Abstract
Gastric cancer remains a major unmet clinical problem with over 1 million new cases worldwide. It is the fourth most commonly occurring cancer in men and the seventh most commonly occurring cancer in women. A major fraction of gastric cancer has been linked to variety of pathogenic infections including but not limited to Helicobacter pylori (H. pylori) or Epstein Barr virus (EBV). Strategies are being pursued to prevent gastric cancer development such as H. pylori eradication, which has helped to prevent significant proportion of gastric cancer. Today, treatments have helped to manage this disease and the 5-year survival for stage IA and IB tumors treated with surgery are between 60 and 80%. However, patients with stage III tumors undergoing surgery have a dismal 5-year survival rate between 18 and 50% depending on the dataset. These figures indicate the need for more effective molecularly driven treatment strategies. This review discusses the molecular profile of gastric tumors, the success, and challenges with available therapeutic targets along with newer biomarkers and emerging targets.
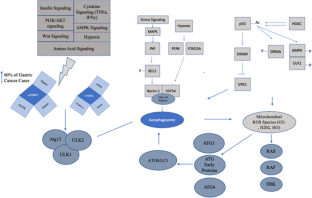
Similar content being viewed by others
References
Prashanth, R., & Barsouk, A. (2019). Epidemiology of gastric cancer: global trends, risk factors and prevention. Przeglad Gastroenterologiczny., 14(1), 26–38. https://doi.org/10.5114/pg.2018.80001.
American Cancer Society. Key statistics about stomach cancer. Cancer.org. Accessed 3/6/2020.
Chen, Y. C., Fang, W. L., Wang, R. F., Liu, C. A., Yang, M. H., Lo, S. S., Wu, C. W., Li, A. F., Shyr, Y. M., & Huang, K. H. (2016). Clinicopathological variation of Lauren classification in gastric cancer. Pathology Oncology Research, 22(1), 197–202. https://doi.org/10.1007/s12253-015-9996-6.
Hu, B., Hajj, N., Sittler, S., Lammert, N., Barnes, R., & Meloni-Ehrig, A. (2012). Gastric cancer: classification, histology and application of molecular pathology. Journal of Gastrointestinal Oncology, 3(3), 251–261. https://doi.org/10.3978/j.issn.2078-6891.2012.021.
Li, Y; Xue, X.W.; Luo, Y.F.; Wu, H.W.; Chen, J; Zhou, W.X. Clinicopathologic features of gastric adenocarcinoma based on the revised Lauren’s classification. Zhonghua Bing Li Xue Za Zhi 2018; 47(7): 486–491. DOI: https://doi.org/10.3760/cma.j.issn.0529-5807.2018.07.002.
Miaozhen, Q., Zhou, Y., Zhang, X., Wang, Z., Wang, F., Shao, J., Lu, J., Jin, Y., Wei, X., Zhang, D., et al. (2014). Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer, 14(823). https://doi.org/10.1186/1471-2407-14-823.
Liang, Y. X., Deng, J. Y., Guo, H. H., et al. (2013). Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World Journal of Gastroenterology, 19(39), 6568–6578. https://doi.org/10.3748/wjg.v19.i39.6568.
Petrelli, F., Berenato, R., Turati, L., Mennitto, A., Steccanella, F., Caporale, M., Dallera, P., de Braud, F., Pezzica, E., Di Bartolomeo, M., et al. (2017). Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. Journal of Gastrointestinal Oncology., 8(1).
Rugge, M., Fassan, M., & Graham, D. Y. (2015). Epidemiology of Gastric Cancer. Gastric Cancer, 23–34.
Han, J. P., Hong, S. J., & Kim, H. K. (2014). Long-term outcomes of early gastric cancer diagnosed as mixed adenocarcinoma after endoscopic submucosal dissection. JGH., 30(2), 316–320. https://doi.org/10.1111/jgh.12838.
Stahl, P., Seeschaaf, C., Lebok, P., et al. (2015). Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol, 15, 7. Published 2015 Feb 5. https://doi.org/10.1186/s12876-015-0231-4.
Bae, J. M., & Kim, E. H. (2016). Epstein-Barr virus and gastric cancer risk: a meta-analysis with meta-regression of case-control studies. Journal of Preventive Medicine and Public Health, 49(2), 97–107. https://doi.org/10.3961/jpmph.15.068.
Wroblewski, L. E., Peek, R. M., & Wilson, K. T. (2010). Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clinical Microbiology Reviews, 23(4), 713–739. https://doi.org/10.1128/CMR.00011-10.
Cats, A., Meuwissen, S. G., Forman, D., Craanen, M. E., & Kuipers, E. J. (1998). Helicobacter pylori: a true carcinogen? European Journal of Gastroenterology & Hepatology, 10(6), 447–450. https://doi.org/10.1097/00042737-199806000-00001.
Conteduca, V., Sansonno, D., Lauletta, G., Russi, S., Ingravallo, G., & Dammacco, F. H. (2012). Pylori infection and gastric cancer: state of the art (Review). International Journal of Oncology. https://doi.org/10.3892/ijo.2012.1701.
Rick, J. R., Goldman, M., Semino-Mora, C., Liu, H., Olsen, C., Rueda-Pedraza, E., Sullivan, C., & Dubois, A. (2010). In situ expression of cagA and risk of gastroduodenal disease in Helicobacter pylori infected children. Journal of Pediatric Gastroenterology and Nutrition, 50(2), 167–172. https://doi.org/10.1097/MPG.0b013e3181bab326.
Hatakeyama, M. (2004). Oncogenic mechanisms of the helicobacter pylori CagA protein. Nature Reviews. Cancer, 4(9), 688–694. https://doi.org/10.1038/nrc1433.
Wroblewski, L. E., Peek Jr., R. M., & Wilson, K. T. (2010). Helicobacter pylori and gastric Cancer: factors that modulate disease risk. Clinical Microbiology Reviews, 713–739. https://doi.org/10.1128/CMR.00011-10.
Jones, K. R., Joo, Y. M., Jang, S., Yoo, Y. J., Lee, H. S., Chung, I. S., Olsen, C. H., Whitmire, J. M., Merrell, S., & Cha, J. H. (2009). Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. Journal of Clinical Microbiology, 959–968. https://doi.org/10.1128/JCM.02330-08.
Kim, K. E. (2003). Gastric cancer in Korean Americans: risks and reductions. Korean Korean Am Stud Bull, 13(1/2), 84–90 PMCID: 1592326.
Lee Y. Association between helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology (New York, N.Y. 1943). 05/2016;150(5):1113–1124.e5 https://doi.org/10.1053/j.gastro.2016.01.028.
Iizas, H., Nanbo, A., Nishikawa, J., & Yoshiyama, H. (2012). Epstein Barr virus (EBV)-associated gastric carcinoma. Viruses., 4(12), 3420–3439. https://doi.org/10.3390/v4123420.
Kim, Y. S., Paik, S. R., Kim, H. K., Yeom, B. W., Kim, I., & Lee, D. (1998). Epstein Barr virus and CD21 expression in gastrointestinal tumors. Pathology, 194(1), 705–711. https://doi.org/10.1016/S0344-0338(98)80130-1.
Kirschner, A. N., Omerovic, J., Popov, B., Longnecker, R., & Jardetzky, T. S. (2006). Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. Journal of Virology, 80(19), 9444–9454. https://doi.org/10.1128/JVI.00572-06.
Xiao, J., Palefsky, J. M., Herrera, R., Berline, J., & Tugizov, S. M. (2008). The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology., 370(2), 430–442. https://doi.org/10.1016/j.virol.2007.09.012.
Vogelaar, I. P., van der Post, R. S., Bisseling, T. M., van Krieken, J. H. J., Ligtenberg, M. J., & Hoogerbrugge, N. (2012). Familial gastric cancer: detection of a hereditary cause helps to understand its etiology. Hereditary Cancer in Clinical Practice, 10(1), 18. https://doi.org/10.1186/1897-4287-10-18.
Josefson, D. (2001). Prophylactic gastrectomy seems to extend life of patients with a family history of stomach cancer. BMJ, 322(7302), 1566 PMCID: PMC1173358.
Ferro, A., Rosato, V., Rota, M., Costa, A. R., Morais, S., Pelucchi, C., Johnson, K. C., Hu, J., Palli, D., Ferraroni, M., et al. (2019). Meat intake and risk of gastric cancer in the stomach cancer pooling (StoP) project. International Journal of Cancer. https://doi.org/10.1002/ijc.32707.
Islami, F., Boffetta, P., Ren, J. S., Pedoeim, L., Khatib, D., & Kamangar, F. (2009). High-temperature beverages and foods and esophageal cancer risk—a systematic review. International Journal of Cancer, 125(3), 491–524. https://doi.org/10.1002/ijc.24445.
