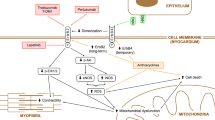
Abstract
Eicosanoids are bioactive lipids that play crucial roles in various pathophysiological conditions, including inflammation and cancer. They include both the COX-derived prostaglandins and the LOX-derived leukotrienes. Furthermore, the epidermal growth factor receptor (EGFR) pathways family of receptor tyrosine kinases also are known to play a central role in the tumorigenesis. Various antitumor modalities have been approved cancer treatments that target therapeutically the COX-2 and EGFR pathways; these include selective COX-2 inhibitors and EGFR monoclonal antibodies. Research has shown that the COX-2 and epidermal growth factor receptor pathways actively interact with each other in order to orchestrate carcinogenesis. This has been used to justify a targeted combinatorial approach aimed at these two pathways. Although combined therapies have been found to have a greater antitumor effect than the administration of single agent, this does not exempt them from the possible fatal cardiac effects that are associated with COX-2 inhibition. In this review, we delineate the contribution of HB-EGF, an important EGFR ligand, to the cardiac dysfunction related to decreased shedding of HB-EGF after COX-2/PGE2 inhibition. A better understanding of the molecular mechanisms underlying these cardiac side effects will make possible more effective regimens that use the dual-targeting approach.

Similar content being viewed by others
References
Greene, E. R., Huang, S., Serhan, C. N., & Panigrahy, D. (2011). Regulation of inflammation in cancer by eicosanoids. Prostaglandins & Other Lipid Mediators, 96(1–4), 27–36. https://doi.org/10.1016/j.prostaglandins.2011.08.004.
Kuhn, H., Banthiya, S., & van Leyen, K. (2015). Mammalian lipoxygenases and their biological relevance. Biochimica et Biophysica Acta, 1851(4), 308–330. https://doi.org/10.1016/j.bbalip.2014.10.002.
Okuyama, T., Ishihara, S., Sato, H., Rumi, M. A., Kawashima, K., Miyaoka, Y., Suetsugu, H., Kazumori, H., Cava, C. F., Kadowaki, Y., Fukuda, R., & Kinoshita, Y. (2002). Activation of prostaglandin E2-receptor EP2 and EP4 pathways induces growth inhibition in human gastric carcinoma cell lines. The Journal of Laboratory and Clinical Medicine, 140(2), 92–102.
Liu, W., Reinmuth, N., Stoeltzing, O., Parikh, A. A., Tellez, C., Williams, S., Jung, Y. D., Fan, F., Takeda, A., Akagi, M., Bar-Eli, M., Gallick, G. E., & Ellis, L. M. (2003). Cyclooxygenase-2 is up-regulated by interleukin-1 beta in human colorectal cancer cells via multiple signaling pathways. Cancer Research, 63(13), 3632–3636.
Zhu, M., Zhu, Y., & Lance, P. (2013). TNFalpha-activated stromal COX-2 signalling promotes proliferative and invasive potential of colon cancer epithelial cells. Cell Proliferation, 46(4), 374–381. https://doi.org/10.1111/cpr.12047.
Glinghammar, B., Inoue, H., & Rafter, J. J. (2002). Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis, 23(5), 839–845.
Eberhart, C. E., Coffey, R. J., Radhika, A., Giardiello, F. M., Ferrenbach, S., & DuBois, R. N. (1994). Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology, 107(4), 1183–1188.
de Groot, D. J., de Vries, E. G., Groen, H. J., & de Jong, S. (2007). Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Critical Reviews in Oncology/Hematology, 61(1), 52–69. https://doi.org/10.1016/j.critrevonc.2006.07.001.
Zhu, Y., Hua, P., & Lance, P. (2003). Cyclooxygenase-2 expression and prostanoid biogenesis reflect clinical phenotype in human colorectal fibroblast strains. Cancer Research, 63(2), 522–526.
Zhu, Y., Zhu, M., & Lance, P. (2012). Stromal COX-2 signaling activated by deoxycholic acid mediates proliferation and invasiveness of colorectal epithelial cancer cells. Biochemical and Biophysical Research Communications, 425(3), 607–612. https://doi.org/10.1016/j.bbrc.2012.07.137.
Zhu, Y., Zhu, M., & Lance, P. (2012). iNOS signaling interacts with COX-2 pathway in colonic fibroblasts. Experimental Cell Research, 318(16), 2116–2127. https://doi.org/10.1016/j.yexcr.2012.05.027.
Zhu, Y., Zhu, M., & Lance, P. (2012). IL1beta-mediated stromal COX-2 signaling mediates proliferation and invasiveness of colonic epithelial cancer cells. Experimental Cell Research, 318(19), 2520–2530. https://doi.org/10.1016/j.yexcr.2012.07.021.
Zhu, Y., Hua, P., Rafiq, S., Waffner, E. J., Duffey, M. E., & Lance, P. (2002). Ca2+− and PKC-dependent stimulation of PGE2 synthesis by deoxycholic acid in human colonic fibroblasts. American Journal of Physiology. Gastrointestinal and Liver Physiology, 283(3), G503–G510. https://doi.org/10.1152/ajpgi.00525.2001.
Chan, A. T., Ogino, S., & Fuchs, C. S. (2009). Aspirin use and survival after diagnosis of colorectal cancer. JAMA, 302(6), 649–658. https://doi.org/10.1001/jama.2009.1112.
