Abstract
Purpose
The contribution of clinical breast exam (CBE) to breast cancer diagnosis in average risk women undergoing regular screening mammography is minimal. To evaluate the role of CBE in high-risk women, we compared BC diagnosis by CBE in BRCA mutation carriers undergoing regular BC surveillance to average to intermediate risk women undergoing regular breast cancer screening.
Methods
A retrospective chart review of all consecutive screening visits of BRCA mutation carriers (January 2012–October 2022) and average to intermediate risk women (November 2016–December 2022) was completed. Women with histologically confirmed BC diagnosis were included. Additional CBE yield for BC diagnosis, defined as the percentage of all BC cases detected by CBE alone, was assessed in both groups.
Results
Overall, 12,997 CBEs were performed in 1,328 BRCA mutation carriers in whom 134 BCs were diagnosed. In 7,949 average to intermediate risk women who underwent 15,518 CBEs, 87 BCs were diagnosed. CBE contributed to BC diagnosis in 3 (2%) BRCA mutation carriers and 3 (4%) non-carriers. In both groups, over 4,000 CBEs were needed in order to diagnose one cancer. In all 3 BRCA mutation carriers BC was palpated during the surveillance round that did not include MRI. In the average to intermediate risk group, 2 of 3 cancers diagnosed following CBE findings were in a different location from the palpable finding.
Conclusions
The contribution of CBE to BC diagnosis is marginal for all women including BRCA mutation carriers. In BRCA mutation carriers, CBE appears redundant during the MRI surveillance round.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With improvements in breast imaging, the role of clinical breast exam (CBE) in screening average risk women is increasingly being questioned; with a recent report suggesting a very low yield in women undergoing regular mammography screening [1]. The American Cancer Society (ACS) does not recommend CBE for BC screening in average risk women at any age [2]. This recommendation is based on lack of level 1 evidence of any proven benefit for CBE either as a stand-alone tool or in conjunction with screening mammography [3]. The National Cancer Comprehensive Network [4] recommends a clinical encounter starting at age 25, which includes a CBE in asymptomatic individuals. Despite lack of proof of efficacy of CBE in average risk women, it may play a role in high-risk women, specifically BRCA mutation carriers. Inclusion of CBE in high-risk surveillance protocols varies worldwide, with some societies recommending annual or bi-annual CBE, while others do not [5, 6]. The ESMO recent recommendations [7] for surveillance of BRCA mutation carriers clearly state that “Clinical breast examination is of no value as a screening tool”, referencing a report by Hettipathirana et al [8]. In their report, 35 cancers were diagnosed in 414 BRCA mutation carriers between 2001 and 2019. Of these, two BCs were detected by CBE alone; however, this was prior to the introduction of MRI into the surveillance protocol in this center in 2009.
We sought to examine the role of CBE in early stage BC diagnosis in high-risk women undergoing regular BC surveillance. As the yield of CBE in women undergoing regular screening mammography is minimal [1], we compared BC detection by CBE alone in BRCA mutation carriers to women at average to intermediate risk undergoing regular breast cancer screening in the same medical center.
Methods
This retrospective chart review was approved by the local ethics committee (SMC-21-8844), and informed consent was waived.
Study population
All consecutive visits of women to the high-risk clinic (January 2012 to October 2022) or to the health screening clinic (November 2016 to December 2022) were identified using MDClone (MDClone LTD, Beer Sheva, Israel), a query tool providing a wide range of patient data.
The High-risk clinic at Sheba Medical Center, Tel Hashomer, provides BC screening to high-risk women, predominantly BRCA pathogenic mutation carriers. Only women with a known pathogenic mutation in BRCA1/2 gene were included in the study group. BRCA mutation carriers with a previous history of BC were included in the cohort, as once completing successful treatment high-risk surveillance is continued as per BRCA surveillance protocol.
The control group consisted of all consecutive women visiting the health screening center at Sheba Medical Center Tel Hashomer. The program is voluntary and provides general health screening services. Most participants receive the service as an employer-provided benefit. Women examined in the health screening clinic were excluded if they had a previous diagnosis of BC (as these women are considered to be at risk for both local recurrence and a new primary and recommendations for surveillance of these women include CBE and more frequent imaging).
Based on their family history the control group included women at average and intermediate risk for breast cancer. Women who reported any family history of breast cancer without a known pathogenic mutation were considered to be at intermediate risk. However, women with a strong family history suggestive of hereditary cancer syndrome were excluded due to their high-risk.
