Abstract
Purpose
One of the most important risk factors for hereditary breast and ovarian cancer is young age. We aim to report the frequency of pathogenic/likely pathogenic variants in breast cancer predisposing genes in young (≤ 40 years old) breast cancer patients who undergone 26-gene inherited cancer panel at our Breast Health Center.
Methods
Medical records of breast cancer patients who were referred to genetic counseling based on NCCN criteria and were ≤ 40 years of age are reviewed. The frequency of germline pathogenic/likely pathogenic variants who undergone 26-gene inherited cancer panel was analyzed.
Results
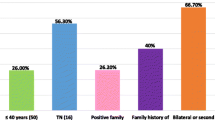
Among 414 breast cancer patients who were ≤ 40 years of age, 308 undergone 26-gene inherited cancer panel and 108 had next generation sequencing (NGS)-based BRCA 1 and 2 genetic testing. Median age was 35 (22–40), Family history in first degree relatives was present in 14% of patients. Forty-five percent of patients met one of the NCCN criteria for genetic testing, 41% of them met two criteria, and 14% of patients fulfilled ≥ 3 criteria. Seventy pathogenic/likely pathogenic variants (PV/LPV) were found in 65 (21%) patients. PV/LPs in BRCA genes and non-BRCA genes represented 53% and 44% of all PV/LPVs, accounting for 12% and 10% of patients in the study cohort respectively. Two PVs were present in 5 patients and eleven PVs were novel. The most common PVs were in BRCA 1 (n:18), BRCA 2 (n:19), ATM (n:7), CHEK2 (n:7) and TP53 (n:5) genes. Thirty-one percent of the patients with triple-negative tumors and 25% of the patients with hormone receptor-positive tumors had PV/LPVs with panel testing. Family history in first degree relatives (p = 0.029), the number of met NCCN criteria (p = 0.036) and axillary nodal involvement (p = 0.000) were more common in patients with PVs. When combined with patient group (n:106) who had only BRCA1 and 2 gene testing, 16% of Turkish breast cancer patients ≤ 40 years of age had PVs in BRCA genes.
Conclusion
One fifth of Turkish breast cancer patients ≤ 40 years of age had at least one PV/LPV in breast cancer predisposing genes with 26-gene inherited cancer panel. The frequency of PV/LPVs was higher in triple-negative young-onset patients compared to hormone receptor and Her-2 positive subtypes. Our findings regarding to frequency PV/LPVs in BRCA 1/2 and non-BRCA genes in young-onset breast cancer patients are in line with the literature.

Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Arnold M, Morgan E, Rumgay H et al (2022) Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66:15–23. https://doi.org/10.1016/j.breast.2022.08.010
Paluch-Shimon S, Cardoso F, Partridge AH et al (2022) ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann Oncol 33(11):1097–1118. https://doi.org/10.1016/j.annonc.2022.07.007
Sopik V (2021) International variation in breast cancer incidence and mortality in young women. Breast Cancer Res Treat 186(2):497–507. https://doi.org/10.1007/s10549-020-06003-8
DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL (2019) Cancer statistics for African Americans, 2019. CA Cancer J Clin 69(3):211–233. https://doi.org/10.3322/caac.21555
Ozmen V, Ozmen T, Dogru V (2019) Breast cancer in Turkey: an analysis of 20,000 patients with breast cancer. Eur J Breast Health 15(3):141–146. https://doi.org/10.5152/ejbh.2019.4890
Dafni U, Tsourti Z, Alatsathianos I (2019) Breast cancer statistics in the European Union: incidence and survival across European Countries. Breast Care (Basel) 14(6):344–353. https://doi.org/10.1159/000503219