Park, S. W., Kim, H. S., Choi, M. S., Jeong, W. J., Heo, D. S., Kim, K. H., & Sung, M. W. (2011). The effects of the stromal cell-derived cyclooxygenase-2 metabolite prostaglandin E2 on the proliferation of colon cancer cells. The Journal of Pharmacology and Experimental Therapeutics, 336(2), 516–523. https://doi.org/10.1124/jpet.110.173278.
Kinzler, K. W., & Vogelstein, B. (1998). Landscaping the cancer terrain. Science, 280(5366), 1036–1037.
Sheng, H., Shao, J., Morrow, J. D., Beauchamp, R. D., & DuBois, R. N. (1998). Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Research, 58(2), 362–366.
Poligone, B., & Baldwin, A. S. (2001). Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. The Journal of Biological Chemistry, 276(42), 38658–38664. https://doi.org/10.1074/jbc.M106599200.
Han, C., Michalopoulos, G. K., & Wu, T. (2006). Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. Journal of Cellular Physiology, 207(1), 261–270. https://doi.org/10.1002/jcp.20560.
Buchanan, F. G., Wang, D., Bargiacchi, F., & DuBois, R. N. (2003). Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. The Journal of Biological Chemistry, 278(37), 35451–35457. https://doi.org/10.1074/jbc.M302474200.
Chang, S. H., Liu, C. H., Conway, R., Han, D. K., Nithipatikom, K., Trifan, O. C., Lane, T. F., & Hla, T. (2004). Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proceedings of the National Academy of Sciences of the United States of America, 101(2), 591–596. https://doi.org/10.1073/pnas.2535911100.
Kamiyama, M., Pozzi, A., Yang, L., DeBusk, L. M., Breyer, R. M., & Lin, P. C. (2006). EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene, 25(53), 7019–7028. https://doi.org/10.1038/sj.onc.1209694.
Watanabe, K., Kawamori, T., Nakatsugi, S., Ohta, T., Ohuchida, S., Yamamoto, H., Maruyama, T., Kondo, K., Ushikubi, F., Narumiya, S., Sugimura, T., & Wakabayashi, K. (1999). Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Research, 59(20), 5093–5096.
Mutoh, M., Watanabe, K., Kitamura, T., Shoji, Y., Takahashi, M., Kawamori, T., Tani, K., Kobayashi, M., Maruyama, T., Kobayashi, K., Ohuchida, S., Sugimoto, Y., Narumiya, S., Sugimura, T., & Wakabayashi, K. (2002). Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Research, 62(1), 28–32.
Fujino, H., Xu, W., & Regan, J. W. (2003). Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. The Journal of Biological Chemistry, 278(14), 12151–12156. https://doi.org/10.1074/jbc.M212665200.
Pozzi, A., Yan, X., Macias-Perez, I., Wei, S., Hata, A. N., Breyer, R. M., Morrow, J. D., & Capdevila, J. H. (2004). Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. The Journal of Biological Chemistry, 279(28), 29797–29804. https://doi.org/10.1074/jbc.M313989200.
Rigas, B., Goldman, I. S., & Levine, L. (1993). Altered eicosanoid levels in human colon cancer. The Journal of Laboratory and Clinical Medicine, 122(5), 518–523.
McLemore, T. L., Hubbard, W. C., Litterst, C. L., Liu, M. C., Miller, S., McMahon, N. A., et al. (1988). Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Research, 48(11), 3140–3147.
Wang, D., & Dubois, R. N. (2004). Cyclooxygenase-2: a potential target in breast cancer. Seminars in Oncology, 31(1 Suppl 3), 64–73.
Hambek, M., Baghi, M., Wagenblast, J., Schmitt, J., Baumann, H., & Knecht, R. (2007). Inverse correlation between serum PGE2 and T classification in head and neck cancer. Head & Neck, 29(3), 244–248. https://doi.org/10.1002/hed.20503.
Park, J. M., Kanaoka, Y., Eguchi, N., Aritake, K., Grujic, S., Materi, A. M., Buslon, V. S., Tippin, B. L., Kwong, A. M., Salido, E., French, S. W., Urade, Y., & Lin, H. J. (2007). Hematopoietic prostaglandin D synthase suppresses intestinal adenomas in ApcMin/+ mice. Cancer Research, 67(3), 881–889. https://doi.org/10.1158/0008-5472.CAN-05-3767.
Kim, J., Yang, P., Suraokar, M., Sabichi, A. L., Llansa, N. D., Mendoza, G., Subbarayan, V., Logothetis, C. J., Newman, R. A., Lippman, S. M., & Menter, D. G. (2005). Suppression of prostate tumor cell growth by stromal cell prostaglandin D synthase-derived products. Cancer Research, 65(14), 6189–6198. https://doi.org/10.1158/0008-5472.CAN-04-4439.
Carpenter, G. (2000). The EGF receptor: a nexus for trafficking and signaling. Bioessays, 22(8), 697–707. https://doi.org/10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1.
Riese 2nd, D. J., & Stern, D. F. (1998). Specificity within the EGF family/ErbB receptor family signaling network. Bioessays, 20(1), 41–48. https://doi.org/10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V.
