Abstract
Purpose
Vaginal oestrogens can be used to treat genitourinary symptoms in women with early breast cancer. Studies evaluating vaginal oestrogens have commonly measured serum oestrogen levels as a surrogate marker of safety, but methods vary. We sought to summarise the data on serum oestrogen measurement in women with breast cancer using vaginal oestrogens to better understand the methods, levels and reliability.
Methods
We searched Medline, Embase, CENTRAL, SCOPUS and CINAHL from inception to October 2023 for clinical studies where serum oestrogen was measured in women with a history of early breast cancer using vaginal oestrogens. Studies with a reported testing methodology were included.
Results
Nine studies met the inclusion criteria for this systematic review. Methods used to measure oestradiol and oestriol in selected studies included mass spectrometry and immunoassays; several studies used more than one with variable concordance. Mass spectrometry detected oestradiol levels down to a lower limit between 1.0 pg/mL and 3.0 pg/mL. Immunoassays such as ELISA (enzyme-linked immunosorbent assay), ECLIA (enhanced chemiluminiscence immunoassay) and RIA (radioimmunoassay) had lower detection limits ranging between 0.8 pg/mL and 10 pg/mL. Studies were heterogeneous in testing techniques used, timing of testing, and the population including with subsequent varying results in the effect on oestrogens reported.
Conclusions
Adopting consistent and standardised methods of measuring oestrogens in clinical trials involving women with early breast cancer on vaginal oestrogens is critical. Serum oestrogens are used as a surrogate marker of safety in this population, and good-quality data are necessary to enable clinicians and patients to feel confident in prescribing and taking vaginal oestrogens. Mass spectrometry, although more expensive, gives more reliable results when dealing with very low levels of oestrogens often found in women on aromatase inhibitors, compared to immunoassays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genitourinary Syndrome of Menopause (GSM) or vulvovaginal atrophy is reported in up to 75% of women after breast cancer (BC) [1]. Symptoms are more prevalent in women with BC compared to peers without [2, 3]. Chemotherapy, treatment-induced early and rapid onset of menopause and endocrine therapy can all contribute to GSM. Changes in genital tissues, with decreasing blood flow and secretions, increased pH, epithelial thinning and loss of elasticity occur from chronic oestrogen depletion [4]. GSM can negatively impact sexual function and health-related quality of life [5], presenting as follows: vaginal dryness; vaginal itch or burning; dysuria; urinary urgency; urinary incontinence; dyspareunia; loss of libido; dysfunction of arousal and orgasm; and increased urinary tract infections [4, 6].
Treatments for GSM include vaginal moisturisers and lubricants, oral or transdermal hormone replacement therapy, vaginal oestrogen therapies, and vaginal laser [7]. For women with a history of BC, particularly hormone receptor-positive (HR+) early breast cancer (EBC), systemic hormone-based therapies are not recommended due to concerns they increase BC recurrences by stimulating tumour growth [4]. However, vaginal oestrogens are considered safe for women with BC based on several studies showing no increased risk of de novo BC, [8,9,10] or recurrences [11,12,13]. International guidelines support use of vaginal oestrogens in women with BC, when non-hormonal interventions are unsuccessful in treating symptoms [14, 15]. Cold et al.’s cohort study [16] has renewed debate regarding vaginal oestrogen safety. Overall, there was no increased BC recurrences or mortality in women with EBC using vaginal oestrogens, but a subgroup analysis showed increased recurrences in women on aromatase inhibitors (AI) but not in women on tamoxifen [16], which may lead to some preferences for tamoxifen as a result.
To definitively establish safety, a large, prospective, randomised trial of vaginal oestrogens in women with BC assessing recurrences and mortality is required. Such a trial would require thousands of women and many years of follow-up, making it is unlikely to be conducted. Instead, measurement of serum oestrogen levels in women using vaginal oestrogens has been a key method of assessing safety.
