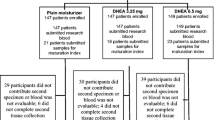
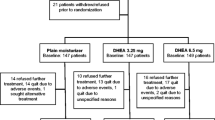
Abstract
Atrophic vaginitis represents a major barrier to compliance with aromatase inhibitor (AI) therapy in breast cancer (BC) survivors. While local estrogen therapy is effective for postmenopausal vaginal dryness, the efficacy of such therapies has not been evaluated systematically in hormone receptor-positive (HR+) BC patients on AI therapy. Furthermore, the potential risk of breast cancer recurrence with vaginal estrogen therapy represents a long-term safety concern for the patients with HR + BC. Unfortunately, there is no standardized assay to measure very low concentrations of estradiol (E2) in these women being treated with AI therapy. This makes it difficult to evaluate even indirectly the potential risk of BC recurrence with vaginal estrogen therapy in HR + BC patients on AI therapy. In this review, we describe available assays to measure very low concentrations of E2, discuss the Food and Drug Administration-approved vaginal estrogen products on the market, and summarize published and ongoing clinical trials evaluating the safety and efficacy of vaginal estrogen in HR + BC patients on AI therapy. In the absence of any randomized controlled clinical trials, this review serves as a summary of available clinical data and ongoing studies to aid clinicians in selecting the best available option for their patients.
Similar content being viewed by others
References
Breast Cancer Facts & Figures 2015–2016. American Cancer Society. http://www.cancer.org/research/cancerfactsstatistics/breast-cancer-facts-figures. Accessed 16 Nov 2015
Clark GM, Osborne CK, McGuire WL (1984) Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol 2(10):1102–1109
Arimidex T, Aoi CTG, Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9(1):45–53. doi:10.1016/S1470-2045(07)70385-6
Crandall C, Petersen L, Ganz PA, Greendale GA (2004) Association of breast cancer and its therapy with menopause-related symptoms. Menopause 11(5):519–530
Yoon J, Malin JL, Tisnado DM, Tao ML, Adams JL, Timmer MJ, Ganz PA, Kahn KL (2008) Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat 108(1):69–77. doi:10.1007/s10549-007-9580-1
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26(4):556–562. doi:10.1200/JCO.2007.11.5451
Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ (2015) Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100(11):3975–4011. doi:10.1210/jc.2015-2236
Rahn DD, Carberry C, Sanses TV, Mamik MM, Ward RM, Meriwether KV, Olivera CK, Abed H, Balk EM, Murphy M, Society of Gynecologic Surgeons Systematic Review G (2014) Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol 124(6):1147–1156. doi:10.1097/AOG.0000000000000526
Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, Clavel-Chapelon F, Fabre A, Hemon B, Rinaldi S, Chajes V, Slimani N, Allen NE, Reeves GK, Bingham S, Khaw KT, Olsen A, Tjonneland A, Rodriguez L, Sanchez MJ, Etxezarreta PA, Ardanaz E, Tormo MJ, Peeters PH, van Gils CH, Steffen A, Schulz M, Chang-Claude J, Kaaks R, Tumino R, Gallo V, Norat T, Riboli E, Panico S, Masala G, Gonzalez CA, Berrino F (2011) Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer 128(1):144–156. doi:10.1002/ijc.25314
Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB (2013) Menopausal hormone therapy and health outcomes during the intervention and extended post stopping phases of the Women’s Health Initiative randomized trials. JAMA 310(13):1353–1368. doi:10.1001/jama.2013.278040
von Schoultz E, Rutqvist LE, Stockholm Breast Cancer Study G (2005) Menopausal hormone therapy after breast cancer: the Stockholm randomized trial. J Natl Cancer Inst 97(7):533–535. doi:10.1093/jnci/dji071