Krause, D. S., & Van Etten, R. A. (2005). Tyrosine kinases as targets for cancer therapy. The New England Journal of Medicine, 353(2), 172–187. https://doi.org/10.1056/NEJMra044389.
Yarden, Y., & Pines, G. (2012). The ERBB network: at last, cancer therapy meets systems biology. Nature Reviews. Cancer, 12(8), 553–563. https://doi.org/10.1038/nrc3309.
Mochizuki, S., & Okada, Y. (2007). ADAMs in cancer cell proliferation and progression. Cancer Science, 98(5), 621–628. https://doi.org/10.1111/j.1349-7006.2007.00434.x.
Kenny, P. A., & Bissell, M. J. (2007). Targeting TACE-dependent EGFR ligand shedding in breast cancer. The Journal of Clinical Investigation, 117(2), 337–345. https://doi.org/10.1172/JCI29518.
Yoshizumi, M., Kourembanas, S., Temizer, D. H., Cambria, R. P., Quertermous, T., & Lee, M. E. (1992). Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells. The Journal of Biological Chemistry, 267(14), 9467–9469.
Cheng, C. Y., Kuo, C. T., Lin, C. C., Hsieh, H. L., & Yang, C. M. (2010). IL-1beta induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. British Journal of Pharmacology, 160(7), 1595–1610. https://doi.org/10.1111/j.1476-5381.2010.00858.x.
Murthy, A., Defamie, V., Smookler, D. S., Di Grappa, M. A., Horiuchi, K., Federici, M., et al. (2010). Ectodomain shedding of EGFR ligands and TNFR1 dictates hepatocyte apoptosis during fulminant hepatitis in mice. The Journal of Clinical Investigation, 120(8), 2731–2744. https://doi.org/10.1172/JCI42686.
Ellis, P. D., Hadfield, K. M., Pascall, J. C., & Brown, K. D. (2001). Heparin-binding epidermal-growth-factor-like growth factor gene expression is induced by scrape-wounding epithelial cell monolayers: involvement of mitogen-activated protein kinase cascades. The Biochemical Journal, 354(Pt 1), 99–106.
Iwamoto, R., & Mekada, E. (2000). Heparin-binding EGF-like growth factor: a juxtacrine growth factor. Cytokine & Growth Factor Reviews, 11(4), 335–344.
Fu, S., Bottoli, I., Goller, M., & Vogt, P. K. (1999). Heparin-binding epidermal growth factor-like growth factor, a v-Jun target gene, induces oncogenic transformation. Proceedings of the National Academy of Sciences of the United States of America, 96(10), 5716–5721.
Johnson, A. C., Murphy, B. A., Matelis, C. M., Rubinstein, Y., Piebenga, E. C., Akers, L. M., Neta, G., Vinson, C., & Birrer, M. (2000). Activator protein-1 mediates induced but not basal epidermal growth factor receptor gene expression. Molecular Medicine, 6(1), 17–27.
Miyamoto, S., Hirata, M., Yamazaki, A., Kageyama, T., Hasuwa, H., Mizushima, H., Tanaka, Y., Yagi, H., Sonoda, K., Kai, M., Kanoh, H., Nakano, H., & Mekada, E. (2004). Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Research, 64(16), 5720–5727. https://doi.org/10.1158/0008-5472.CAN-04-0811.
Yotsumoto, F., Yagi, H., Suzuki, S. O., Oki, E., Tsujioka, H., Hachisuga, T., Sonoda, K., Kawarabayashi, T., Mekada, E., & Miyamoto, S. (2008). Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochemical and Biophysical Research Communications, 365(3), 555–561. https://doi.org/10.1016/j.bbrc.2007.11.015.
McCarthy, S. A., Samuels, M. L., Pritchard, C. A., Abraham, J. A., & McMahon, M. (1995). Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes & Development, 9(16), 1953–1964.
Svensson, K. J., Kucharzewska, P., Christianson, H. C., Skold, S., Lofstedt, T., Johansson, M. C., et al. (2011). Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 108(32), 13147–13152. https://doi.org/10.1073/pnas.1104261108.
Szalad, A., Katakowski, M., Zheng, X., Jiang, F., & Chopp, M. (2009). Transcription factor Sp1 induces ADAM17 and contributes to tumor cell invasiveness under hypoxia. Journal of Experimental & Clinical Cancer Research, 28, 129. https://doi.org/10.1186/1756-9966-28-129.
Nakai, K., Yoneda, K., Moriue, T., Igarashi, J., Kosaka, H., & Kubota, Y. (2009). HB-EGF-induced VEGF production and eNOS activation depend on both PI3 kinase and MAP kinase in HaCaT cells. Journal of Dermatological Science, 55(3), 170–178. https://doi.org/10.1016/j.jdermsci.2009.06.002.
Sauer, L., Gitenay, D., Vo, C., & Baron, V. T. (2010). Mutant p53 initiates a feedback loop that involves Egr-1/EGF receptor/ERK in prostate cancer cells. Oncogene, 29(18), 2628–2637. https://doi.org/10.1038/onc.2010.24.
Wu, W. K., Sung, J. J., Lee, C. W., Yu, J., & Cho, C. H. (2010). Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Letters, 295(1), 7–16. https://doi.org/10.1016/j.canlet.2010.03.015.