Hu, M. L., Yeh, K. T., Lin, P. M., Hsu, C. M., Hsiao, H. H., Liu, Y. C., Lin, H. Y., Lin, S. F., & Yang, M. Y. (2014). Deregulated expression of circadian rhythm clock genes in gastric cancer. BMC Gastroenterology, 14, 67. https://doi.org/10.1186/1471-230x-14-67.
Praud, D; Rota, M; Pelucchi, C; Bertuccio, P; Rosso, T; Galeone, C; Zhang, Z.F; Matsuo, K Ito, H; Hu, J; et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) project. European Journal of Cancer Prevention 2018; 27(2): 124–133. DOI: https://doi.org/10.1097/CEJ.0000000000000290.
Amin, M. B., Greene, F. L., Edge, S. B., Compton, C. C., Gershenwald, J. E., Brookland, R. K., Meyer, L., Gress, D. M., Byrd, D. R., & Winchester, D. P. (2017). The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: a Cancer Journal for Clinicians, 67(2), 93–99. https://doi.org/10.3322/caac.21388.
Bollschweiler, E., Berlth, F., Baltin, C., Mönig, S., & Hölscher, A. H. (2014). Treatment of early gastric cancer in the Western world. World Journal of Gastroenterology, 20(19), 5672–5678. https://doi.org/10.3748/wjg.v20.i19.5672.
Wakahara, T., Ueno, N., Maeda, T., Kanemitsu, K., Yoshikawa, T., Tsuchida, S., & Toyokawa, A. (2018). Impact of gastric cancer surgery in elderly patients. Oncology, 94(2), 79–84. https://doi.org/10.1159/000481404.
Cha, J. H., & Jang, J. S. (2020). Correlation between healing type of lesion and recurrence in gastric neoplastic lesions after endoscopic submucosal dissection. The Turkish Journal of Gastroenterology, 31(1), 36–41. https://doi.org/10.5152/tjg.2020.18764.
Sasako, M., Sakuramoto, S., Katai, H., Kinoshita, T., Furukawa, H., Yamaguchi, T., Nashimoto, A., Fujii, M., Nakajima, T., & Ohashi, Y. (2011). Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. Journal of Clinical Oncology, 29(33), 4387–4393. https://doi.org/10.1200/JCO.2011.36.5908.
Gastric Cancer. National Comprehensive Cancer Network (NCCN) Guidelines. Verison 1. (2020). March 19, 2020. In Available from https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
Jayanathan, M., Erwin, R. P., Molacek, N., Fluck, M., Hunsinger, M., Wild, J., Arora, T. K., Shabahang, M. M., Franko, J., & Blansfield, J. A. (2019). MAGIC versus MacDonald treatment regimes for gastric cancer: Trends and predictors of multimodal therapy for gastric cancer using the National Cancer Database. American Journal of Surgery. https://doi.org/10.1016/j.amjsurg.2019.06.005.
Schwartz, G.K.; Christman, K; Saltz, L; Casper, E; Quan, V; Bertino, J; Martin, D.S.; Colofiore, J; Kelsen, D. A phase I trial of modified, dose intensive FAMTX regimen (high dose 5-fluorouracil + doxorubicin + high dose methotrexate + leucovorin) with oral uridine rescue. ACS Journals. 1996. DOI: https://doi.org/10.1002/(SICI)1097-0142(19961101)79:9<1998::AID-CNCR21>3.0.CO;2-T.
Webb, A., Cunningham, D., Scarffe, J. H., Harper, P., Norman, A., Joffe, J. K., Hughes, M., Mansi, J., Findlay, M., et al. (1997). Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. Journal of Clinical Oncology, 15(1), 261–267.
Cunningham, D., Starling, N., Rao, S., Iveson, T., Nicolson, M., Coxon, F., Middleton, G., Daniel, F., Oates, J., & Norman, A. R. (2008). Capecitabine and oxaliplatin for advanced esophagogastric cancer. The New England Journal of Medicine, 358, 36–46.
Al-Batran, S. E., Homann, N., Pauligk, C., Goetze, T. O., Meiler, J., Kasper, S., Koop, H. G., Mayer, F., Haag, G. M., Luley, K., et al. (2019). Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, rescectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomized, phase 2/3 trial. Lancet., 393(10184), 1948–1957. https://doi.org/10.1016/S0140-6736(18)32557-1.
Masuishi, T., Kadowaki, S., Kondo, M., Komori, A., Sugiyama, K., Mitani, S., Honda, K., Narita, Y., Taniguchi, H., Ura, T., et al. (2017). FOLFOX as first-line therapy for gastric cancer with severe peritoneal metastasis. Anticancer Res, 37(12), 7037–7042. https://doi.org/10.21873/anticanres.12174.
Ahn, H. S., Jeong, S. H., Son, Y. G., Lee, H. J., Im, S. A., Bang, Y. J., Kim, H. H., & Yang, H. K. (2014). Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. The British Journal of Surgery, 101(12), 1560–1565. https://doi.org/10.1002/bjs.9632.
Al-Batran, S.-E., Hofheinz, R. D., Schmalenberg, H., Strumberg, D., Goekkurt, E., Angermeier, S., Zander, T., Potenberg, J., Koop, H. G., Pink, D., et al. (2020). Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7): results of the phase II-portion—a multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. Journal of Clinical Oncology, 38(15).
Hofheinz, R. D., Haag, G. M., Ettrich, T. J., Borchert, K., Kretzschmar, A., Teschendorf, C., Siegler, G. M., Ebert, M. P., Goekkurt, E., Welslau, M., et al. (2020). Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma: final results of the PETRARCA multicenter randomized phase II trail of the AIO. Journal of Clinical Oncology, 35(18).
Bang Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Kim, Y.H; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. The Lancet (British edition). 2012;379(9813):315–321. DOI: https://doi.org/10.1016/S0140-6736(11)61873-4.
Bang, Y. J., Van Cutsem, E., Feyereislova, A., Chung, H. C., Shen, L., Sawaki, A., Lordick, F., Ohtsu, A., Omuro, Y., Satoh, T., et al. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. The Lancet., 376(9742), 687–697. https://doi.org/10.1016/S0140-6736(10)6121-X.
Abrahao-Machado, L. F. (2016). Scapulatempo-Neto; C. HER2 testing in gastric cancer: an update. World Journal of Gastroenterology, 22(19), 4619–4625. https://doi.org/10.3748/wjg.v22.i19.4619.
An Cutsem, E., Muro, K., Cunningham, D., Bodoky, G., Sobrero, A., Cascinu, S., Ajani, J., Al-Batran, S. E., Wainberg, Z. A., Wijayawardana, S. R., et al. (2020). Biomarker analyses of second-line Ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. European Journal of Cancer, 127, 150–157. https://doi.org/10.1016/j.ejca.2019.10.026.
FDA grands accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA. Accessed July 28, 2020.G.
Fuchs, C. S., Doi, T., Jang, R. W., Muro, K., Satoh, T., Machado, M., Sun, W., Jalal, S. I., Shah, M. A., Metges, J. P., et al. (2018). Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE- 059 trial. JAMA Oncology, 4, e180013.
FDA approves pembrolizumab for adults and children with TMB-H solid tumors. (2020). FDA. Accessed July, 25.
Makiyama, A., Arimizu, K., Hirano, G., Makiyama, C., Matsushita, Y., Shirakawa, T., Ohmura, H., Komoda, M., Uchino, K., Inadomia, K., et al. (2018). Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer, 21(3), 464–472. https://doi.org/10.1007/s10120-017-0759-9.
Bando, H; Doi, T; Muro, K; Yasui, H; Nishina, T; Yamaquchi, K; Takahashi, S; Nomura, S; Kumo, H; Shitara, K; et al. A multicenter phase II study of TAS-102 monotherapy in patients with pretreated advanced gastric cancer (EPOC1201 https://doi.org/10.1016/j.ejca.2016.04.009.
Hansson, L. E., Sparén, P., & Nyrén, O. (1999). Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Annals of Surgery, 230(2), 162–169. https://doi.org/10.1097/00000658-199908000-00005.
Ferguson, F. M., & Gray, N. S. (2018). Kinase inhibitors: the road ahead. Nature Reviews. Drug Discovery, 17, 353–377.
Clinicaltrials.gov. A Phase II Trial of STI571 in the Treatment of Metastatic Gastric Cancer. Identifier: NCT00068380.
Bang, Y. J., Kang, Y. K., Kang, W. K., Boku, N., Chung, H. C., Chen, J. S., Doi, T., Sun, Y., Shen, L., Qin, S., et al. (2011). Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Investigational New Drugs, 29(6), 1449–1458. https://doi.org/10.1007/s10637-010-9438-y.