Holmberg L, Iversen OE, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J, Jassem J, Dobaczewska D, Fjosne HE, Peralta O, Arriagada R, Holmqvist M, Maenpaa J, Group HS (2008) Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst 100(7):475–482. doi:10.1093/jnci/djn058
Folkerd EJ, Lonning PE, Dowsett M (2014) Interpreting plasma estrogen levels in breast cancer: caution needed. J Clin Oncol 32(14):1396–1400. doi:10.1200/JCO.2013.53.9411
Handelsman DJ, Newman JD, Jimenez M, McLachlan R, Sartorius G, Jones GR (2014) Performance of direct estradiol immunoassays with human male serum samples. Clin Chem 60(3):510–517. doi:10.1373/clinchem.2013.213363
Ankarberg-Lindgren C, Norjavaara E (2008) A purification step prior to commercial sensitive immunoassay is necessary to achieve clinical usefulness when quantifying serum 17beta-estradiol in prepubertal children. Eur J Endocrinol 158(1):117–124. doi:10.1530/EJE-07-0403
Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72(8):666–671. doi:10.1016/j.steroids.2007.05.003
Grebe SK, Singh RJ (2011) LC-MS/MS in the clinical laboratory—where to from here? Clin Biochem Rev 32(1):5–31
Gao W, Stalder T, Kirschbaum C (2015) Quantitative analysis of estradiol and six other steroid hormones in human saliva using a high throughput liquid chromatography-tandem mass spectrometry assay. Talanta 143:353–358. doi:10.1016/j.talanta.2015.05.004
Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG (2010) Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomark Prev 19(1):292–300. doi:10.1158/1055-9965.EPI-09-0643
Wooding KM, Hankin JA, Johnson CA, Chosich JD, Baek SW, Bradford AP, Murphy RC, Santoro N (2015) Measurement of estradiol, estrone, and testosterone in postmenopausal human serum by isotope dilution liquid chromatography tandem mass spectrometry without derivatization. Steroids 96:89–94. doi:10.1016/j.steroids.2015.01.007
Bruce SJ, Rey F, Beguin A, Berthod C, Werner D, Henry H (2014) Discrepancy between radioimmunoassay and high performance liquid chromatography tandem-mass spectrometry for the analysis of androstenedione. Anal Biochem 455:20–25. doi:10.1016/j.ab.2014.03.021
Gynoflor® Vaginal Tablets [product information] (2012) Medinova Ltd (Switzerland), Zurich, April 2012
Ovestin Ovula [product information] (2011) Merck & Dohme (Australia) Pty Limited, South Granvill, October 2011
Wills S, Ravipati A, Venuturumilli P, Kresge C, Folkerd E, Dowsett M, Hayes DF, Decker DA (2012) Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Pract 8(3):144–148. doi:10.1200/JOP.2011.000352
Donders G, Neven P, Moegele M, Lintermans A, Bellen G, Prasauskas V, Grob P, Ortmann O, Buchholz S (2014) Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor((R))) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat 145(2):371–379. doi:10.1007/s10549-014-2930-x
Pfeiler G, Glatz C, Konigsberg R, Geisendorfer T, Fink-Retter A, Kubista E, Singer CF, Seifert M (2011) Vaginal estriol to overcome side-effects of aromatase inhibitors in breast cancer patients. Climacteric 14(3):339–344. doi:10.3109/13697137.2010.529967
Kendall A, Dowsett M, Folkerd E, Smith I (2006) Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 17(4):584–587. doi:10.1093/annonc/mdj127
Erekson EA, Yip SO, Wedderburn TS, Martin DK, Li FY, Choi JN, Kenton KS, Fried TR (2013) The vulvovaginal symptoms questionnaire: a questionnaire for measuring vulvovaginal symptoms in postmenopausal women. Menopause 20(9):973–979. doi:10.1097/GME.0b013e318282600b
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Meghana V. Trivedi and Polly Niravath are the Co-senior authors.
Rights and permissions
About this article
Cite this article
Sulaica, E., Han, T., Wang, W. et al. Vaginal estrogen products in hormone receptor-positive breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res Treat 157, 203–210 (2016). https://doi.org/10.1007/s10549-016-3827-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3827-7