Abstract
Purpose
There is an ongoing search for new drugs and drug targets to treat diseases like Alzheimer’s disease, cancer and type 2 diabetes (T2D). Both obesity and T2D are characterized by the development of a cardiomyopathy associated with increased hypertension and compensatory left ventricular hypertrophy.
Small, specific glycogen synthase kinase-3 (GSK-3) inhibitors were developed to replace lithium chloride for use in psychiatric disorders. In addition, they were advocated as treatment for T2D since GSK-3 inhibition improves blood glucose handling. However, GSK-3 is a regulator of hypertrophic signalling in the heart via phosphorylation of NFATc3 and β-catenin respectively. In view of this, we hypothesized that chronic inhibition of GSK-3 will induce myocardial hypertrophy or exacerbate existing hypertrophy.
Methods
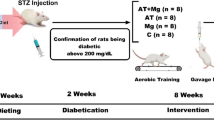
Rats with obesity-induced prediabetes were treated orally with GSK-3 inhibitor (CHIR118637 (CT20026)), 30 mg/kg/day for the last 8 weeks of a 20-week diet high in sugar content vs a control diet. Biometric and biochemical parameters were measured, echocardiography performed and localization and co-localization of NFATc3 and GATA4 determined in cardiomyocytes.
Results
Obesity initiated myocardial hypertrophy, evidenced by increased ventricular mass (1.158 ± 0.029 vs 0.983 ± 0.03 g) and enlarged cardiomyocytes (18.86 ± 2.25 vs 14.92 ± 0.50um2) in association with increased end-diastolic diameter (EDD = 8.48 ± 0.11 vs 8.15 ± 0.10 mm). GSK-3 inhibition (i) increased ventricular mass only in controls (1.075 ± 0.022 g) and (ii) EDD in both groups (controls: 8.63 ± 0.07; obese: 8.72 ± 0.15 mm) (iii) localized NFATc3 and GATA4 peri-nuclearly.
Conclusion
Indications of onset of myocardial hypertrophy in both control and obese rats treated with a GSK-3 inhibitor were found. It remains speculation whether these changes were adaptive or maladaptive.




Similar content being viewed by others
References
Alexander JK. The cardiomyopathy of obesity. Prog Cardiovasc Dis. 1985;27:325–34.
Licata G, Scaglione R, Barbagallo M, Parrinello G, Capuana G, Lipari R, Merlino G, Ganguzza A. Effect of obesity on left ventricular functional studies by radionuclide angiocardiography. Int J Obes. 1991;15:295–302.
Devitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation. 1981;64:477–82.
Messerli FH, Ventura HO, Reisin E, Dreslinki GR, Dunn FG, Mac Phee AA, Frohlich ED. Borderline hypertension and obesity: two prehypertensive states with elevated cardiac output. Circulation. 1982;66:55–60.
Opie LH. The heart: Physiology from cell to circulation. Second ed. New York: Raven Press; 1991. p. p184–5 .p396–400
Ahuja P, Sdek P, Maclellan WD. Cardiac myocyte cell cycle control in development, disease and regeneration. Physiol Rev. 2007;87:521–44.
Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227.
Haq S, Choukroun G, Ranu H, et al. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–29.
Antos CL, McKinsey TA, Frey N, et al. Activated glycogen synthase-3β suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99:907–12.
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28.
Huisamen B, Lochner A. GSK-3 protein and the heart – friend of foe? SA Heart. 2010;7:48–57.
Vollenweider P, Ménard B, Nicod P. Insulin resistance, defective insulin receptor substrate 2-associated phosphatidylinositol-3' kinase activation, and impaired atypical protein kinase C (zeta/lambda) activation in myotubes from obese patients with impaired glucose tolerance. Diabetes. 2002;51(4):1052–9.
Huisamen B. Protein kinase B in the diabetic heart – an invited publication. J Mol Cell Biochem. 2003;249:31–8.
Henriksen EJ, Dokken BB. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr Drug Targets. 2006;7:1435–41.
Van Wauwe J, Haefner B. Glycogen synthase kinase-3 as drug target: from wallflower to center of attention. Drug News Perspect. 2003;16:557–65.
Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–80.
Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–9.
Wagman AS, Johnson KW, Bussiere DE. Discovery and development of GSK3 inhibitors for the treatment of type 2 diabetes. Curr Pharm Des. 2004;10:1105–37.