The primary oestrogens are oestrone (E1), oestradiol (E2) and oestriol (E3). Available vaginal oestrogen preparations contain oestradiol and oestriol, so measurement of these oestrogens in the serum is of greatest interest. Oestradiol is the most frequently tested oestrogen. Oestriol is less potent and shorter acting than oestradiol and cannot be converted back into oestradiol [17, 18]. The problem with measuring oestrogen levels is the lack of clearly defined safe levels of serum oestrogen for women with BC. The normal range for median oestradiol in postmenopausal women ranges from 5 to 27 pg/ml [19, 20]. For women on AIs, which suppress oestradiol by up to 99% [20], serum oestradiol levels are typically lower with mean levels reported between 2 and 10 pg/ml [19, 20], and with ultrasensitive testing median oestradiol levels as low as < 0.1 pg/ml [20]. Previous studies used multiple methodologies for measuring oestrogens with varying sensitivity and reliability, making interpretation challenging. For example, results from pooled individual patient data from nine prospective case–control studies revealed an increased relative risk of BC recurrence of 1.29 (95% CI, 1.15–1.44, p < 0.001) for every doubling of oestradiol (E2) concentration [21]. One study defined a prolonged rise of oestradiol as > 10 pg/mL and > 10 pg/mL above baseline on 2 consecutive blood tests > 2 weeks apart [22].
Any method used to measure serum oestrogens in clinical trials and practice must meet these criteria: ability to detect very low levels; high specificity; and reproducibility [23]. Commercially available oestradiol testing is often unable to detect very low levels of oestradiol (E2) induced by AI. Mass spectrometry (liquid chromatography mass spectrometry [LC-MS] or gas chromatography mass spectrometry [GC-MS]) and immunoassays have been used in clinical trials, but do not always show concordance [24]. Mass spectrometry appears more accurate at detecting low levels of oestrogens [20, 23, 25]. However, access and cost prohibit use outside clinical trials.
Our systematic review aimed to (i) identify methods used to test oestradiol, oestriol and other hormones; (ii) summarise E2 and E3 levels reported; (iii) assess the quality of reported assays, in women with HR+ EBC using vaginal oestrogens.
Methods
Our systematic review was conducted according to PRISMA guidelines [26]. Searches were conducted in October 2023 using five databases: Medline, Embase, CENTRAL (Cochrane Register of Controlled Trials), SCOPUS, and CINAHL (Cumulative Index to Nursing and Allied Health Literature). Table 3 shows search terms used. Reference lists of relevant studies were hand searched and a Google Scholar search performed to identify additional studies.
Eligible studies included randomised controlled trials (RCTs) and other clinical intervention studies. Study inclusion criteria were postmenopausal women with a history of HR+ EBC receiving any form of vaginal oestrogen treatment. To be included, oestradiol and/or oestriol had to be measured and techniques and testing parameters reported. Studies involving systemic (oral or transdermal) oestrogen treatments were excluded. Conference abstracts were permitted where required information was accessible.
Following database searches, studies were imported to Covidence [27], and duplicates identified. Two authors (AP; JC) independently screened titles and abstracts to determine eligibility. Full manuscripts were independently reviewed by both authors, with disagreements reviewed by BK.
Data extracted included design, duration, inclusion criteria, menopausal status and age, use and type of endocrine therapy, type of vaginal oestrogen (including dose, frequency, duration), control group, sample size, oestrogen testing timepoints, oestrogens measured (for each: level, testing method, unit of measurement, detection limits, normal range), other sex hormones measured. Study quality and risk of bias were to be assessed if possible.