Gotink, K.J.; Broxterman, H.J.; Labots, M; de Haas, R.R.; Dekker, H; Honeywell, R.J.; Rudek, M.A.; Beerepoot, L.V.; Musters, R.J; Jansen, G; et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clinical Cancer Research 2011; 17(23):7337–7346. DOI: https://doi.org/10.1158/1078-0432.CCR-11-1667.
Guo, T; Hajdu, M; Agaram, N.P.; Shinoda, H; Veach, D; Clarkson, B.D.; Maki, R.G.; Singer, S; DeMatteo, R.P.; Besmer, P; et al. Mechanisms of sunitinib resistance in gastrointestinal stromal tumors harboring KITAY502-3ins mutation: an in vitro mutagenesis screen for drug-resistance. Clinical Cancer Research 2009; 15(22): 6862–6870. DOI: https://doi.org/10.1158/1078-0432.CCR-09-1315.
Clinicaltrials.gov. Docetaxel with or without vandetanib in treating patients with metastatic stomach cancer. Identifier: NCT00683787.
Boland, P. M., Meyer, J. E., Berger, A. C., Cohen, S. J., Neuman, T., Cooper, H. S., Olszanski, A. J., Davey, M., Cheng, J. D., Lebenthal, A., et al. (2018). Induction therapy for locally advanced, resectable esophagogastric cancer: A phase I trial of vandetanib (ZD6474), paclitaxel, carboplatin, 5-fluorouracil and radiotherapy followed by resection. American Journal of Clinical Oncology, 40(4), 393–398. https://doi.org/10.1097/COC.0000000000000171.
Xie, L., Su, X., Zhang, L., Yin, X., Tang, L., Zhang, X., Xu, Y., Gao, Z., Liu, K., Zhou, M., et al. (2013). FGFR2 gene amplification in gastric cancer predicts sensitivity to the FGFR inhibitor AZD4547. Clinical Cancer Research, 19(9), 2572–2583. https://doi.org/10.1158/1078-0432.CCR-12-3898.
Mitani, S., & Kawakami, H. (2020). Emerging targeted therapies for HER2 positive gastric cancer that can overcome Trastuzumab resistance. Cancers (Basel)., 12(2), 400. https://doi.org/10.3390/cancers12020400.
Bhat, K. M. R., & Setaluri, V. (2007). Microtubule-associated proteins as targets in cancer chemotherapy. Clin Cancer Res, 13(10). https://doi.org/10.1158/1078-043.CCR-06-3040.
Huang, M., Ma, X., Shi, H., Hu, L., Fan, Z., Pang, L., Zhu, F., Yang, X., Xu, W., Liu, B., et al. (2017). FAM83D, a microtubule-associated protein, promotes tumor growth and progression of human gastric cancer. Oncotarget, 8(43), 74479–74493. https://doi.org/10.18632/oncotarget.20157.
Cochran, J. C., Gatial III, J. E., Kapoor, T. M., & Gilbert, S. P. (2005). Monastrol inhibition of the mitotic kinesin Eg5. JBC., 280(13), 12658–12667. https://doi.org/10.1074/jbc.M413140200.
Torgovnick, A., & Schumacher, B. (2015). DNA repair mechanisms in cancer development and therapy. Frontiers in Genetics, 6, 157. https://doi.org/10.3389/fgene.2015.00157.
Marin, J. J., Al-Abdulla, R., Lozano, E., Briz, O., Bujanda, L., Banales, J. M., & Macias, R. I. (2016). Mechanisms of resistance to chemotherapy in gastric cancer. Anti-Cancer Agents in Medicinal Chemistry, 16(3), 318–334.
Meehan, R. S., & Chen, A. P. (2016). New treatment option for ovarian cancer: PARP inhibitors. Gynecologic Oncology Research and Practice., 7(3).
Nijman, S. M. B. (2011). Synthetic lethality: general principles, utility and detection using genetic screens in human cells. FEBS Letters, 585(1), 1–6. https://doi.org/10.1016/j.febslet.2010.11.024.
Mo, M; Yang, J; Zhu, X; Zhu, J. Use of olaparib in patients with advanced gastric cancer. Oncology. 2018; 19(2): 75. DOIL https://doi.org/10.1016/S1470-2045(18)30023-8.
Bang, Y.J.; Xu, R.H.; Chin, K; Lee, K.W.; Park, S.H.; Rha, S.Y.; Shen, L; Qin, S; Xu, N; Im, S.A; et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): A double-blind, randomized, placebo-controlled, phase 3 trial. Oncology. 2017; 18(12): 1637–1651. DOI: https://doi.org/10.1016/S1470-2045(17)30682-4.
Cechini, M., LoRusso, P., Shyr, Y., Ivy, P., Sklar, J., Dooley, K., & Lacy, J. (2018). NCI 10066: A phase I-II study of Olaparib in combination with ramucirumab in metastatic gastric and gastroesophageal junction (GEJ) adenocarcinoma. Journal of Clinical Oncology, 36(15).
Jang, J. Y., Kang, Y. J., Sung, B., Kim, M. J., Park, C., Kang, D., Moon, H. R., Chung, H. Y., & Kim, N. D. (2018). MHY440, a novel topoisomerase I inhibitor, induces cell cycle arrest and apoptosis via a ROS-dependent DNA damage signaling pathway in AGS human gastric Cancer cells. Molecules, 24(1), E96. https://doi.org/10.3390/molecules24010096.
Yi, H., Yan, X., Luo, Q., Yuan, L., Li, B., Pan, W., Zhang, L., Chen, H., Wang, J., Zhang, Y., et al. (2018). A novel small molecule inhibitor of MDM2-p53 (APG-115) enhances radiosensitivity of gastric adenocarcinoma. Journal of Experimental & Clinical Cancer Research, 37(1), 97. https://doi.org/10.1186/s13046-018-0765-8.
Wu, Q. N., Liao, Y. F., Lu, Y. X., Wang, Y., Lu, J. H., Zeng, Z. L., Huang, Q. T., Sheng, H., Yun, J. P., Xie, D., et al. (2018). Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Letters, 412, 243–255. https://doi.org/10.1016/j.canlet.2017.10.007.
Fok, J. H., Ramos-Montoya, A., Vazquez-Chantada, M., Wijnhoven, P. W. G., Follia, V., James, N., Farrington, P. M., Karmokar, A., Willis, S. E., Cairns, J., et al. (2019). AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nature Communications, 10(5065).
Tamura, T., Ohira, M., Tanaka, H., Muquruma, K., Toyokawa, T., Kubo, N., Sakurai, K., Amano, R., Kimura, K., Shibutani, M., et al. (2015). Programmed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric Cancer. Anticancer Research, 35(10), 5369–5376.
B; Mondaca, S; Sanchez, C; Galindo, H; Nervi, B; Ibanez, C; et al. High proportion of potential candidates for immunotherapy in a Chilean cohort of gastric cancer patients: results of the FORCE1 Study. Cancers (Basel). 2019; 11(9): E1275. DOI: https://doi.org/10.3390/cancers11091275.
Lee, H. S., Chang, M. S., Yang, H. K., Lee, B. L., & Kim, W. H. (2004). Epstein-Barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with Epstein-Barr virus-negative carcinoma. Clinical Cancer Research, 10, 1698–1705.
Fuchs, C.S.; Doi, T; Jang, R.W.; Muro, K; Satoh, T; Machado, M; Sun, W; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical keynote-059 trial. JAMA Oncology 2018; 4(5):e180013. DOI: https://doi.org/10.1001/jamaoncol.2018.0013.
Xing, X., Guo, J., Ding, G., Li, B., Dong, B., Feng, Q., Li, S., Zhang, J., Ying, X., Cheng, X., et al. (2017). Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology, 7(3), e1356114 DOI: 1080/2162402X.2017.1356144.
Kang, Y. K., Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K., Chung, H. C., Chen, J. S., Muro, K., Kang, W. K., et al. (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomized, double-blind, placebo controlled, phase 3 trial. The Lancet., 390(10111), 2461–2471. https://doi.org/10.1016/S0140-6736(17)31827-5.
Hara, H., Shoji, H., Takahari, D., Esaki, T., Machida, N., Nagashima, K., Aoki, K., Honda, K., Miyamoto, T., Boku, N., et al. (2019). Phase I/II study of ramucirumab plus nivolumab in patients in second-line treatment for advanced gastric adenocarcinoma (NivoRam study). Journal of Clinical Oncology, 37(4).
Xu, Y. H., Li, Z. L., & Qiu, S. F. (2018). IFN-y induces gastric cancer cell proliferation and metastasis through upregulation of integrin B3-mediated NF-kB signaling. Translational Oncology, 11(1), 182–192. https://doi.org/10.1016/j.tranon.2017.11.008.