Buchanan, F. G., Gorden, D. L., Matta, P., Shi, Q., Matrisian, L. M., & DuBois, R. N. (2006). Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America, 103(5), 1492–1497. https://doi.org/10.1073/pnas.0510562103.
Pai, R., Soreghan, B., Szabo, I. L., Pavelka, M., Baatar, D., & Tarnawski, A. S. (2002). Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nature Medicine, 8(3), 289–293. https://doi.org/10.1038/nm0302-289.
Yang, C. C., Tu, H. F., Wu, C. H., Chang, H. C., Chiang, W. F., Shih, N. C., Lee, Y. S., Kao, S. Y., & Chang, K. W. (2016). Up-regulation of HB-EGF by the COX-2/PGE2 signaling associates with the cisplatin resistance and tumor recurrence of advanced HNSCC. Oral Oncology, 56, 54–61. https://doi.org/10.1016/j.oraloncology.2016.03.010.
Ohtsu, H., Dempsey, P. J., & Eguchi, S. (2006). ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. American Journal of Physiology. Cell Physiology, 291(1), C1–C10. https://doi.org/10.1152/ajpcell.00620.2005.
Oshima, H., Popivanova, B. K., Oguma, K., Kong, D., Ishikawa, T. O., & Oshima, M. (2011). Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis. Cancer Science, 102(4), 713–719. https://doi.org/10.1111/j.1349-7006.2011.01847.x.
Pai, R., Nakamura, T., Moon, W. S., & Tarnawski, A. S. (2003). Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. The FASEB Journal, 17(12), 1640–1647. https://doi.org/10.1096/fj.02-1011com.
Zhang, X., Chen, Z. G., Choe, M. S., Lin, Y., Sun, S. Y., Wieand, H. S., Shin, H. J., Chen, A., Khuri, F. R., & Shin, D. M. (2005). Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clinical Cancer Research, 11(17), 6261–6269. https://doi.org/10.1158/1078-0432.CCR-04-2102.
Roberts, H. R., Smartt, H. J., Greenhough, A., Moore, A. E., Williams, A. C., & Paraskeva, C. (2011). Colon tumour cells increase PGE(2) by regulating COX-2 and 15-PGDH to promote survival during the microenvironmental stress of glucose deprivation. Carcinogenesis, 32(11), 1741–1747. https://doi.org/10.1093/carcin/bgr210.
Cherukuri, D. P., Chen, X. B., Goulet, A. C., Young, R. N., Han, Y., Heimark, R. L., et al. (2007). The EP4 receptor antagonist, L-161,982, blocks prostaglandin E2-induced signal transduction and cell proliferation in HCA-7 colon cancer cells. Experimental Cell Research, 313(14), 2969–2979. https://doi.org/10.1016/j.yexcr.2007.06.004.
Kurtova, A. V., Xiao, J., Mo, Q., Pazhanisamy, S., Krasnow, R., Lerner, S. P., Chen, F., Roh, T. T., Lay, E., Ho, P. L., & Chan, K. S. (2015). Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature, 517(7533), 209–213. https://doi.org/10.1038/nature14034.
Krausova, M., & Korinek, V. (2014). Wnt signaling in adult intestinal stem cells and cancer. Cellular Signalling, 26(3), 570–579. https://doi.org/10.1016/j.cellsig.2013.11.032.
Castellone, M. D., Teramoto, H., Williams, B. O., Druey, K. M., & Gutkind, J. S. (2005). Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science, 310(5753), 1504–1510. https://doi.org/10.1126/science.1116221.
McGee, J., & Fitzpatrick, F. (1985). Enzymatic hydration of leukotriene A4. Purification and characterization of a novel epoxide hydrolase from human erythrocytes. The Journal of Biological Chemistry, 260(23), 12832–12837.
Dreyling, K. W., Hoppe, U., Peskar, B. A., Morgenroth, K., Kozuschek, W., & Peskar, B. M. (1986). Leukotriene synthesis by human gastrointestinal tissues. Biochimica et Biophysica Acta, 878(2), 184–193.
Hennig, R., Ding, X. Z., Tong, W. G., Schneider, M. B., Standop, J., Friess, H., Büchler, M. W., Pour, P. M., & Adrian, T. E. (2002). 5-Lipoxygenase and leukotriene B(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. The American Journal of Pathology, 161(2), 421–428. https://doi.org/10.1016/S0002-9440(10)64198-3.
Larre, S., Tran, N., Fan, C., Hamadeh, H., Champigneulles, J., Azzouzi, R., et al. (2008). PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins & Other Lipid Mediators, 87(1–4), 14–19. https://doi.org/10.1016/j.prostaglandins.2008.05.001.
Chen, X., Li, N., Wang, S., Wu, N., Hong, J., Jiao, X., Krasna, M. J., Beer, D. G., & Yang, C. S. (2003). Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. Journal of the National Cancer Institute, 95(14), 1053–1061.
Jeong, C. H., Bode, A. M., Pugliese, A., Cho, Y. Y., Kim, H. G., Shim, J. H., Jeon, Y. J., Li, H., Jiang, H., & Dong, Z. (2009). [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Research, 69(13), 5584–5591. https://doi.org/10.1158/0008-5472.CAN-09-0491.