Surveillance and screening protocols
The surveillance protocol for BRCA pathogenic mutation carriers includes CBE every 6 months starting at the age of 25; and annual breast MRI starting at the age of 25 years, alternating with annual ultrasound (US) or mammography (starting at age 30). Pregnant and breast feeding women undergo CBE and US every 3 months. Women that are post risk-reducing bilateral mastectomy continue to undergo CBE every 6 months, and a baseline MRI. After assessment of residual breast tissue individual recommendations are given.
The breast screening protocol at the screening center includes a yearly CBE by a surgeon and imaging according to personal risk. This usually includes a yearly screening mammogram from age 40 and breast ultrasound (US) or contrast enhanced spectral mammography (CESM) based on mammographic density. There is no upper age limit for CBE or screening mammography. Some of the women undergo regular breast screening by their HMO and complete only part of the breast health exam during their visit at the screening center. In women with findings on CBE, further evaluation is recommended as indicated by the surgeon and/or the breast radiologist.
Women presenting with a symptomatic cancer, i.e., complaining of symptoms that were related to the cancer, were considered to have an interval cancer.
Breast imaging
Mammography was performed with a digital mammography system (Senographe Essential and Senographe Pristina; GE Healthcare, New York, NY, USA). Standard craniocaudal and mediolateral oblique projections of each breast were acquired. CESM studies were performed using a digital mammography system (Senographe Essential, GE Healthcare; Chalfont St-Giles, UK). Whole breast and bilateral axillae US examinations were performed by certified fellowship-trained breast radiologists, using Siemens Acuson S2000 ultrasound system with a linear transducer 18-6MHz, (Siemens Medical Solutions USA, Inc., Mountain View, CA, USA). MRI was performed on 1.5-T MRI (Signa Excite HDX, GE Healthcare) using a dedicated double breast coil.
The imaging was considered abnormal if the Breast Imaging-Reporting and Data System (BIRADS) classification was 0, 3, 4, or 5, or if the text included a recommendation for further work-up.
Data collection
Using ICD-9 codes, the charts of women with a diagnosis of BC were identified. Further data were manually extracted from the electronic medical records (Chameleon, version 5.12.2.43395, Elad Health, Israel).
Women that were diagnosed with breast cancer after a visit to one of the clinics were included in the final cohort.
Data retrieved from the charts included demographics, family history of cancer, time from previous imaging, clinical and imaging findings, tumor features and treatment.
Based on the chart review, the mode of diagnosis was determined. If a palpable finding led to further work-up and a subsequent diagnosis of BC, this was considered to be BC diagnosed by CBE. If the finding on CBE correlated with a finding on standard imaging, this was not considered BC diagnosed by CBE. As the tests are performed in parallel with some women first undergoing a CBE while others undergo imaging first, charts were manually reviewed in order to determine if a finding on CBE led to the diagnosis of BC (i.e., second look US, etc.).
Analysis
Descriptive statistics of the study population were summarized. The two groups were compared using chi-square test for categorical data, and the Student’s t-test for continuous variables. All tests were 2-sided and significance was set at 0.05. The additional cancer yield of CBE (defined as the percentage of all cancer cases detected by CBE only) and number needed to screen by CBE in order to diagnose one cancer were calculated. These proportions were calculated separately for the two groups. Rate precision was determined with 95% confidence intervals (CI), which were derived by using the Wilson score interval for binomial parameters with continuity correction.
Results
During the study period, 1,328 BRCA mutation carriers (BRCA1 – 773; BRCA2- 550; BRCA1 + BRCA2 – 5) had 12,997 documented CBEs. After excluding BCs that were diagnosed prior to the first high-risk clinic visit, the cohort included 134 BC cases in the BRCA mutation carrier group (Fig. 1, Table 1). In 39 (29%) of these women, there was at least one previous diagnosis of breast cancer. Five were post bilateral mastectomy. Five had a history of ovarian cancer. Seventy (52%) underwent bilateral salpingo-oopherectomy prior to the current diagnosis. Mean time from previous imaging was 0.56 years (range 0.03–1.47 years). Findings on CBE are summarized in Fig. 1.
Six (4.5%) women presented with interval cancers, median age was 36 years (range 28–67). Median time from previous imaging was 3 months (range 0.11–0.66). Time from previous MRI ranged between 7.5 months and 3 years; one woman had never had a previous MRI. Average tumor size was 18 mm (range 10–46mm). Four were node negative, one was node positive, and for one this data was missing. Two were triple negative, two were luminal, one was HER2neu positive and data were missing for the last.
Three cancers (2%; 95% CI 0.7–6) were diagnosed secondary to CBE findings, none of these women had an MRI during this round (Table 2). In the screening rounds not including MRI three of the BCs were diagnosed by CBE (7.7%; 95% CI 2.6–20.3).