Ham, I. H., Oh, H. J., Jin, H., Bae, C. A., Jeon, S. M., Choi, K. S., Son, S. Y., Han, S. U., Brekken, R. A., Lee, D., & Hur, H. (2019). Targeting interleukin-6 as a stragety to overcome stroma-induced resistance to chemotherapy in gastric cancer. Molecular Cancer, 18(68).
Zhang, Z., Zhang, J., Miao, L., Liu, K., Yang, S., Pan, C., & Jiao, B. (2012). Interleukin-11 promotes the progress of gastric carcinoma via abnormally expressed Versican. International Journal of Biological Sciences, 8(3), 383–393. https://doi.org/10.7150/ijbs.3579.
Wang, Y., Wu, H., Wu, Bian, Z., & Gao, Q. (2014). Interleukin 17A promotes gastric cancer invasiveness via NF-kB mediated matrix metalloproteinases 2 and 9 expression. PLoS One. https://doi.org/10.1371/journal.pone.0096678.
Khawar, M. B., Abbasi, M. H., & Sheikh, N. (2016). IL-32: A novel pluripotent inflammatory interleukin, towards gastric inflammation, gastric cancer, and chronic rhino sinusitis. Mediators of Inflammation.
Lazar, D. C., Avram, M. F., Romosan, I., Cornianu, M., Taban, S., & Goldis, A. (2018). Prognostic significance of tumor immune microenvironment and immunotherapy: Novel insights and future perspectives in gastric cancer. World Journal of Gastroenterology, 24(32), 3583–3616. https://doi.org/10.3748/wjg.v24.i32.3583.
Zhan, X., Wang, B., Li, L., Li, J., Wang, H., Chen, L., Jiang, H., Wu, M., Xiao, J., Peng, X., et al. (2019). Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. Journal of Clinical Oncology, 37(15).
Clinicaltrials.gov. Intraperitoneal infusion of EpCAM CAR-T cell in advanced gastric cancer with peritoneal metastasis. Identifier: NCT03563326.
Lu, J., Xu, Y., Wu, Y., Huang, X. Y., Xie, J. W., Wang, J. B., Lin, J. X., Zheng, C. H., Huang, A. M., & Huang, C. M. (2019). Tumor-inflitrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC Cancer, 19(920).
Lee, S., & Margolin, K. (2013). Tumor-infiltrating lymphocytes in melanoma. Current Oncology Reports, 14(5), 468–474. https://doi.org/10.1007/s11912-012-0257-5.
Clinicaltrials.gov. Adoptive Transfer of Tumor Infiltrating Lymphocytes for Advanced Solid Cancers. Identifier: NCT03935893.
Lin, H. J. (2015). Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel), 7(4), 2443–2458.
Zhang, Q; Peng, C. Cancer-associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer. Oncology Letters 2018; 15(1): 691–698. DOI: https://doi.org/10.3892/ol.2017.7385.
Sathe, A., Grimes, S. M., Lau, B. T., Chen, J., Suarez, C., Huang, R. J., Poultsides, G. A., & Ji, H. P. (2020). Single cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-19-3231.
Uppal, A., Dehal, A., Chang, S. C., Barrak, D., Naeini, Y., Jalas, J. R., & Bilchik, A. J. (2020). The immune microenvironment impacts survival in Western patients with gastric adenocarcinoma. Journal of Gastrointestinal Surgery, 24(1), 28–38. https://doi.org/10.1007/s11605-019-04403-w.
Jiang, Y; Xie, J; Huang, W; Chen, H; Xi, S; Han, Z; Huang, L; Lin, T; Zhao, L.Y.; Hu, Y.F.; et al. Tumor immune microenvironment and chemosensitivity signature for predicting response to chemotherapy in gastric cancer. Cancer Immunol Res. 2019; 7(12): 2065–73. DOI: https://doi.org/10.1158/2326-6066. CIR-19-0311.
Protti, L. F., Soto-Molinari, R., Calderon-Osorno, M., Mora, J., & Alpizar-Alpizar, W. (2019). Gastric cancer in the era of immune checkpoint blockade. Journal of Oncology. https://doi.org/10.1155/2019/107910.
Kuroda, K., Yashiro, M., Sera, T., Yamamoto, Y., Kushitani, Y., Sugimoto, A., Kushiyama, S., Nishimura, S., Togano, S., Okuno, T., et al. (2019). The clinicopathological significance of thrombospondin-4 expression in the tumor microenvironment of gastric cancer. PLoS One, 14(11), e0224727. https://doi.org/10.1371/journal.pone.0223727.
Wu, X., Tao, P., Zhou, Q., Li, J., Zhenjia, Y., Wang, X., Li, J., Li, C., Yan, M., Zhu, Z., et al. (2017). IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget, 8(13), 20741–20750. https://doi.org/10.18632/oncotarget.15119.
Shiga, K., Hara, M., Nagasaki, T., Sato, T., Takahashi, H., & Takeyama, H. (2015). Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel)., 7(4), 2443–2458. https://doi.org/10.3390/cancers7040902.
Yuan, L. W., Yamashita, H., & Seto, Y. (2016). Glucose metabolism in gastric cancer: The cutting edge. World Journal of Gastroenterology, 22(6), 2046–2059. https://doi.org/10.3748/wjg.v22.i6.2046.
Zhang, L., & Li, S. (2020). Lactic acid promotes macrophage polarization through MCT-HIFa signaling in gastric cancer. Experimental Cell Research, 111846. https://doi.org/10.1016/j.yexcr.2020.111846.
Zhou, Q., Wu, X., Wang, X., Yu, Z., Pan, T., Li, Z., Chang, X., Jin, Z., Li, J., Zhu, Z., Liu, B., & Su, L. (2020). The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-a/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene, 39(7), 1414–1428. https://doi.org/10.1038/s41388-019-1078-x.
Kemi, N., Eskuri, M., & Kauppila, J. H. (2019). Tumour-stroma ratio and 5-year mortality in gastric adenocarcinoma: a systematic review and meta-analysis. Scientific Reports, 9(1), 16018. https://doi.org/10.1038/s41598-019-52606-7.
Liu, Y., Chen, X., Chen, X., Yang, X., Song, Q., & Wu, H. (2019). High SALM3 expression in tumor cells and fibroblasts is correlated with poor prognosis in gastric cancer patients. Disease Markers, 8282414. https://doi.org/10.1155/2019/8282414.
Jiang, C., Zhou, Y., Huang, Y., Wang, Y., Wang, W., & Kuai, X. (2019). Overexpression of ADAMTS-2 in tumor cells and stroma is predictive of poor clinical prognosis in gastric cancer. Human Pathology, 84, 44–51. https://doi.org/10.1016/j.humpath.2018.08.030.
Ding, X., Ji, J., Jiang, J., Cai, Q., Wang, C., Shi, M., Yu, Y., Zhu, Z., & Zhang, J. (2018). HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death & Disease, 9(9), 867. https://doi.org/10.1038/s41419-018-0922-1.
Yang, T., Chen, M., Yang, X., Zhang, X., Zhang, Z., Sun, Y., Xu, B., Hua, J., He, Z., & Song, Z. (2017). Down-regualtion of KLF-5 in cancer-associated fibroblasts inhibits gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biology & Therapy, 18(10), 806–815. https://doi.org/10.1080/15384047.2017.1373219.
Wang, X., Zhou, Q., Yu, Z., Wu, X., Chen, X., Li, J., Li, C., Yan, M., Zhu, Z., Liu, B., & Su, L. (2017). Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin B1-FAK signaling pathway. International Journal of Cancer, 141(5), 998–1010. https://doi.org/10.1002/ijc.30801.
Fujii, S., Fujihara, A., Natori, K., Abe, A., Kuboki, Y., Higuchi, Y., Aizawa, M., Kuwata, T., Kinoshita, T., Yasui, W., et al. (2015). TEM1 expression in cancer-associated fibroblasts is correlated with a poor prognosis in patients with gastric cancer. Cancer Medicine, 4(11), 1667–1678. https://doi.org/10.1002/cam4.515.
Muz, B., de la Puente, P., Azab, F., & Azab, A. K. (2015). The role of hypoxia in cancer progression, angiogenesis, metastasis and resistance to therapy. Hypoxia (Auckl)., 3, 83–92. https://doi.org/10.2147/HP.S93413.
Sun, Z., Liu, C., Jiang, W. G., & Ye, L. (2020). Deregulated bone morphogenic proteins and their receptors are associated with disease progression of gastric cancer. Comput Struct Biotechnol., 18, 177–188. https://doi.org/10.1016/j.csbj.2019.12.014.
Sun, Z., Cai, S., Liu, C., Cui, Y., Ji, J., Jiang, W. G., & Ye, L. (2020). Increased expression of Gremlin1 promotes proliferation and epithelial mesenchymal transition in gastric cancer cells and correlates with poor prognosis of patients with gastric cancer. Cancer Genomics & Proteomics, 17(1), 49–60. https://doi.org/10.21873/cgp.20167.