Ohd, J. F., Wikstrom, K., & Sjolander, A. (2000). Leukotrienes induce cell-survival signaling in intestinal epithelial cells. Gastroenterology, 119(4), 1007–1018.
Matsuyama, M., Hayama, T., Funao, K., Kawahito, Y., Sano, H., Takemoto, Y., Nakatani, T., & Yoshimura, R. (2007). Overexpression of cysteinyl LT1 receptor in prostate cancer and CysLT1R antagonist inhibits prostate cancer cell growth through apoptosis. Oncology Reports, 18(1), 99–104.
Braccioni, F., Dorman, S. C., O'Byrne, P. M., Inman, M. D., Denburg, J. A., Parameswaran, K., et al. (2002). The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. The Journal of Allergy and Clinical Immunology, 110(1), 96–101.
Chung, J. W., Kim, G. Y., Mun, Y. C., Ahn, J. Y., Seong, C. M., & Kim, J. H. (2005). Leukotriene B4 pathway regulates the fate of the hematopoietic stem cells. Experimental & Molecular Medicine, 37(1), 45–50. https://doi.org/10.1038/emm.2005.6.
Wada, K., Arita, M., Nakajima, A., Katayama, K., Kudo, C., Kamisaki, Y., & Serhan, C. N. (2006). Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. The FASEB Journal, 20(11), 1785–1792. https://doi.org/10.1096/fj.06-5809com.
Boehmler, A. M., Drost, A., Jaggy, L., Seitz, G., Wiesner, T., Denzlinger, C., Kanz, L., & Mohle, R. (2009). The CysLT1 ligand leukotriene D4 supports alpha4beta1- and alpha5beta1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. Journal of Immunology, 182(11), 6789–6798. https://doi.org/10.4049/jimmunol.0801525.
Modat, G., Muller, A., Mary, A., Gregoire, C., & Bonne, C. (1987). Differential effects of leukotrienes B4 and C4 on bovine aortic endothelial cell proliferation in vitro. Prostaglandins, 33(4), 531–538.
Kim, G. Y., Lee, J. W., Cho, S. H., Seo, J. M., & Kim, J. H. (2009). Role of the low-affinity leukotriene B4 receptor BLT2 in VEGF-induced angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology, 29(6), 915–920. https://doi.org/10.1161/ATVBAHA.109.185793.
Steiner, D. R., Gonzalez, N. C., & Wood, J. G. (2001). Leukotriene B(4) promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J Appl Physiol (1985), 91(3), 1160–1167. https://doi.org/10.1152/jappl.2001.91.3.1160.
Tsopanoglou, N. E., Pipili-Synetos, E., & Maragoudakis, M. E. (1994). Leukotrienes C4 and D4 promote angiogenesis via a receptor-mediated interaction. European Journal of Pharmacology, 258(1–2), 151–154.
Tong, W. G., Ding, X. Z., Talamonti, M. S., Bell, R. H., & Adrian, T. E. (2005). LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochemical and Biophysical Research Communications, 335(3), 949–956. https://doi.org/10.1016/j.bbrc.2005.07.166.
Mezhybovska, M., Wikstrom, K., Ohd, J. F., & Sjolander, A. (2006). The inflammatory mediator leukotriene D4 induces beta-catenin signaling and its association with antiapoptotic Bcl-2 in intestinal epithelial cells. The Journal of Biological Chemistry, 281(10), 6776–6784. https://doi.org/10.1074/jbc.M509999200.
Ihara, A., Wada, K., Yoneda, M., Fujisawa, N., Takahashi, H., & Nakajima, A. (2007). Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. Journal of Pharmacological Sciences, 103(1), 24–32.
Yoo, M. H., Song, H., Woo, C. H., Kim, H., & Kim, J. H. (2004). Role of the BLT2, a leukotriene B4 receptor, in Ras transformation. Oncogene, 23(57), 9259–9268. https://doi.org/10.1038/sj.onc.1208151.
Paruchuri, S., Hallberg, B., Juhas, M., Larsson, C., & Sjolander, A. (2002). Leukotriene D(4) activates MAPK through a Ras-independent but PKCepsilon-dependent pathway in intestinal epithelial cells. Journal of Cell Science, 115(Pt 9), 1883–1893.
Paruchuri, S., Broom, O., Dib, K., & Sjolander, A. (2005). The pro-inflammatory mediator leukotriene D4 induces phosphatidylinositol 3-kinase and Rac-dependent migration of intestinal epithelial cells. The Journal of Biological Chemistry, 280(14), 13538–13544. https://doi.org/10.1074/jbc.M409811200.
Choe, M. S., Zhang, X., Shin, H. J., Shin, D. M., & Chen, Z. G. (2005). Interaction between epidermal growth factor receptor- and cyclooxygenase 2-mediated pathways and its implications for the chemoprevention of head and neck cancer. Molecular Cancer Therapeutics, 4(9), 1448–1455. https://doi.org/10.1158/1535-7163.MCT-04-0251.
Mendelsohn, J., & Baselga, J. (2006). Epidermal growth factor receptor targeting in cancer. Seminars in Oncology, 33(4), 369–385. https://doi.org/10.1053/j.seminoncol.2006.04.003.
Oxnard, G. R., Arcila, M. E., Chmielecki, J., Ladanyi, M., Miller, V. A., & Pao, W. (2011). New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clinical Cancer Research, 17(17), 5530–5537. https://doi.org/10.1158/1078-0432.CCR-10-2571.