Results
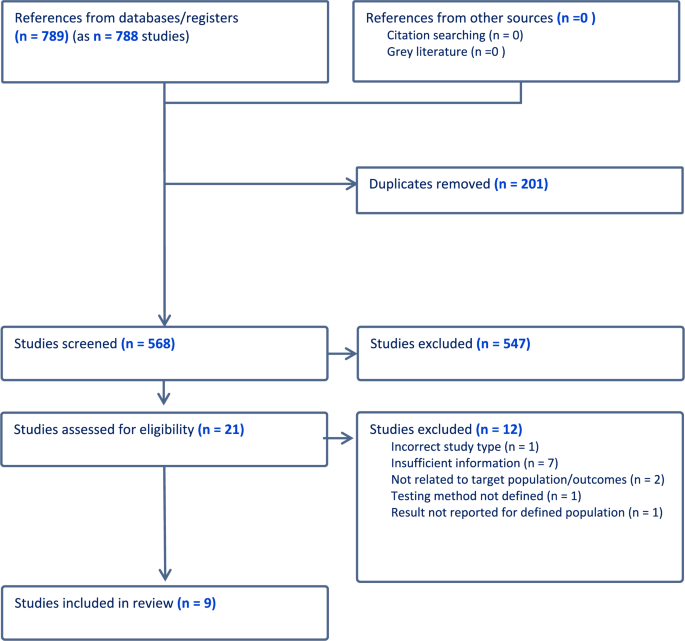
The PRISMA flow diagram (Fig. 1) depicts 789 citations identified, of which 201 were duplicates and 547 excluded on title and abstract screening. After full-text review, nine studies [22, 28,29,30,31,32,33,34,35,36] met the inclusion criteria (See Table 1). Of these, three were RCTs, two case–control studies, and four single-arm studies. Oestrogen testing methodology is outlined in Table 2.
Methods used to measure oestradiol and oestriol in selected studies included mass spectrometry and immunoassays; several studies used more than one with variable concordance [22, 33]. Mass spectrometry detected oestradiol levels down to a lower limit between 1.0 pg/mL and 3.0 pg/mL [22, 29, 33,34,35,36]. Immunoassays such as ELISA (enzyme-linked immunosorbent assay), ECLIA (enhanced chemiluminiscence immunoassay) and RIA (radioimmunoassay) had lower detection limits ranging between 0.8 pg/mL and 10 pg/mL [22, 28, 30, 32,33,34, 36].
The highest quality and largest study was Sanchez-Rovira et al.’s trial of vaginal oestriol [35]. Sixty-one women with HR+ EBC taking an AI for > 6 months were randomised to a 0.005% oestriol gel (50mcg oestriol per application) or placebo moisturising gel daily for 3 weeks then twice weekly for 9 weeks. Serum oestrogen levels were measured at baseline, 3, 8, and 12 weeks via ultrasensitive LC-MS (detection limit of 3 pg/mL for oestradiol and 1 pg/mL for oestriol) [37]. There was no significant rise in E2 detected. A small rise in E3 was detected in the treatment group in the first 3 weeks returning to same level as the placebo group by 12 weeks (median, [interquartile range IQR], E3 = 0.5 pg/mL [0.5 to 7.3] in the treatment group and 0.5 pg/mL [0.5 to 0.5] placebo group, p = 0.140). Oestrone, follicle stimulating hormone (FSH) and luteinising hormone (LH) had no significant changes detected at any timepoint.
The REVIVE study [33], an ongoing open-label RCT, reported serum hormone levels for the first eight participants. This study compares a vaginal oestradiol ring (Estring®) which releases approximately 7.5mcg oestradiol daily for 90 days to a polycarbophil-based vaginal moisturiser (Replens ™) in women with HR+ EBC taking an AI. Two different ELISA kits were used to quantify oestradiol (E2) with a detection limit of 3 pg/mL. There was concordance between two ELISA kit measurements for 5/6 patients, but one had different results: E2 < 3 pg/mL versus E2 67 pg/mL. Samples from three patients were analysed using LC-MS/MS with a detection limit of 1 pg/mL. The values of E2 between different testing methods (ELISA and LC-MS/MS) varied markedly. One patient had higher readings of E2 on both ELISA kits compared to LC-MS/MS, and the degree of elevation varied between the two kits. Another patient had elevation in E2 at 2 weeks on LC-MS/MS, not detected on ELISA. The third patient was taking exemestane, an AI known to cause aberrant results via immunoassays due to cross-reactivity [20, 38]. Her results showed the anticipated rise in E2 on ELISA, but corresponding LC-MS/MS testing showed an elevated level of E2 at baseline but levels < 2 pg/mL at all other timepoints, attributed to a laboratory error. Although the RCT design is sound, this sub-study is fraught with issues: small sample size (N = 8), heterogeneity of testing methodologies, and discordant results.