In the Health screening center 7,949 average to intermediate risk women had 15,518 CBEs done during the study period. The final cohort included 87 women diagnosed with BC after a visit to the clinic with a documented CBE within 6 months of the diagnosis (Fig. 1).
Most of these women (54; 62%) had no known family history of BC (Table 1). Four women with no known family history were subsequently identified as BRCA mutation carriers.
The imaging, pathology and treatment characteristics are summarized in Table 1.
The median age of women presenting with an interval cancer was 64.3 years (range 41–81). Median time from previous imaging was 0.8 years (range 0.73–10).
There were 3 (4%; 95% CI 1–9) cancers that were diagnosed secondary to an abnormal CBE (Table 2). One was found on MRI; the other 2 on US. In two of these cases, the palpable finding was in a different location from the cancer diagnosed. Nonetheless, the CBE initiated the work-up.
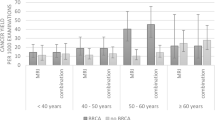
As expected, when compared to the average to intermediate risk group, BRCA mutation carriers were diagnosed with BC at an earlier age; mainly by MRI; and more often with invasive, high grade triple negative cancers. They underwent more often mastectomies and received systemic chemotherapy (Table 1).
In both groups, diagnosis of cancer by CBE only was a rare event; over 4,000 exams were needed in order to diagnose one BC.
Discussion
In order to assess the yield of CBE in high-risk women we compared the additional cancer yield of CBE in BRCA carriers to average to intermediate risk women undergoing regular screening mammography. In the current study, the additional yield of CBE to BC diagnosis in both BRCA mutation carriers and in average to intermediate risk population was marginal at best. Notably, the number of CBE needed in order to find one breast cancer was over 4,000 in BRCA mutation carriers. These results are in line with previous studies in which the additional yield of CBE to BC detection ranged from 0 to 6% (Table 3). The incorporation of breast MRI in the surveillance protocol of BRCA mutation carriers resulted in a decrease in the proportion of women presenting with interval cancers from 35–50% [9] to 0–19% [10,11,12,13, 15, 16] (Table 3).
In 2014, Roeke et al. [17] systematically reviewed the additional cancer yield of CBE in women at increased risk of BC, and reported that it ranged between 0 and 4% in 7 prospective studies. The recent ESMO guidelines removed the previous recommendation for CBE in surveillance of BRCA mutation carriers, while recommending in BRCA1 mutation carriers imaging every 6 months preferably by MRI [7]. The National Institute of Health and Care Excellence (NICE) continues to recommend breast awareness and annual MRI [18], while NCCN continues to recommend CBE from age 25 and annual MRI [19].
Based on the results of the current study, and given the well- established superiority of MRI over other breast imaging modalities [20], combined with the fact that all 3 BC cases in BRCA mutation carriers identified by CBE were in women not undergoing MRI at the same screening round, it appears safe to forgo the CBE at the time surveillance MRI is performed.
This study has several limitations. Data on visits to the high-risk clinic and the health screening center were based on computer queries and therefore errors in coding may have resulted in inaccurate estimation of the total number of women and of the total number of visits to these clinics. Although charts of women subsequently diagnosed with breast cancer were manually reviewed, the total number of women and of visits was based on retrieval of visit codes. This may impact the accuracy of the estimation of the number needed to screen in order to detect one BC. As the estimates of the additional cancer yield of CBE are based on women diagnosed with breast cancer, these estimates are minimally affected by coding errors. The BRCA cohort included women after a previous diagnosis of BC (as these women are recommended to continue the same surveillance scheme), whereas we excluded women with a history of BC in the average to intermediate risk group. Women that underwent bilateral mastectomy were included in the BRCA cohort despite controversy regarding their continued need for increased surveillance. With the increasing prevalence of risk-reducing mastectomy, CBE may play a bigger role in the surveillance of these women, however our numbers are too small to reach meaningful conclusions. The surgeons performing the CBEs were a heterogenous group, with varying experience and abilities. CBE is a skill which is almost impossible to quantify and analyze objectively. The population analyzed herein is from a single medical center in Israel and may not reflect the reality in other medical centers in the country. The compliance of average risk women to BC screening is below 80% in Israel and this may have impacted the results as well.
In summary, based on a large population of BRCA mutation carriers, it appears that CBE has a marginal contribution to the diagnosis of BC, specifically during the screening round that does not include MRI. It seems safe not to perform CBE during the screening visit that includes an MRI. In average to intermediate risk women undergoing regular BC screening the yield of CBE is very low, and may not be justified at all.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due privacy concerns. Upon request the analyses generated from the data are available from the corresponding author.