Wu, J. Y., Yu, C. J., Chang, Y. C., Yang, C. H., Shih, J. Y., & Yang, P. C. (2011). Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clinical Cancer Research, 17(11), 3812–3821. https://doi.org/10.1158/1078-0432.CCR-10-3408.
Laurent-Puig, P., Cayre, A., Manceau, G., Buc, E., Bachet, J. B., Lecomte, T., Rougier, P., Lievre, A., Landi, B., Boige, V., Ducreux, M., Ychou, M., Bibeau, F., Bouché, O., Reid, J., Stone, S., & Penault-Llorca, F. (2009). Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. Journal of Clinical Oncology, 27(35), 5924–5930. https://doi.org/10.1200/JCO.2008.21.6796.
Sartore-Bianchi, A., Di Nicolantonio, F., Nichelatti, M., Molinari, F., De Dosso, S., Saletti, P., et al. (2009). Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One, 4(10), e7287. https://doi.org/10.1371/journal.pone.0007287.
Lu, Y., Shi, C., Qiu, S., & Fan, Z. (2016). Identification and validation of COX-2 as a co-target for overcoming cetuximab resistance in colorectal cancer cells. Oncotarget, 7(40), 64766–64777. https://doi.org/10.18632/oncotarget.8649.
Rothwell, P. M., Wilson, M., Elwin, C. E., Norrving, B., Algra, A., Warlow, C. P., & Meade, T. W. (2010). Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet, 376(9754), 1741–1750. https://doi.org/10.1016/S0140-6736(10)61543-7.
Burn, J., Bishop, D. T., Chapman, P. D., Elliott, F., Bertario, L., Dunlop, M. G., Eccles, D., Ellis, A., Evans, D. G., Fodde, R., Maher, E. R., Moslein, G., Vasen, H. F. A., Coaker, J., Phillips, R. K. S., Bulow, S., Mathers, J. C., & for the International CAPP consortium. (2011). A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prevention Research (Philadelphia, Pa.), 4(5), 655–665. https://doi.org/10.1158/1940-6207.CAPR-11-0106.
Kim, B., & Giardiello, F. M. (2011). Chemoprevention in familial adenomatous polyposis. Best Practice & Research. Clinical Gastroenterology, 25(4–5), 607–622. https://doi.org/10.1016/j.bpg.2011.08.002.
Oshima, M., Dinchuk, J. E., Kargman, S. L., Oshima, H., Hancock, B., Kwong, E., Trzaskos, J. M., Evans, J. F., & Taketo, M. M. (1996). Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell, 87(5), 803–809.
Gupta, G. P., Nguyen, D. X., Chiang, A. C., Bos, P. D., Kim, J. Y., Nadal, C., Gomis, R. R., Manova-Todorova, K., & Massagué, J. (2007). Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature, 446(7137), 765–770. https://doi.org/10.1038/nature05760.
Limasale, Y. D., Tezcaner, A., Ozen, C., Keskin, D., & Banerjee, S. (2015). Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. International Journal of Pharmaceutics, 479(2), 364–373. https://doi.org/10.1016/j.ijpharm.2015.01.016.
Banu, N., Buda, A., Chell, S., Elder, D., Moorghen, M., Paraskeva, C., Qualtrough, D., & Pignatelli, M. (2007). Inhibition of COX-2 with NS-398 decreases colon cancer cell motility through blocking epidermal growth factor receptor transactivation: possibilities for combination therapy. Cell Proliferation, 40(5), 768–779. https://doi.org/10.1111/j.1365-2184.2007.00459.x.
Dittmann, K. H., Mayer, C., Ohneseit, P. A., Raju, U., Andratschke, N. H., Milas, L., & Rodemann, H. P. (2008). Celecoxib induced tumor cell radiosensitization by inhibiting radiation induced nuclear EGFR transport and DNA-repair: a COX-2 independent mechanism. International Journal of Radiation Oncology, Biology, Physics, 70(1), 203–212. https://doi.org/10.1016/j.ijrobp.2007.08.065.
Chen, L., He, Y., Huang, H., Liao, H., & Wei, W. (2008). Selective COX-2 inhibitor celecoxib combined with EGFR-TKI ZD1839 on non-small cell lung cancer cell lines: in vitro toxicity and mechanism study. Medical Oncology, 25(2), 161–171. https://doi.org/10.1007/s12032-007-9015-1.
Kim, J., Kim, N., Park, J. H., Chang, H., Kim, J. Y., Lee, D. H., Kim, J. M., Kim, J. S., & Jung, H. C. (2013). The effect of helicobacter pylori on epidermal growth factor receptor-induced signal transduction and the preventive effect of celecoxib in gastric cancer cells. Gut Liver, 7(5), 552–559. https://doi.org/10.5009/gnl.2013.7.5.552.
Qian, M., Qian, D., Jing, H., Li, Y., Ma, C., & Zhou, Y. (2014). Combined cetuximab and celecoxib treatment exhibits a synergistic anticancer effect on human oral squamous cell carcinoma in vitro and in vivo. Oncology Reports, 32, 1681–1688. https://doi.org/10.3892/or.2014.3334.