Lal H, Ahmad F, Woodgett J, Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ Res. 2015;116:138–49.
Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antor CL, Olson EN, Solott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signalling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49.
Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–8.
Cheng H, Woodgett J, Maamari M, Force T. Targeting GSK-3 family members in the heart: a very sharp double-edged sword. J Mol Cell Cardiol. 2011;51:607–13.
Xia Y, Rao J, Yao A, Zhang F, Li G, Wang X, Lu L. Lithium exacerbates hepatic ischemia/reperfusion injury by inhibiting GSK-3/NF-KB-mediated protective signalling in mice. Eur J Pharmacol. 2012;697:117–25.
Liu A, Fang H, Dahmen U, Dirsch O. Chronic lithium treatment protects against liver ischemia/reperfusion injury in rats. Liver Transpl. 2013;19:762–72.
Flepisi TB, Lochner A, Huisamen B. The consequences of long-term glycogen synthase kinase-3 inhibition on normal and insulin resistant rat hearts. Cardiovasc Drugs Ther. 2013;27:381–92.
Cline GW, Johnson K, Regittnig W, Perret P, Tozzo E, Xiao L, et al. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes. 2002;51:2903–10.
Marais E, Genade S, Salie R, Huisamen B, Maritz S, Moolman JA, Lochner A. The temporal relationship between p38 MAPK and HSP27 activation in ischaemic and pharmacological preconditioning. Basic Res Cardiol. 2005;100:35–47.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anals Biochem. 1976;72:248–54.
Huisamen B, Genis A, Marais E, Lochner A. Pre-treatment with a DPP-4 inhibitor is infarct sparing in hearts from obese, pre-diabetic rats. Cardiovasc Drugs Ther. 2011;25:13–20.
Bird SD, Doevendans PA. Van Rooijen MA, Brutel de la Riviere a, Hassink RJ, Passier, R, mummery CL. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58:423–34.
Tokudome T, Horio T, Kishimoto I, Soeki T, Mori K, Kawano Y, Kohno M, Garbers DL, Nakao K, Kangawa K. Calcineurin–nuclear factor of activated T cells pathway–dependent cardiac remodeling in mice deficient in guanylyl cyclase a, a receptor for atrial and brain natriuretic peptides. Circulation. 2005;111:3095–104.
Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res. 2011;50:171–82.
Wensley I, Salaveria K, Bulmer AC, Donner DG, Du Toit EF. Myocardial structure, function and ischaemic tolerance in a rodent model of obesity with insulin resistance. Exp Physiol. 2013;11:1552–64.
Dokken BB, Sloniger JA, Henriksen EJ. Acute selective glycogen synthase kinase-3 inhibition enhances insulin signaling in prediabetic insulin resistant rat skeletal muscle. Am J Physiol Endocrinol Metal. 2005;288:E1188–94.
Rao R, Hao CM, Redha R, Wasserman DH, McGuinness OP, Breyer MD. Glycogen synthase kinase 3 inhibition improves insulin-stimulated glucose metabolism but not hypertension in high-fat C57BL/6 J mice. Diabetologia. 2007;50:452–60.
Kaidanovich-Beilin O, Eldar FH. Long-term treatment with novel glycogen synthase kinase-3 inhibitor improves glucose homeostasis in ob/ob mice: molecular characterization in liver and muscle. J Pharmacol Exp Ther. 2006;316:17–24.
Hargreaves M. Interactions between muscle glycogen and blood glucose during exercise. Exerc Sport Sci Rev. 1997;25:21–39.
Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci U S A. 1997;94:9660–4.
Stypmann J, Engelen MA, Troatz C, Rothenburger M, Eckard L, Tiemann K. Echocardiographic assessment of global left ventricular function in mice. Lab Anim. 2009;43:127–37.
Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–9.
Abe Y, Ono K, Kawamura T, Wada H, Kita T, Shimatsu A, Hasegawa K. Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am J Physiol Heart Circ Physiol. 2007;292:H2387–96.
C.R B, C.M S, C.W T, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huisamen, B., Hafver, T.L., Lumkwana, D. et al. The Impact of Chronic Glycogen Synthase Kinase-3 Inhibition on Remodeling of Normal and Pre-Diabetic Rat Hearts. Cardiovasc Drugs Ther 30, 237–246 (2016). https://doi.org/10.1007/s10557-016-6665-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6665-2