Melisko et al. [22] conducted a randomised open-label phase 2 study comparing vaginal testosterone cream to a vaginal oestradiol ring (Estring®) in 76 women with HR + EBC on AI for > 30 days. The vaginal testosterone cream was used daily for 2 weeks then three times per week for 10 weeks for the first 24 participants then changed to three times per week for the 12-week study duration for the subsequent 12 participants. Both RIA and ultrasensitive LC-MS methods were used to measure oestradiol (E2) with a detection limit of 3 pmol/L (0.82 pg/mL) for RIA and no reported detection limit for LC-MS. The authors predefined < 10 pg/mL as the expected postmenopausal level of oestradiol, and the primary safety endpoint was defined as < 25% of participants having a persistent elevation of E2 (> 10 pg/mL and at least > 10 pg/mL above baseline on two consecutive blood tests more than two weeks apart) and was met for both arms in this study. 63 participants had E2 measured with both methods and surprisingly of these, 25 (40%) had baseline E2 above the 10 pg/mL threshold with LC-MS and 9 (14%) with RIA. In the oestradiol ring arm, 4/35 participants had a transient rise in E2 (range 11–29 pg/mL) but none had persistent elevation. No differences were detected in mean E2 levels between LC-MS and RIA at baseline (17.7 [SD 28.5] pg/mL and 17.9 [SD 44.1] pg/mL, respectively) or at week 4 (7.8 [SD 15.0] pg/mL and 2.9 [SD 13.4] pg/mL, respectively). There was some variability of reported E2 levels between RIA and LC-MS on review of matched samples, but discordant E2 elevation was rare (2/63 participants with matched samples). Similar to REVIVE [33], Melisko et al. found that LC-MS was more likely than RIA to detect an early rise in E2, 19% of participants had elevated E2 at 4 weeks with LC–MS vs. 1.5% with RIA. This study had a reasonable sample size, with most patients (63/76) having matched samples, allowing greater reliability of concordance assessment for testing methods. Although very discordant results were rare, there was still variability and RIA failed to detect early rises in E2 which were detected with LC-MS.
A prospective, open-label, single-arm study by Kendall et al. which showed a persistent rise in oestradiol (E2) levels, [32] is often used as a basis for concern regarding safety of vaginal oestrogens. In this study, six women with HR+ EBC on AI for > 6 months used a 25mcg vaginal oestradiol pessary (Vagifem®) daily for two weeks, then twice weekly for 10 weeks. Oestradiol (E2) was measured using RIA with a detection limit of 3 pmol/L (0.82 pg/mL). One participant on exemestane had high baseline levels of E2 attributed to cross-reactivity with immunoassays [38]. E2 levels increased at two weeks in 5/6 participants (from median 0.82 pg/mL at baseline to 19.61 pg/mL) and decreasing to median 4.36 pg/mL at 4 weeks and stayed at this level for most participants. However, two participants had persistently raised E2 when tested between 7 and 10 weeks (37.32 pg/mL and 59.65 pg/mL), one of whom was on exemestane. This study has several limitations including a very small sample size, no randomisation, heterogeneity in testing schedules, and variable reported oestrogen levels. The study also used a 25mcg oestradiol pessary, which has been subsequently replaced by a lower dose 10mcg preparation (Vagifem Low®).
Donders et al. [29] enrolled 16 women with HR+ EBC on AI for > 6 months to take a vaginal tablet containing 100 million acidophilus KS400 and 0.03 mg oestriol (Gynoflor®) daily for 4 weeks, then three times per week for 8 weeks. The authors used a highly sensitive GC-MS to quantify oestradiol (E2) and oestriol (E3) with a detection limit of 1 pg/mL and 10 pg/mL, respectively. As expected, there was no rise in E2 (as oestriol does not back convert to oestradiol [17, 18]), but there was a small transient rise in E3, most pronounced after the first dose. Oestrone, LH, FSH and sex hormone-binding globulin (SBHG) were tested, with no change detected except in FSH which had a small but significant decrease at 4 weeks. Again, this study is limited by a very small sample size and its single-arm design.