Li, N., Li, H., Su, F., Li, J., Ma, X., & Gong, P. (2015). Relationship between epidermal growth factor receptor (EGFR) mutation and serum cyclooxygenase-2 level, and the synergistic effect of celecoxib and gefitinib on EGFR expression in non-small cell lung cancer cells. International Journal of Clinical and Experimental Pathology, 8(8), 9010–9020.
Valverde, A., Penarando, J., Canas, A., Lopez-Sanchez, L. M., Conde, F., Hernandez, V., et al. (2015). Simultaneous inhibition of EGFR/VEGFR and cyclooxygenase-2 targets stemness-related pathways in colorectal cancer cells. PLoS One, 10(6), e0131363. https://doi.org/10.1371/journal.pone.0131363.
Jalili, A., Pinc, A., Pieczkowski, F., Karlhofer, F. M., Stingl, G., & Wagner, S. N. (2008). Combination of an EGFR blocker and a COX-2 inhibitor for the treatment of advanced cutaneous squamous cell carcinoma. Journal der Deutschen Dermatologischen Gesellschaft, 6(12), 1066–1069. https://doi.org/10.1111/j.1610-0387.2008.06861.x.
Kao, J., Genden, E. M., Chen, C. T., Rivera, M., Tong, C. C., Misiukiewicz, K., et al. (2011). Phase 1 trial of concurrent erlotinib, celecoxib, and reirradiation for recurrent head and neck cancer. Cancer, 117(14), 3173–3181. https://doi.org/10.1002/cncr.25786.
Fu, S., Rivera, M., Ko, E. C., Sikora, A. G., Chen, C. T., Vu, H. L., et al. (2011). Combined inhibition of epidermal growth factor receptor and cyclooxygenase-2 as a novel approach to enhance radiotherapy. Journal of Cell Science and Therapy, 1(2).
Shin, D. M., Zhang, H., Saba, N. F., Chen, A. Y., Nannapaneni, S., Amin, A. R., et al. (2013). Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: preclinical and clinical studies. Clinical Cancer Research, 19(5), 1244–1256. https://doi.org/10.1158/1078-0432.CCR-12-3149.
Reckamp, K. L., Koczywas, M., Cristea, M. C., Dowell, J. E., Wang, H. J., Gardner, B. K., Milne, G. L., Figlin, R. A., Fishbein, M. C., Elashoff, R. M., & Dubinett, S. M. (2015). Randomized phase 2 trial of erlotinib in combination with high-dose celecoxib or placebo in patients with advanced non-small cell lung cancer. Cancer, 121(18), 3298–3306. https://doi.org/10.1002/cncr.29480.
Kearney, P. M., Baigent, C., Godwin, J., Halls, H., Emberson, J. R., & Patrono, C. (2006). Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ, 332(7553), 1302–1308. https://doi.org/10.1136/bmj.332.7553.1302.
Gomez Cerezo, J., Lubomirov Hristov, R., Carcas Sansuan, A. J., & Vazquez Rodriguez, J. J. (2003). Outcome trials of COX-2 selective inhibitors: global safety evaluation does not promise benefits. European Journal of Clinical Pharmacology, 59(2), 169–175. https://doi.org/10.1007/s00228-003-0579-1.
Mukherjee, D., Nissen, S. E., & Topol, E. J. (2001). Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA, 286(8), 954–959.
Pratico, D., & Dogne, J. M. (2009). Vascular biology of eicosanoids and atherogenesis. Expert Review of Cardiovascular Therapy, 7(9), 1079–1089. https://doi.org/10.1586/erc.09.91.
Iwamoto, R., & Mekada, E. (2006). ErbB and HB-EGF signaling in heart development and function. Cell Structure and Function, 31(1), 1–14.
Nanba, D., Mammoto, A., Hashimoto, K., & Higashiyama, S. (2003). Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. The Journal of Cell Biology, 163(3), 489–502. https://doi.org/10.1083/jcb.200303017.
Kinugasa, Y., Hieda, M., Hori, M., & Higashiyama, S. (2007). The carboxyl-terminal fragment of pro-HB-EGF reverses Bcl6-mediated gene repression. The Journal of Biological Chemistry, 282(20), 14797–14806. https://doi.org/10.1074/jbc.M611036200.
Yeyati, P. L., Shaknovich, R., Boterashvili, S., Li, J., Ball, H. J., Waxman, S., Nason-Burchenal, K., Dmitrovsky, E., Zelent, A., & Licht, J. D. (1999). Leukemia translocation protein PLZF inhibits cell growth and expression of cyclin A. Oncogene, 18(4), 925–934. https://doi.org/10.1038/sj.onc.1202375.
Barna, M., Merghoub, T., Costoya, J. A., Ruggero, D., Branford, M., Bergia, A., Samori, B., & Pandolfi, P. P. (2002). Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Developmental Cell, 3(4), 499–510.
McConnell, M. J., Chevallier, N., Berkofsky-Fessler, W., Giltnane, J. M., Malani, R. B., Staudt, L. M., & Licht, J. D. (2003). Growth suppression by acute promyelocytic leukemia-associated protein PLZF is mediated by repression of c-myc expression. Molecular and Cellular Biology, 23(24), 9375–9388.
Shaffer, A. L., Yu, X., He, Y., Boldrick, J., Chan, E. P., & Staudt, L. M. (2000). BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity, 13(2), 199–212.
