/nrcardio.2017.162
Jarr K-U, Ye J, Kojima Y, et al. The pleiotropic benefits of statins include the ability to reduce CD47 and amplify the effect of pro-efferocytic therapies in atherosclerosis. Nat Cardiovasc Res. 2022;1(3):253–62. https://doi.org/10.1038/s44161-022-00023-x
Fernández-Ruiz I. Statins promote efferocytosis in atherosclerotic plaques. Nat Rev Cardiol. 2022;19(5):286. https://doi.org/10.1038/s41569-022-00699-5
Tajbakhsh A, Gheibihayat SM, Askari H, et al. Statin-regulated phagocytosis and efferocytosis in physiological and pathological conditions. Pharmacol Ther. 2022;238:108282. https://doi.org/10.1016/j.pharmthera.2022.108282
Li S, Sun Y, Liang C-P, et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105(11):1072–82. https://doi.org/10.1161/CIRCRESAHA.109.199570
Code Availability
Not applicable
Funding
This work was supported by the “Topping Up Project” of First Teaching Hospital of Tianjin University of Traditional Chinese Medicine.
Author information
Authors and Affiliations
Contributions
XL and SP contributed to the conception of and writing of the manuscript. YJ and LW contributed to the analysis and revision of the manuscript. SP and YL helped perform the analysis with constructive discussions.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoyu Liu and Shuchao Pang share first authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Pang, S., Jiang, Y. et al. The Role of Macrophages in Atherosclerosis: Participants and Therapists. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07513-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07513-5