A short study by Pfeiler et al. [34] assessed use of oestriol 0.5 mg pessaries (Ovestin®) daily for two weeks in 10 women with HR + EBC on anastrozole. Oestradiol (E2) was quantified at baseline and 2 weeks using both ECLIA (detection limit of 10 pg/mL) and GC-MS (unreported detection limit). No significant change was reported for E2 or E3, but mean FSH and LH levels decreased significantly from baseline to 2 weeks (LH − 10.8%, p = 0.02; FSH − 12.8%, p = 0.01). This is a very small, single-arm study of two-week duration; thus, the duration of effect on FSH and LH remains unknown. Both ECLIA and GC-MS were performed, but detection limits were much higher for ECLIA than in other studies, so small rises in E2 could have been missed.
Biglia et al.’s [28] non-randomised three-arm study compared 0.25 mg oestriol cream, 12.5mcg oestradiol pessaries (Vagifem®), and a vaginal moisturiser (Replens™) in 26 women with HR + EBC on endocrine treatment. Unlike other studies reviewed, women on AI were not permitted in the vaginal oestrogen groups (but permitted in moisturiser group). RIA was used to quantify oestradiol (E2) with a detection limit of 5 pg/mL. No significant difference was found in E2 levels from baseline to week 12 or additionally, in E3, E1, LH, FSH, testosterone and SHBG. Although there were no reported issues with E2 RIA testing, the lower detection limit of 5 pg/mL is not as sensitive as methods used in other studies. Given most patients were not on an AI, the impact of small rises in oestradiol is less concerning.
Wills et al.’s prospective case–control study enrolled 48 women with HR+ EBC or an increased risk of developing BC (all on endocrine therapy) and compared cases of those on vaginal oestrogens for > 3 months (25mcg oestradiol pessary twice weekly or vaginal oestradiol ring inserted every 90 days), with a no vaginal oestrogen control group [30]. Oestradiol (E2) levels were measured using RIA with a detection limit of 3 pmol/L (0.82 pg/mL). For women using oestrogen pessaries for > 3 months, pre-insertion levels of E2 were not elevated compared to controls, although 12 h post-insertion E2 levels were raised. For those using the oestrogen ring for > 3 months, pre-insertion mean E2 levels were already elevated compared to controls suggesting a persistent elevation in E2 in this group. This study implemented a novel approach testing oestrogen levels pre- and post-insertion in women who had used vaginal oestrogens for > 3 months, with the intent of capturing whether persistent elevation in oestrogens occurred before insertion and after.
Streff et al. conducted a prospective study of vaginal oestradiol rings (Estring®) in women with HR+ EBC on AI [36]. This study included 8 prospective participants and 6 retrospective participants who had oestradiol (E2) quantification via tandem mass spectrometry or ECLIA with a variety of laboratories and reference ranges. Baseline E2 levels were in the expected range, but after commencing the oestradiol ring, 6/8 prospective participants had a transient rise in E2 (at week 4) which returned to baseline levels by week 16. The study quality was low due to small sample size, heterogeneous testing methods and no clear definition of the detection limits used.
Due to the heterogeneity of studies included in this systematic review, a formal bias assessment was not possible.
Discussion
We identified a variety of testing methodologies used to quantify serum oestradiol (E2) and oestriol (E3) from included studies. Some used multiple techniques with mixed concordance across testing methods. Overall, the quality of studies was poor with considerable heterogeneity of testing techniques, timing and populations. There was also a mixture of concordant and discordant results when more than one methodology was used. This creates a confusing landscape to determine the impact vaginal oestrogens may have on oestrogen levels in women with BC. In addition, the impact of small, and often transient elevations in serum oestrogen levels on outcomes of women with BC remains unknown.