Yoshida, T., Fukuda, T., Hatano, M., Koseki, H., Okabe, S., Ishibashi, K., Kojima, S., Arima, M., Komuro, I., Ishii, G., Miki, T., Hirosawa, S., Miyasaka, N., Taniguchi, M., Ochiai, T., Isono, K., & Tokuhisa, T. (1999). The role of Bcl6 in mature cardiac myocytes. Cardiovascular Research, 42(3), 670–679.
Senbonmatsu, T., Saito, T., Landon, E. J., Watanabe, O., Price Jr., E., Roberts, R. L., et al. (2003). A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. The EMBO Journal, 22(24), 6471–6482. https://doi.org/10.1093/emboj/cdg637.
Cook, M., Gould, A., Brand, N., Davies, J., Strutt, P., Shaknovich, R., Licht, J., Waxman, S., Chen, Z., & Gluecksohn-Waelsch, S. (1995). Expression of the zinc-finger gene PLZF at rhombomere boundaries in the vertebrate hindbrain. Proceedings of the National Academy of Sciences of the United States of America, 92(6), 2249–2253.
Yamazaki, S., Iwamoto, R., Saeki, K., Asakura, M., Takashima, S., Yamazaki, A., Kimura, R., Mizushima, H., Moribe, H., Higashiyama, S., Endoh, M., Kaneda, Y., Takagi, S., Itami, S., Takeda, N., Yamada, G., & Mekada, E. (2003). Mice with defects in HB-EGF ectodomain shedding show severe developmental abnormalities. The Journal of Cell Biology, 163(3), 469–475. https://doi.org/10.1083/jcb.200307035.
Iwamoto, R., Yamazaki, S., Asakura, M., Takashima, S., Hasuwa, H., Miyado, K., Adachi, S., Kitakaze, M., Hashimoto, K., Raab, G., Nanba, D., Higashiyama, S., Hori, M., Klagsbrun, M., & Mekada, E. (2003). Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proceedings of the National Academy of Sciences of the United States of America, 100(6), 3221–3226. https://doi.org/10.1073/pnas.0537588100.
Gann, P. H., Manson, J. E., Glynn, R. J., Buring, J. E., & Hennekens, C. H. (1993). Low-dose aspirin and incidence of colorectal tumors in a randomized trial. Journal of the National Cancer Institute, 85(15), 1220–1224.
Baron, J. A., Cole, B. F., Sandler, R. S., Haile, R. W., Ahnen, D., Bresalier, R., McKeown-Eyssen, G., Summers, R. W., Rothstein, R., Burke, C. A., Snover, D. C., Church, T. R., Allen, J. I., Beach, M., Beck, G. J., Bond, J. H., Byers, T., Greenberg, E. R., Mandel, J. S., Marcon, N., Mott, L. A., Pearson, L., Saibil, F., & van Stolk, R. U. (2003). A randomized trial of aspirin to prevent colorectal adenomas. The New England Journal of Medicine, 348(10), 891–899. https://doi.org/10.1056/NEJMoa021735.
Benamouzig, R., Deyra, J., Martin, A., Girard, B., Jullian, E., Piednoir, B., Couturier, D., Coste, T., Little, J., & Chaussade, S. (2003). Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology, 125(2), 328–336.
Liao, X., Lochhead, P., Nishihara, R., Morikawa, T., Kuchiba, A., Yamauchi, M., Imamura, Y., Qian, Z. R., Baba, Y., Shima, K., Sun, R., Nosho, K., Meyerhardt, J. A., Giovannucci, E., Fuchs, C. S., Chan, A. T., & Ogino, S. (2012). Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. The New England Journal of Medicine, 367(17), 1596–1606. https://doi.org/10.1056/NEJMoa1207756.
Tougeron, D., Sha, D., Manthravadi, S., & Sinicrope, F. A. (2014). Aspirin and colorectal cancer: back to the future. Clinical Cancer Research, 20(5), 1087–1094. https://doi.org/10.1158/1078-0432.CCR-13-2563.
Liska, F., Mancini, M., Krupkova, M., Chylikova, B., Krenova, D., Seda, O., et al. (2014). Plzf as a candidate gene predisposing the spontaneously hypertensive rat to hypertension, left ventricular hypertrophy, and interstitial fibrosis. American Journal of Hypertension, 27(1), 99–106. https://doi.org/10.1093/ajh/hpt156.
Thomas, C. M., Yong, Q. C., Seqqat, R., Chandel, N., Feldman, D. L., Baker, K. M., & Kumar, R. (2013). Direct renin inhibition prevents cardiac dysfunction in a diabetic mouse model: comparison with an angiotensin receptor antagonist and angiotensin-converting enzyme inhibitor. Clinical Science (London, England), 124(8), 529–541. https://doi.org/10.1042/CS20120448.
Funding
This work was supported by grants MOST-105-2314-B-010-029-MY3 from the Ministry of Science and Technology and MOHW106-TDU-B-211-113001 from the Ministry of Health and Welfare of Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, CC., Chang, KW. Eicosanoids and HB-EGF/EGFR in cancer. Cancer Metastasis Rev 37, 385–395 (2018). https://doi.org/10.1007/s10555-018-9746-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-018-9746-9