Bae, W. J., Ahn, J. M., Byeon, H. E., Kim, S., & Lee, D. (2019). PTPRD-inactivation-induced CXCL8 promotes angiogenesis and metastasis in gastric cancer and is inhibited by metformin. Journal of Experimental & Clinical Cancer Research, 38(1), 484. https://doi.org/10.1186/s13046-019-1469-4.
Liu, R., Deng, X., Peng, Y., Feng, W., Xiong, R., Zou, Y., Lei, X., Zheng, X., Xie, Z., & Tang, G. (2020). Synthesis and biological evaluation of novel 5,6,7-trimethyloxy flavonoid salicylate derivates as potential anti-tumor agents. Bioorganic Chemistry, 96, 103652. https://doi.org/10.1016/j.bioorg.2020.103652.
Zhang, L., Liu, L., Zhan, S., Chen, L., Wang, Y., Zhang, Y., Du, J., Wu, Y., & Gu, L. (2018). Arsenic trioxide suppressed migration and angiogenesis by targeting FOXO3a in gastric cancer cells. International Journal of Molecular Sciences, 19(12), E3739. https://doi.org/10.3390/ijms19123739.
Lei, Z., Chai, N., Tian, M., Zhang, Y., Wang, G., Liu, J., Tian, Z., Yi, X., Chen, D., Li, X., et al. (2018). Novel peptide GX1 inhibits angiogenesis by specifically binding to transflutaminase-2 in the tumorous endothelial cells of gastric cancer. Cell Death & Disease, 9(6), 579. https://doi.org/10.1038/s41419-018-0594-x.
Uhlik, M. T., Liu, J., Falcon, B. L., Iyer, S., Stewart, J., Celikkaya, H., O’Mahony, M., Sevinsky, C., Lowes, C., Douglass, L., et al. (2016). Stromal-based signatures for the classification of gastric cancer. Cancer Research, 76(9). https://doi.org/10.1158/0008-5472.CAN-16-0022.
Ismail, N. A., Ragab, S. H., ElBaky, A. A., Shoeib, A. R. S., Alhosary, Y., & Fekry, D. (2011). Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Archives of Medical Science, 7(3), 501–507. https://doi.org/10.5114/aoms.2011/23418.
Bull, M. J., & Plummer, N. T. (2014). Part 1: the human gut microbiome in health and diseases. Interreg Mediterranean (Encinitas)., 13(6), 17–22.
Cheng, H. Y., Ning, M. X., Chen, D. K., & Ma, W. T. (2019). Interactions between the gut microbiota and the host innate immune response against pathogens. Frontiers in Immunology, 10, 607. https://doi.org/10.3389/fimmu.2019.00607.
Oliphant, K., & Allen-Vercoe, E. (2019). Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome., 7(91).
Abenavoli, L; Scarpellini, E; Colica, C; Boccuto, L; Salehi, B; Sharifi-rad J; Aiello, V; Romano, B; De Lorenzo, A; Izzo, A.A.; et al. Gut microbiota and obesity: a role for probiotics. Nutrients 2019;11(11):2690. DOI: https://doi.org/10.3390/nu11112690.
Galland, L. (2014). The gut microbiome and the brain. Journal of Medicinal Food, 17(12), 1261–1272. https://doi.org/10.1089/jmf.2014.7000.
Shin, A., Preidis, G. A., Shulman, R., & Kashyap, P. (2018). The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clinical Gastroenterology and Hepatology, 17(2), 256–274. https://doi.org/10.10165/j.cgh.2018.08.054.
Harsch, I. A., & Konturek, P. C. (2018). The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: New insights into “old” diseases. Med Sci (Basel)., 6(2), 32. https://doi.org/10.3390/medsci6020032.
Gazerani, P. (2019). Probiotics for Parkinson’s disease. International Journal of Molecular Sciences, 20(17), 4121. https://doi.org/10.3390/ijms20174121.
Nagano, T., Otoshi, T., Hazama, D., Kiriu, T., Umezawa, K., Katsurada, N., & Nishimura, Y. (2019). Novel cancer therapy targeting microbiome. Oncotargets and Therapy, 12, 3619–3624. https://doi.org/10.2147/OTT.S207546.
Liu, X., Shao, L., Liu, X., Ji, F., Mei, Y., Cheng, Y., Liu, F., Yan, C., Li, L., & Lin, Z. (2019). Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine, 40, 336–348. https://doi.org/10.1016/j.ebiom.2018.12.034.
Hu, Y. L., Pang, W., Huang, Y., Zhang, Y., & Zhang, C. J. (2018). The gastric microbiome is perturbed in advanced gastric adenocarcinoma identified through shotgun metagenomics. Frontiers in Cellular and Infection Microbiology, 8, 433. https://doi.org/10.3389/fcimb.2018.00433.
Spanguolo, C., Russo, G. L., Orhan, I. E., Habtemariam, S., Dagila, M., Sureda, A., Nabavi, S. F., Devi, K. P., Loizzo, M. R., Tundis, R., et al. (2015). Genistein and cancer: current status, challenges, and future directions. Advances in Nutrition, 6(4), 408–419. https://doi.org/10.3945/an.114.008052.
Wang, Y., Liu, H., Peng, Y., Tong, L., Feng, L., & Ma, K. (2018). Characterization of the diethyl phthalate-degrading bacterium Sphingobium yanoikuyae SHJ. Data Breif, 20, 1758–1763. https://doi.org/10.1016/j.dib.2018.09.033.
Zhao, Q., Hu, H., Wang, W., Peng, H., & Zhang, X. (2015). Genome sequencing of Sphingobium yanoikuyae B1, a polycyclic aromatic hydrocarbon-degrading strain. Genome Announcements, 3(1), e01522–e01514. https://doi.org/10.1128/genomeA.01522-14.
Terzi, H. A., Demiray, T., Koroglu, M., Cakmak, G., Ciftci, I. H., Ozbek, A., & Altindis, M. (2016). Intra-abdominal abscess and primary peritonitis caused by Streptococcus anginosus., 9(6), e33863. https://doi.org/10.5812/jjm.33863.
Downes, J; Wade, W.G. Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. International Journal of Systematic and Evolutionary Microbiology 2006; 56(Pt 4): 751–754. DOI: https://doi.org/10.1099/ijs.0.64041-0.
Kim, K. S., Rowlinson, M. C., Bennion, R., Liu, C., Talan, D., Summanen, P., & Finegold, S. M. (2010). Characterization of Slackia exigua isolated from human wound infections, including abscesses of intestinal origin. Journal of Clinical Microbiology, 48(4), 1070–1075. https://doi.org/10.1128/JCM.01576-09.
Baghban, A., & Gupta, S. (2016). Parvimonas micra: a rare cause of native joint septic arthritis. Anaerobe., 39, 26–27. https://doi.org/10.1016/j.anaerobe.2016.02.004.
Khan, M. S., Ishaq, M., Hinson, M., Potugari, B., & Rehman, A. U. (2019). Parvimonas micra bacteremia in a patient with colonic carcinoma. Caspian Journal of Internal Medicine, 10(4), 472–475. https://doi.org/10.22088/cjim.10.4.472.
Coker, O. O., Dai, Z., Nie, Y., Zhao, G., Cao, L., Nakatsu, G., Wu, W. K. K., Wong, S. H., Chen, Z., Sung, J. J. Y., & Yu, J. (2017). Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut.
Rousse, J. M., Bermond, D., Piemont, Y., Tournoud, C., Heller, R., Kehrli, P., Harlay, M. L., Monteil, H., & Jaulhac, B. (2002). Dialister pneumosintes associated with human brain abscesses. Journal of Clinical Microbiology, 40(10), 3871–3873. https://doi.org/10.1128/JCM.40.10.3871-3873.2002.
Contreras, A., Doan, N., Chen, C., Rusitanonta, T., Flynn, M. J., & Slots, J. (2001). The Importance of Dialister pneumosintes in human periodontitis. Oral Microbiology and Immunology. https://doi.org/10.1034/j.1399-302x.2000.150410.x.
Schulz, C., Schutte, K., Mayerle, J., & Malfertheiner, P. (2019). The role of the gastric bacterial microbiome in gastric cancer and beyond. Therapeutic Advances in Gastroenterology. https://doi.org/10.1177/1756284819894062.
Liou, J. M., Lee, Y. C., El-Omar, E. M., & Wu, M. S. (2019). Efficacy and long-term safety of H. pylori eradication for gastric cancer prevention. Cancers (Basel), 11(5), E593. https://doi.org/10.3390/cancers11050593.
Romano, M., & Cuomo, A. (2004). Eradication of helicobacter pylori: a clinical update. MedGenMed, 6(1), 19.