Folkerd et al. [39] cautioned against false interpretations from studies testing serum oestrogens with potentially erroneous quantification methods. The REVIVE study highlights challenges of using different testing methodologies for measurement of low levels of oestradiol (E2), with discordant oestrogen levels between methods [33].
Immunoassay methodologies (RIA, ECLIA, ELISA) can detect low levels of oestrogens, but are prone to inaccuracies [39], and cannot detect levels as low as mass spectrometry. Caution is required for patients taking exemestane, an AI known to falsely elevate oestradiol levels when immunoassays are used [38]. Immunoassays appear less likely to detect early rises in oestradiol levels in women using vaginal oestrogens compared to mass spectrometry [22, 33]. A recent study of women with EBC on letrozole showed no concordance between oestrogen levels measured with immunoassays compared to mass spectrometry [40]. Given multiple confounding issues with results from sensitive immunoassays, mass spectrometry appears the most reliable testing methodology in women with BC on vaginal oestrogens, particularly those on AI.
Vaginal oestrogen formulations varied considerably between included studies. Kendall et al. [32] and Wills et al. [30] used 25mcg oestradiol tablets (Vagifem®), replaced by lower dosage 10mcg formulation (Vagifem® Low), which remains effective for symptom relief with less systemic absorption [41, 42]. Oestradiol is also the oestrogen used in the ring (Estring®), whereas the other formulations were oestriol based, a less potent oestrogen which cannot be converted to oestradiol [17, 18].
Oestrogen levels increase after initiation of vaginal oestrogens due to a denuded vaginal epithelium, tapering over time as the epithelium recovers and absorption decreases [43, 44]. This transient rise was demonstrated in several studies [22, 29, 30, 32, 35, 36]. When considering the impact of vaginal oestrogens on BC recurrence risk, transient raised oestrogen levels are less concerning than a persistent rise. Oestrogen levels should be measured after vaginal oestrogens have been used for a few months. Of studies showing a persistent rise in oestrogen [30, 32], both assessed women using higher dose vaginal oestradiol products than typically recommended in women with BC. Wills et al. [30] showed persistent elevations of oestradiol (E2) at 60 days post-insertion in women using an oestradiol ring; however, in the oestradiol 25mcg tablet group, there were no elevations in oestradiol after 3 months of treatment, suggesting that any rise in E2 post-oestradiol tablet insertion is not persistent. Contrastingly, Kendall et al. [32] found persistently elevated E2 in two patients using 25mcg vaginal oestradiol tablets for 7–10 weeks, but no week 12 test was performed. This may reflect a true significant rise in E2 but may be due to inaccuracies of RIA.
Several studies in our systematic review are of low quality, and reasons include small sample size; retrospective design; mixed methodologies for testing oestrogens; inconsistencies in timing of oestrogen testing; lack of randomisation; and frequent lack of a control group. Despite significant limitations, these studies are often used to justify withholding vaginal oestrogens from women experiencing GSM because concerns use could increase BC recurrence risk.
It is essential that larger, more robust, and reliable studies are performed for this population. This includes a more uniform approach to oestrogen testing in clinical trials and practice. A better understanding of oestrogen levels and fluctuations over time in postmenopausal women with EBC not using vaginal oestrogens is essential; future studies should include a control group. Larger RCTs are needed to demonstrate the impact of longer-term use of vaginal oestrogens on oestrogen levels, given factors potentially influencing them including adherence, testing methodology, sample storage, timing of testing, and cross-reactivity with immunoassays. Relying on small studies to suggest a safety signal is problematic.
Limitations of our systematic review include the small number of included studies and heterogeneity of their design, size, intervention, and testing methods. Consequently, we were unable to perform a formal quality and bias assessment.