References
Menes TS, Coster D, Coster D, Shenhar-Tsarfaty S (2021) Contribution of clinical breast exam to cancer detection in women participating in a modern screening program. BMC Womens Health 21(1):368
https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html. Accessed 14 Aug 2023
Oeffinger KC, Fontham ETH, Etzioni R et al (2015) Breast cancer screening for women at average risk: guideline update from the American cancer society. JAMA 314(15):1599–1614
Network NCC. NCCN Guidelines Version 1.2022 Breast cancer screening and diagnosis. Accessed 30 May 2023
Madorsky-Feldman D, Sklair-Levy M, Perri T et al (2016) An international survey of surveillance schemes for unaffected BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 157(2):319–327
Bernstein-Molho R, Friedman E, Evron E (2022) Controversies and open questions in management of cancer-free carriers of germline pathogenic variants in BRCA1/BRCA2. Cancers (Basel) 14(19):4592. https://doi.org/10.3390/cancers14194592.PMID:36230512;PMCID:PMC9559251
Sessa C, Balmaña J, Bober SL ESMO Guidelines Committee et al (2023) Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes ESMO Clinical Practice Guideline. Ann Oncol 34(1):33–47. https://doi.org/10.1016/j.annonc.2022.10.004
Hettipathirana T, Macdonald C, Xie J, Moodie K, Michael C, Phillips KA (2021) The value of clinical breast examination in a breast cancer surveillance program for women with germline BRCA1 or BRCA2 mutations. Med J Aust 215(10):460–464. https://doi.org/10.5694/mja2.51226
Warner E (2018) Screening BRCA1 and BRCA2 mutation carriers for breast cancer. Cancers (Basel) 10(12):477
Warner E, Plewes DB, Hill KA et al (2004) Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 292(11):1317–1325
Kriege M, Brekelmans CT, Boetes C Magnetic Resonance Imaging Screening Study Group et al (2004) Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 351(5):427–437
Trop I, Lalonde L, Mayrand MH, David J, Larouche N, Provencher D (2010) Multimodality breast cancer screening in women with a familial or genetic predisposition. Curr Oncol 17(3):28–36
Rijnsburger AJ, Obdeijn IM, Kaas R et al (2010) BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC Screening Study. J Clin Oncol 28(36):5265–5273
Maurice A, Evans DG, Affen J, Greenhalgh R, Duffy SW, Howell A (2012) Surveillance of women at increased risk of breast cancer using mammography and clinical breast examination: further evidence of benefit. Int J Cancer 131(2):417–425. https://doi.org/10.1002/ijc.26394
Fakkert IE, Jansen L, Meijer K et al (2011) Breast cancer screening in BRCA1 and BRCA2 mutation carriers after risk reducing salpingo-oophorectomy. Breast Cancer Res Treat 129(1):157–164. https://doi.org/10.1007/s10549-011-1423-4
Mihalco SP, Keeling SB, Murphy SF, O’Keeffe SA (2020) Comparison of the utility of clinical breast examination and MRI in the surveillance of women with a high risk of breast cancer. Clin Radiol 75(3):194–199
Roeke T, van Bommel AC, Gaillard-Hemmink MP, Hartgrink HH, Mesker WE, Tollenaar RA (2014) The additional cancer yield of clinical breast examination in screening of women at hereditary increased risk of breast cancer: a systematic review. Breast Cancer Res Treat 147(1):15–23. https://doi.org/10.1007/s10549-014-3074-8
https://www.nice.org.uk/guidance/cg164/chapter/recommendations#surveillance-and-strategies-for-early-detection-of-breast-cancer. Accessed 14 Aug 2023
https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed 14 Aug 2023
Kuhl CK, Schrading S, Leutner CC et al (2005) Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 23(33):8469–8476
Funding
Open access funding provided by Tel Aviv University. Not applicable.
Author information
Authors and Affiliations
Contributions
TM and DFM collected the data DFM contributed to the design TM analyzed and interpreted the data and drafted the Manuscript DZ, MSL, EF, RF, RBM and DFM contributed to the interpretation of the data and critically revised the manuscript All authors reviewed the manuscript
Corresponding author
Ethics declarations
Competing interests
The authors have not disclosed any competing interests.
Ethical approval
This retrospective chart review was approved by the local ethical committee (SMC-21-8844), and informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Menes, T.S., Zippel, D., Sklair-Levy, M. et al. Clinical breast exam contribution to breast cancer diagnosis in BRCA mutation carriers vs. average to intermediate risk women. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07345-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07345-3