Hamilton-Miller, J. M. (2003). The role of probiotics in the treatment and prevention of helicobacter pylori infection. International Journal of Antimicrobial Agents, 22(4), 360–366. https://doi.org/10.1016/s0924-8579(03)00153-5.
Drago, L. (2019). Probiotics and colon cancer. Microorganisms, 7(3), 66. https://doi.org/10.3390/microorganisms7030066.
Quaresma, M., Damasceno, S., Monteiro, C., Lima, F., Mendes, T., Lima, M., Justino, P., Barbosa, A., Souza, M., Souza, E., et al. (2019). Probiotic mixture containing Lactobacillus spp. and Bifidobacterium spp. attenuates 5-fluorouracil-induced intestinal mucositis in mice. Nutrition and Cancer, 1–11. https://doi.org/10.1080/01635581.2019.167519.
Prisciandaro, L. D., Geier, M. S., Butler, R. N., Cummins, A. G., & Howarth, G. S. (2011). Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy- induced intestinal mucositis. Critical Reviews in Food Science and Nutrition, 51(3), 239–247. https://doi.org/10.1080/10408390903551747.
Cousin, F. J., Jouan-Lanhouet, S., Dimanche-Boitrel, M. T., Corcos, L., & Jan, G. (2012). Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric Cancer cells. PLoS One, 7(3), e31892. https://doi.org/10.1371/journal.pone.0031892.
Shimizu, N., Wakatsuki, T., Murakami, A., Yoshioka, H., Hamazoe, R., Kanayama, H., Maeta, M., & Koga, S. (1987). Carcinoembryonic antigen in gastric cancer patients. Oncology., 44, 240–244. https://doi.org/10.1159/000226486.
Tierman, J. P., Perry, S. L., Verghese, E. T., West, N. P., Yeluri, S., Jayne, D. G., & Hughes, T. A. (2013). Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Journal of Cancer, 108(3), 662–667. https://doi.org/10.1038/bjc.2012.605.
Pavai, S., & Yap, S. F. (2003). The clinical significance of elevated levels of serum CA 19-9. The Medical Journal of Malaysia, 58(5), 667–672.
Wu, J., Li, G., Wang, Z., Yao, Y., Chen, R., Pu, X., & Wang, J. (2015). Circulating MicroRNA-21 is a potential diagnostic biomarker in gastric Cancer. Disease Markers, 435656. https://doi.org/10.1155/2015/435656.
Iwasaki, H., Shimura, T., Yamada, T., Okuda, Y., Natsume, M., Kitagawa, M., Horike, S., & Kataoka, H. (2019). A novel urinary microRNA biomarker panel for detecting gastric cancer. Journal of Gastroenterology, 54, 1061–1069.
Sexton, R., Mahdi, Z., Chaudhury, R., Beydoun, R., Aboukameel, A., Khan, H. Y., Baloglu, E., Senapedis, W., Landesman, Y., Tesfaye, A., Kim, S., Philip, P. A., & Azmi, A. S. (2019). Targeting nuclear exporter protein XPO1/CRM1 in gastric cancer. International Journal of Molecular Sciences, 20(19), E4826. https://doi.org/10.3390/ijms20194826.
Shi, Y., Wang, Z., Zhu, X., Chen, L., Ma, Y., Wang, J., Yang, X., & Liu, Z. (2020). Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. International Journal of Clinical Oncology, 25(1), 89–99. https://doi.org/10.1007/s10147-019-01532-9.
Mei, J. W., Yang, Z. Y., Xiang, H. G., Bao, R., Ye, Y. Y., Ren, T., Wang, X. F., & Shu, Y. J. (2019). MicroRNA-1275 inhibits cell migration and invasion in gastric cancer by regulating vimentin and E-cadherin via JAZF1. BMC Cancer, 19(1), 740. https://doi.org/10.1186/s12885-019-5929-1.
Novikova, I. V., Hennelly, S. P., & Sanbonmatsu, K. Y. (2012). Sizing up long non-coding RNAs do lncRNAs have secondary and tertiary structure? Bioarchitecture., 2(6), 189–199. https://doi.org/10.4161/bioa.22592.
Yao, X. M., Tang, J. H., Zhu, H., & Jing, Y. (2017). High expression of LncRNA CASC15 is a risk factor for gastric cancer prognosis and promote the proliferation of gastric cancer. European Review for Medical and Pharmacological Sciences, 21, 5661–5667.
Qi, D., Wang, Q., Wu, M., & Zhang, X. (2018). Comprehensive bioinformatics analysis of lncRNAs in gastric cancer. Oncology Letters, 1279–1291. https://doi.org/10.3892/ol.2018.9707.
Fei, Z. H., Yu, X. J., Zhou, M., Su, H. F., Zheng, Z., & Xie, C. Y. (2016). Upregulated expression of long-noncoding RNA LINC00982 regulates cell proliferation and its clinical relevance in patients with gastric cancer. Tumour Biology, 37(2), 1983–1993. https://doi.org/10.1007/s13277-015-3979-9.
Lin, X., Xia, Y., Hu, D., Mao, Q., Yu, Z., Zhang, H., Li, C., Chen, G., Liu, F., Zhu, W., et al. (2019). Transcriptome-wide piRNA profiling in human gastric cancer. Oncol Rep, 41(5), 3089–3099. https://doi.org/10.3892/or.2019.7073.
Martinez, V. D., Enfield, K. S. S., Rowbotham, D. A., & Lam, W. L. (2015). An atlas of gastric PIWI-interacting RNA transcriptomes and their utility for identifying signatures of gastric cancer recurrence. Europe PMC., 19(2), 660–665. https://doi.org/10.1007/s10120-015-0487-y.
Cabral, G. F., Azevedo dos Santos Pinheiro, J., Vidal, A. F., Santos, S., & Ribeiro-dos-Santos, A. (2020). piRNAs in gastric cancer: a new approach towards translational research. MDPI, 21(6), 2126. https://doi.org/10.3390/ijms21062126.
Kang, R., Zeh, H. J., Lotze, M. T., & Tang, D. (2011). The Beclin 1 network regulates autophagy and apoptosis. Cell Death and Differentiation, 18(4), 571–580. https://doi.org/10.1038/cdd.2010.191.
Morris, D. H., Yip, C. K., Shi, Y., Chait, B. T., & Wang, Q. J. (2015). Beclin-1VSP34 complex architecture: understanding the nuts and bolts of therapeutic targets. Front Biol (Beijing)., 10(5), 398–426. https://doi.org/10.1007/s11515-015-1374-y.
Galluzzi, L., Baehrecke, E. H., Ballabio, A., Boya, P., Bravo-San Pedro, J. M., Cecconi, F., Choi, A. M., Chu, C. T., Codogno, P., Colombo, M. I., et al. (2017). Molecular definitions of autophagy and related processes. The EMBO Journal, 36(13), 1811–1836. https://doi.org/10.15252/embj.201796697.
Fei, B., Ji, F., Chen, X., Liu, Z., Li, S., Mo, Z., & Fang, X. (2016). Expression and clinical significance of Beclin-1 in gastric cancer tissues of various clinical stages. Oncology Letters, 11(3), 2271–2277. https://doi.org/10.3892/ol.2016.4183.
Giatromanolaki, A., Koukourakis, M. I., Georgiou, I., Kouroupi, M., & Siviridis, E. (2018). LC3A, LC3B and Beclin-1 expression in gastric cancer. Anticancer Research, 38(12), 6827–6833. https://doi.org/10.21873/anticanres.13056.
Levy, J. M. M., Towers, C. G., & Thorburn, A. (2017). Targeting autophagy in cancer. Nature Reviews. Cancer, 17(9), 528–542. https://doi.org/10.1038/nrc.2017.53.
Wesselborg, S., & Stork, B. (2015). Autophagy signal transduction by ATG proteins: from hierarchies to networks. Cellular and Molecular Life Sciences, 72, 4721–4757. https://doi.org/10.1007/s00018-015-2034-8.
Suzuki, K; Noda, T; Ohsumi, Y. Interrelationships among ATG proteins during autophagy in Saccharomyces cerevisiae. 2004. https://doi.org/10.1002/yea.1152.
Yu, L., Chen, Y., & Tooze, S. A. (2018). Autophagy pathway: cellular and molecular mechanisms. Autophagy., 14(2), 207–215. https://doi.org/10.1080/15548627.2017.1378838.
Cherra III, S. J., Kulich, S. M., Uechi, G., Balasubramani, M., Mountzouris, J., Day, B. W., & Chu, C. T. (2010). Regulation of the autophagy protein LC3 phosphorylation. Journal of Cellular Biochemistry, 190(4), 553–539. https://doi.org/10.1083/jcb.201002108.
Roach, P. J. (2011). AMPKà ULK1à Autophagy. Molecular and Cellular Biology, 31(15), 3082–3084. https://doi.org/10.1128/MCB.05565-11.