Conclusion
Inconsistent measurement and reporting of serum E2 and E3 levels in women with EBC on endocrine therapy creates uncertainty in safety of vaginal oestrogens, generating reluctance in clinicians to prescribe and patients to use vaginal oestrogens, even when symptoms are severe. More robust and standardised methods of measuring E2/E3 are critical to improve our understanding of the impact of vaginal oestrogens on serum oestrogen levels. In the absence of prospective randomised trials assessing BC outcomes, measurement of serum oestrogens remains the main method of assessing safety. Good quality data are required to increase confidence of women and clinicians worldwide.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AI:
-
Aromatase inhibitor
- BC:
-
Breast Cancer
- E1:
-
Oestrone
- E2:
-
Oestradiol
- E3:
-
Oestriol
- EBC:
-
Early breast cancer
- ECLIA:
-
Enhanced chemiluminiscence immunoassay
- ELISA:
-
Enzyme-linked immunosorbent assay
- FSH:
-
Follicle stimulating hormone
- GC-MS:
-
Gas chromatography mass spectrometry
- GSM:
-
Genitourinary syndrome of menopause
- HR+ :
-
Hormone receptor positive
- LC-MS:
-
Liquid chromatography mass spectrometry
- LH:
-
Lutenising hormone
- RCT:
-
Randomised controlled trial
- RIA:
-
Radioimmunoassay
- SBHG:
-
Sex hormone-binding globulin
- VE:
-
Vaginal oestrogen
References
Mazzarello S et al (2015) Management of urogenital atrophy in breast cancer patients: a systematic review of available evidence from randomized trials. Breast Cancer Res Treat 152(1):1–8
Lester J, Bernhard L, Ryan-Wenger N (2012) A self-report instrument that describes urogenital atrophy symptoms in breast cancer survivors. West J Nurs Res 34(1):72–96
Goodwin PJ et al (1999) Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17(8):2365–2370
Lester J et al (2015) Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 5(2):50–66
Salvatore S et al (2015) Sexual function after fractional microablative CO(2) laser in women with vulvovaginal atrophy. Climacteric 18(2):219–225
Portman DJ, Gass ML (2014) Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 21(10):1063–1068
Biglia N et al (2015) Genitourinary syndrome of menopause in breast cancer survivors: are we facing new and safe hopes? Clin Breast Cancer 15(6):413–420
Collaborative Group on Hormonal Factors in Breast Cancer (2019) Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. The Lancet
Bhupathiraju SN, Bhupathiraju SN, Grodstein F, Stampfer MJ, Willett WC, Crandall CJ, Shifren JL, Manson JE et al (2019) Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause 26(6):603–61
Crandall CJ et al (2018) Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause 25(1):11–20
Dew JE, Wren BG, Eden JA (2003) A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric 6(1):45–52
O’Meara ES et al (2001) Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J Natl Cancer Inst 93(10):754–762
Le Ray I et al (2012) Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat 135(2):603–609
Faubion SS et al (2022) The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 29(7):767–794
Stuenkel CA et al (2015) Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 100(11):3975–4011
Cold S et al (2022) Systemic or vaginal hormone therapy after early breast cancer: a Danish Observational Cohort Study. J Natl Cancer Inst 114(10):1347–1354
Head KA (1998) Estriol: safety and efficacy. Altern Med Rev 3(2):101–113
van der Vies J (1982) The pharmacology of oestriol. Maturitas 4(4):291–299
Wang S et al (2005) Recombinant cell ultrasensitive bioassay for measurement of estrogens in postmenopausal women. J Clin Endocrinol Metab 90(3):1407–1413
Santen RJ et al (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72(8):666–671
Key T et al (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94(8):606–616
Melisko ME et al (2017) Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer a randomized clinical trial. JAMA Oncol 3(3):313–319
Rosner W et al (2013) Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab 98(4):1376–1387
Dowsett M, Folkerd E (2004) Deficits in plasma oestradiol measurement in studies and management of breast cancer. Breast Cancer Res 7(1):1
Jaque J et al (2013) Deficiencies in immunoassay methods used to monitor serum Estradiol levels during aromatase inhibitor treatment in postmenopausal breast cancer patients. Springerplus 2(1):5