Backer, J. M. (2008). The regulation and function of class III PI3Ks: novel roles for Vps34. The Biochemical Journal, 410(1), 1–17. https://doi.org/10.1042/BJ20071427.
Chen, M. B., Ji, X. Z., Liu, Y. Y., Zeng, P., Xu, X. Y., Ma, R., Guo, Z. D., Lu, J. W., & Feng, J. F. (2017). Ulk1 over-expression in human gastric cancer is correlated with patients’ T classification and cancer relapse. Oncotarget., 8(20), 33704–33712. https://doi.org/10.18632/Oncotarget.16734.
Wang, X., Wu, W. K. K., Gao, J., Li, Z., Dong, B., Lin, X., Li, Y., Li, Y., Gong, J., Qi, C., Peng, Z., Yu, J., & Shen, L. (2019). Autophagy inhibition enhances PD-L1 expression in gastric cancer. Journal of Experimental & Clinical Cancer Research, 38, 140. https://doi.org/10.1186/s13046-019-1148-5.
Akin, D., Wang, S. K., Habibzadegah-Tari, P., Law, B., Ostrov, D., Li, M., Yin, X. M., Kim, J. S., Horenstein, N., & Dunn, W. A. (2014). A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy., 10(11), 2021–2035. https://doi.org/10.4161/auto.32229.
Kim, J. S., Bae, G. E., Kim, K. H., Lee, S. I., Chung, C., Lee, D., Lee, T. H., Kwon, I. S., & Yeo, M. K. (2019). Prognostic significance of LC3B and p62/SQSTM1 expression in gastric adenocarcinoma. Anticancer Research, 39(12), 6711–6722. https://doi.org/10.21873/anticanres.13886.
Sui, X., Chen, R., Wang, Z., Huang, Z., Kong, N., Zhang, M., Han, W., Lou, F., Yang, J., Zhang, Q., Wang, X., He, C., & Pan, H. (2013). Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death & Disease, 4(10), 838. https://doi.org/10.1038/cddis.2013.350.
Jung, C. H., Ro, S. H., Cao, J., Otto, N. M., & Kim, D. H. (2010). mTOR regulation of autophagy. FEBS Letters, 584(7), 1287–1295. https://doi.org/10.1016/j.febslet.2010.01.017.
Laplante, M., & Sabatini, D. M. (2009). mTOR signaling at a glance. Journal of Cell Science, 122, 3589–3594. https://doi.org/10.1242/jcs.051011.
Jung, C. H., Jun, C. B., Ro, S. H., Kim, Y. M., Otto, N. M., Cao, J., Kundu, M., & Kim, D. H. (2009). ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Molecular Biology of the Cell, 20, 1992–2003. https://doi.org/10.1091/mbc.E08-12-1249.
Ganley, I.G.; Lam du, H; Wang, J; Ding, X; Chen, S; Jiang, X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. Journal of Biological Chemistry . 2009; 284:12297–12305. DOI: https://doi.org/10.0174/jbc.M900573200.
Tian, T., Li, X., & Zhang, J. (2019). mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. International Journal of Molecular Sciences, 20(3), 755. https://doi.org/10.3390/ijms20030755.
Galluzzi, L., Pietrocola, F., Bravo-San Pedro, J. M., Amaravadi, R. K., Baehrecke, E. H., Cecconi, F., Codogno, P., Debnath, J., Gewirtz, D. A., Karantza, V., et al. (2015). Autophagy in malignant transformation and cancer progression. The EMBO Journal, 34, 856–880. https://doi.org/10.15252/embj.201490784.
Amaravadi, R., Kimmelman, A. C., & White, E. (2016). Recent insights into the function of autophagy in cancer. Genes & Development, 30, 1913–1930. https://doi.org/10.1101/gad.287524.116.
Riquelme, I., Tapia, O., Espinoza, J. A., Leal, P., Buchegger, K., Sandoval, A., Bizama, C., Araya, J. C., Peek, R. M., & Roa, J. C. (2016). The gene expression status of the PI3K/AKT/mTOR pathway in gastric Cancer tissues and cell lines. Pathology Oncology Research, 22, 797–805. https://doi.org/10.1007/s12253-016-0066-5.
Byeon, S. J., Han, N., Choi, J., Kim, M. A., & Kim, W. H. (2014). Prognostic implication of TSC1 and mTOR expression in gastric carcinoma. Journal of Surgical Oncology, 109, 812–817. https://doi.org/10.1002/jso.23585.
Tian, T., Li, X., & Zhang, J. (2019). mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. International Journal of Molecular Sciences, 20(3), 755. https://doi.org/10.3390/ijms20030755.
Fukamachi, H., Kim, S. K., Koh, J., Lee, H. S., Sasaki, Y., Yamashita, K., Nishikawaji, T., Shimada, S., Akiyama, Y., Byeon, S. J., et al. (2019). A subset of diffuse-type gastric cancer is susceptible to mTOR inhibitors and checkpoint inhibitors. Journal of Experimental & Clinical Cancer Research, 38(127).
Greenfield, L. K., & Jones, N. L. (2013). Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends in Microbiology, 21(11), 602–612. https://doi.org/10.1016/j.tim.2013.09.004.
Ohtsu, A; Ajani, J.A.; Bai, Y.X.; Bang, Y.J.; Chung, H.C.; Pan, H.M.; Sahmoud, T; Shen, L; Yeh, K.H.; Chin, K; et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double blind, phase III GRANITE-1 study. Gastrointestinal Cancer. 2013; 31: 3935–3943. DOIL https://doi.org/10.1200/JCO.2012.48.3552.
Rosich, L., Xargay-Torrent, S., Lopez-Guerra, M., Campo, E., Colomer, D., & Roue, G. (2012). Counteracting autophagy overcomes resistance to everolimus in mantle cell lymphoma. Cancer Therapy: Preclinical., 18(19). https://doi.org/10.1158/1078-0432.CCR-12-0351.
Wang, W., Lin, L., Zhou, Y., Ye, Q., Yang, X., Jiang, J., Ye, Z., Gao, F., Tan, X., Zhang, G., et al. (2019). Hydroxychloroquine enhances the antitumor effects of BC001 in gastric cancer. International Journal of Oncology, 55(2), 405–414. https://doi.org/10.3892/ijo.2019.4824.
Rong, L., Li, Z., Leng, X., Li, H., Ma, Y., Chen, Y., & Song, F. (2020). Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomedicine & Pharmacotherapy, 122, 109726.
Liu, J., Zhang, Y., Qu, J., Xu, L., Hou, K., Zhang, J., Qu, X., & Liu, Y. (2011). B-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer, 11(183).
Sun, X., Zhang, X., Zhai, H., Zhang, D., & Ma, S. (2019). Chicoric acid (CA) induces autophagy in gastric cancer through promoting endoplasmic reticulum (ER) stress regulated by AMPK. Biomedicine & Pharmacotherapy, 118, 109144. https://doi.org/10.1016/j.biopha.2019.109144.
Zhang, Y., Liu, S., Feng, Q., Huang, X., Wang, X., Peng, Y., Zhao, Z., & Liu, Z. (2018). Perialdehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. Journal of Cellular Biochemistry. https://doi.org/10.1002/jcb.27491.
Zhi, X., Li, B., Li, Z., Zhang, J., Yu, J., Zhang, L., & Xu, Z. (2019). Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. International Journal of Oncology, 54(5), 1625–1638. https://doi.org/10.3892/ijo.2019.4753.
Ji, C. H., & Kwon, Y. T. (2017). Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Biology, Medicine. https://doi.org/10.14348/molcells.2017.0115.
Mrakovcic, M., Kleinheinz, J., & Frohlich, L. F. (2017). Histone deacetylase inhibitor-induced autophagy in tumor cells: implications for p53. International Journal of Molecular Sciences, 18(9), 1883. https://doi.org/10.3390/ijms18091883.
Peracchio, C., Alabiso, O., Valente, G., & Isidoro, C. (2012). Involvement of autophagy in ovarian cancer: a working hypothesis. Journal of Ovarian Research, 5, 22. https://doi.org/10.1186/1757-2215-5-22.
Zhou, J., Liao, W., Yang, J., Ma, K., Li, X., Wang, Y., Wang, D., Wang, L., Zhang, Y., Yin, Y., Zhao, Y., & Zhu, W. G. (2012). FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy, 8(12), 1712–1723. https://doi.org/10.46161/auto.21830.
Avivar-Valderas, A., Salas, E., Bobrovnikova-Marjon, E., Diehl, J. A., Nagi, C., Debnath, J., & Aguirre-Ghiso, J. A. (2011). PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Molecular and Cellular Biology, 31(17), 3616–3629. https://doi.org/10.1128/MCB.05164-11.
Chen, Y. F., Liu, H., Luo, X. J., Zhao, Z., Zou, Z. Y., Li, J., Lin, X. J., & Liang, Y. (2017). The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Critical Reviews in Oncology/Hematology, 112, 21–30. https://doi.org/10.1016/j.critevonc.2017.02.004.
Li, Z. Y., Yang, Y., Ming, M., & Liu, B. (2011). Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochemical and Biophysical Research Communications, 414(1), 5–8. https://doi.org/10.1016/j.bbrc.2011.09.046.
Zou, P., Xia, Y., Chen, T., Zhang, J., Wang, Z., Chen, W., Chen, M., Kanchana, K., Yang, S., & Liang, G. (2015). Selective killing of gastric cancer cells by small molecule targeting ROS-mediated ER stress activation. Molecular Carcinogenesis. https://doi.org/10.1002/mc.22351.
Wang, T., Gao, J., Yu, J., & Shen, L. (2013). Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenographs. Chinese Journal of Cancer Research, 25(5), 505–513. https://doi.org/10.3978/j.issn.1000-9604.2013.08.14.
Li, S.J.; Sun, S.J.; Gao, J; Sun, F.B. Wogonin induces Beclin-1/PI3K and reactive oxygen species-mediated autophagy in human pancreatic cells. Oncology Letters 2016; 12(6): 5059–5067. DOI: https://doi.org/10.3892/ol.2016.5367.
Aviles-Jimenez, F., Vazquez-Jimenez, F., Medrano-Guzman, R., Mantilla, A., & Torres, J. (2014). Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Scientific Reports, 4, 4202. https://doi.org/10.1038/srep04202.
Hu, Y. L., Pang, W., Huang, Y., Zhang, Y., & Zhang, C. J. (2018). The gastric Microbioe is perturbed in advanced gastric adenocarcinoma identified through shotgun metagenomics. Frontiers., 8, 433. https://doi.org/10.3389/fcimb.2018.00433.
Liu, X., Nie, W., Liang, J., & Li, Y. (2017). Interaction of Helicobacter pylori with other microbiota species in the development of gastric cancer. Clinical Microbiology.
Dias, Jacome, E; Libanio, D; Borges-Canha, M; Galaghar, A; Pimentel-Nunes, P. Gastric microbiota and carcinogenesis: the role of non-Helicobacter pylori bacteria—a systematic review. Revistia Espanola de Enfermedades Digestivas. 2016. DOI: https://doi.org/10.17235/reed.2016.4261/2016.
Salazar, C. R., Sun, J., Li, Y., Francois, F., Corby, P., Perez-Perez, G., Dasanayake, A., Pei, Z., & Chen, Y. (2013). Association between selected Oral pathogens and gastric precancerous lesions. PLoS One, 8(1), e51604. https://doi.org/10.1371/journal.pone.0051604.
Hsieh, Y. Y., Tung, S. Y., Pan, H. Y., Yen, C. W., Xu, H. W., Lin, Y. J., Deng, Y. F., Hsu, W. T., Wu, C. S., & Li, C. (2018). Increased abundance of Clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Scientific Reports, 8(158).
Roberts, P. J., Dickinson, R. J., Whitehead, A., Laughton, C. R., & Foweraker, J. E. (2002). The culture of lactobacilli species in gastric carcinoma. Journal of Clinical Pathology, 55, 477–480.
Weng, M. T., Chiu, Y. T., Wei, P. Y., Chiang, C. W., & Fang, H. L. (2019). Wei, S.C. microbiota and gastrointestinal cancer. Journal of the Formosan Medical Association, 118(1), S32–S41. https://doi.org/10.1016/j.fma.2019.01.002.
Wang, L. L., Yu, X. J., Zhan, S. H., Jia, S. J., Tian, Z. B., & Dong, Q. J. (2014). Participation of microbiota in the development of gastric cancer. World Journal of Gastroenterology, 20(17), 4948–4952. https://doi.org/10.3748/wjg.v20.i17.4948.
Zhao, Y., Gao, X., Guo, J., Yu, D., Xiao, Y., Wang, H., & Li, Y. (2019). Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter., 24, e12567. https://doi.org/10.1111/hel.12567.
Zhang, L., Fu, L., Zhang, S., Zhang, J., Zhao, Y., Zheng, Y., He, G., Yang, S., Ouyang, L., & Liu, B. (2017). Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chemical Science, 8(4), 2687–2601. https://doi.org/10.1039/c6sc05368h.
Fu, X., Huang, Z., Hong, L., Lu, J. H., Feng, D., Yin, X. M., & Li, M. (2019). Targeting ATG4 in cancer therapy. Cancers., 11(5), 649. https://doi.org/10.3390/cancers11050649.
Xie, J., Wang, X., & Proud, C. G. (2016). mTOR inhibitors in cancer therapy. F1000Res, 5, F1000 Faculty Rev-2078. https://doi.org/10.12688/f1000research.9207.1.
Rebecca, V. W., Nicastri, M. C., Fennelly, C., Chude, C. L., Barber-Rotenberg, J. S., Ronghe, A., McAfee, Q., McLaughlin, N. P., Zhang, G., Goldman, A. R., et al. (2018). PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. AACR Journals. Published. https://doi.org/10.1158/2159-8290.CD-18-0706.
Zhou, J., Li, G., Zheng, Y., Shen, H. M., Hu, X., Ming, Q. L., Huang, S., Li, P., & Gao, N. (2015). A novel autophagy/mitophagy inhibitor Liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy., 11(8), 1259–1279. https://doi.org/10.1080/15548627.2015.1056970.
Mahameed, M., Wilhelm, T., Darawshi, O., Obiedat, A., Tommy, W. S., Chintha, C., Schubert, T., Samali, A., Chevet, E., et al. (2019). The unfolded protein response modulators GSK2606414 and KIRA6 are potent KIT inhibitors. Cell Death & Disease, 10(300).
Axten, J. M., Romeril, S. P., Shu, A., Ralph, J., Medina, J. R., Feng, Y., Li, W. H. H., Grant, S. W., Heerding, D. A., et al. (2013). Discovery of GSK2656157: An optimized PERK inhibitor selected for preclinical development. ACS Medicinal Chemistry Letters. https://doi.org/10.1021/ml400228e.
Li, J., Tu, H. J., Li, J., Dai, G., Dai, Y. C., Wu, Q., Shi, Q. Z., Cao, Q., & Li, Z. J. (2007). N-acetyl cysteine inhibits human signet ring cell gastric cancer cell line (SJ-89) cell growth by inducing apoptosis and DNA synthesis arrest. European Journal of Gastroenterology & Hepatology, 19(9), 769–774. https://doi.org/10.1097/MEG.0b013e3292202bda.
Klenke, S., Akdeli, N., Stelmach, P., Heukamp, L., Schulte, J. H., & Bachmann, H. S. (2019). The small molecule Bcl-2/mcl-1 inhibitor TW-37 shows single-agent cytotoxicity in neuroblastoma cell lines. BMC Cancer, 19(1), 243. https://doi.org/10.1186/s12885-019-5439-1.
Chen, X., Mao, G., Chen, H., Liu, S., Wang, S., Li, X., Ye, Y., Wu, H., & Liu, J. (2018). TW37 enhances the pro-apoptosis and anti-migratory ability of gefitinib in non-small cell lung cancer. Molecular and Cellular Biology (Noisy-le-grand)., 64(4), 6–10.
Krasniqi, E., Pellicori, S., & Formica, V. (2015). Emerging role of S-1 in gastric cancer. Indian Journal of Medical and Paediatric Oncology, 36(4), 219–228. https://doi.org/10.4103/0971-5851.171542.
Manero, F., Gautier, F., Gallenne, T., Cauquil, N., Gree, D., Catron, P. F., Geneste, O., Gree, R., Vallette, F. M., & Juin, P. (2006). The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Research, 66(5), 2757–2764.
Chang, J., Wang, Y., Shao, L., Laberge, R. M., Demaria, M., Campisi, J., Janakiraman, K., Sharpless, N. E., Ding, S., Feng, W., et al. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22(1), 78–83.
Zhang, H., Zhong, X., Zhang, X., Shang, D., Zhou, Y. I., & Zhang, C. (2016). Enhanced anticancer effect of ABT-737 in combination with naringenin on gastric cancer cells. Experimental and Therapeutic Medicine, 11(2), 669–673.
Funding
Work in the lab of ASA is supported by NIH R37CA215427 and SKY Foundation Inc.
Author information
Authors and Affiliations
Contributions
RS, MNH, MD, and ASA: concept design, literature search, writing, and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sexton, R.E., Al Hallak, M.N., Diab, M. et al. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev 39, 1179–1203 (2020). https://doi.org/10.1007/s10555-020-09925-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09925-3