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. www.covidence.org. Accessed 2023
Biglia N et al (2010) Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: a preliminary study. Gynecol Endocrinol 26(6):404–412
Donders G et al (2014) Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor()) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat 145(2):371–379
Wills S et al (2012) Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Pract 8(3):144–149
Hirschberg AL et al (2020) Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: a phase II, randomized, double-blind, placebo-controlled trial. Menopause 27(5):526–534
Kendall A et al (2006) Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 17(4):584–587
Niravath P et al (2017) Challenges of measuring accurate estradiol levels in aromatase inhibitor-treated postmenopausal breast cancer patients on vaginal estrogen therapy. Pharmacol Res Perspect 5(4):7
Pfeiler G et al (2011) Vaginal estriol to overcome side-effects of aromatase inhibitors in breast cancer patients. Climacteric 14(3):339–344
Sanchez-Rovira P et al (2020) A phase II prospective, randomized, double-blind, placebo-controlled and multicenter clinical trial to assess the safety of 0.005% estriol vaginal gel in hormone receptor-positive postmenopausal women with early stage breast cancer in treatment with aromatase inhibitor in the adjuvant setting. Oncologist 25(12):e1846-1854
Streff A, Streff A, Chu-Pilli M, Stopeck A, Chalasani P et al (2021) Changes in serum estradiol levels with Estring in postmenopausal women with breast cancer treated with aromatase inhibitors. Support Care Cancer 29:187
Sanchez Rovira P et al (2016) A phase II prospective, randomized, double-blind, placebo-controlled multi-centre clinical trial to assess the safety of 0.005% estriol vaginal gel in hormone receptor-positive postmenopausal women with early stage breast cancer in treatment with aromatase inhibitors (AIs) in the adjuvant setting-Initial safety results. Eur J Cancer 2:S44
Mandic S et al (2017) Falsely elevated serum oestradiol due to exemestane therapy. Ann Clin Biochem 54(3):402–405
Folkerd EJ, Lonning PE, Dowsett M (2014) Interpreting plasma estrogen levels in breast cancer: caution needed. J Clin Oncol 32(14):1396–1400
Faltinová M et al (2023) Effects of letrozole on serum estradiol and estrone in postmenopausal breast cancer patients and tolerability of treatment: a prospective trial using a highly sensitive LC-MS/MS (liquid chromatography-tandem mass spectrometry) method for estrogen measurement. Breast Cancer Res Treat 201(3):425–435
Goldfarb SB et al (2012) Limited absorption of low dose 10 μg intravaginal 17-β estradiol (vagifem) in postmenopausal women with breast cancer on aromatase inhibitors. Cancer Res. https://doi.org/10.1158/0008-5472.SABCS12-P2-12-05
Goldfarb SB et al (2013) Use of intravaginal 17-β estradiol to improve sexual function and menopausal symptoms in postmenopausal women with breast cancer on aromatase inhibitors. J Clin Oncol. https://doi.org/10.1200/jco.2013.31.15_suppl.9610
Heimer G, Englund D (1984) Estriol: absorption after long-term vaginal treatment and gastrointestinal absorption as influenced by a meal. Acta Obstet Gynecol Scand 63(6):563–567
Buhling KJ et al (2012) Systemic bioavailability of estriol following single and repeated vaginal administration of 0.03 mg estriol containing pessaries. Arzneimittelforschung 62(8):378–83
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr Antonia Pearson and Jill Chen. The first draft of the manuscript was written by Antonia Pearson, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author TP has received conference support from GSK and Novartis Author JC declares they have no financial interests. Author HD has received speaker and consultant honoraria from MDS, BMS, Janssen. Author BEK reports: honoraria for speaking from Novartis, Eisai, MSD Oncology, Gilead; payment for advisory board participation from Gilead and Novartis; support for conferences from Novartis, Pfizer and MSD Oncology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pearson, A., Chen, J., Dhillon, H.M. et al. Measuring serum oestrogen levels in breast cancer survivors using vaginal oestrogens: a systematic review. Breast Cancer Res Treat 206, 215–226 (2024). https://doi.org/10.1007/s10549-024-07364-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07364-0