Petrucelli N, Daly MB, Pal T (1998) BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. 1998 Sept 4 (Updated 2022 May 26). In: Adam MP, Mirzaa GM, Pagon RA et al (eds) GeneReviews® (Internet). University of Washington, Seattle, WA, 1993–2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1247/
Antoniou A, Pharoah PD, Narod S et al (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies [published correction appears in Am J Hum Genet. 2003 Sept;73(3):709]. Am J Hum Genet 72(5):1117–1130. https://doi.org/10.1086/375033
NCCN Guidelines for Genetics/Familial High-Risk Assessment: Breast and Ovarian V.1.2019—web teleconference on 04/10/18
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Geredeli C, Yasar N, Sakin A (2019) Germline mutations in BRCA1 and BRCA2 in breast cancer patients with high genetic risk in Turkish population. Int J Breast Cancer 2019:9645147. https://doi.org/10.1155/2019/9645147
Yazici H, Bitisik O, Akisik E, Cabioglu N, Saip P et al (2000) BRCA1 and BRCA2 mutations in Turkish breast/ovarian families and young breast cancer patients. Br J Cancer 83(6):737–742. https://doi.org/10.1054/bjoc.2000.1332
Bilgic DG, Gumus AA, Bilgic A, Cam FS (2022) Mutations of BRCA 1/2 genes in the west of Turkey and genotype-phenotype correlations. Clin Lab. https://doi.org/10.7754/Clin.Lab.2021.210425
Yazici H, Kilic S, Akdeniz D et al (2018) Frequency of rearrangements versus small indels mutations in BRCA1 and BRCA2 genes in Turkish patients with high risk breast and ovarian cancer. Eur J Breast Health 14(2):93–99. https://doi.org/10.5152/ejbh.2017.3799
Akin Duman T, Ozturk FN (2023) Frequency and distribution of BRCA1/BRCA2 large genomic rearrangements in Turkish population with breast cancer (published online ahead of print, 2023 Mar 3). J Hum Genet. https://doi.org/10.1038/s10038-023-01140-6
Bisgin A, Ozemri SS, Dogan ME, Yildiriö MS et al (2022) Germline landscape of BRCAs by 7-site collaborations as a BRCA consortium in Turkey. Breast 65:15–22. https://doi.org/10.1016/j.breast.2022.06.005
Kang E, Seong MW, Park SK et al (2015) The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res Treat 151(1):157–168. https://doi.org/10.1007/s10549-015-3377-4
ElBiad O, Laraqui A, El Boukhrissi F et al (2022) Prevalence of specific and recurrent/founder pathogenic variants in BRCA genes in breast and ovarian cancer in North Africa. BMC Cancer. https://doi.org/10.1186/s12885-022-09181-4
Herzog JS, Chavarri-Guerra Y, Castillo D et al (2021) Genetic epidemiology of BRCA1- and BRCA2-associated cancer across Latin America. NPJ Breast Cancer 7(1):107. https://doi.org/10.1038/s41523-021-00317-6
de Sanjosé S, Léoné M, Bérez V et al (2003) Prevalence of BRCA1 and BRCA2 germline mutations in young breast cancer patients: a population-based study. Int J Cancer 106(4):588–593. https://doi.org/10.1002/ijc.11271
Bakkach J, Mansouri M, Derkaoui T et al (2020) Contribution of BRCA1 and BRCA2 germline mutations to early onset breast cancer: a series from north of Morocco. BMC Cancer 20(1):859. https://doi.org/10.1186/s12885-020-07352-9
John EM, Miron A, Gong G et al (2007) Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA 298(24):2869–2876. https://doi.org/10.1001/jama.298.24.2869
van der Merwe NC, Ntaita KS, Stofberg H et al (2022) Implementation of multigene panel testing for breast and ovarian cancer in South Africa: a step towards excellence in oncology for the public sector. Front Oncol 12:938561. https://doi.org/10.3389/fonc.2022.938561
Awadelkarim KD, Aceto G, Veschi S et al (2007) BRCA1 and BRCA2 status in a Central Sudanese series of breast cancer patients: interactions with genetic, ethnic and reproductive factors. Breast Cancer Res Treat 102(2):189–199. https://doi.org/10.1007/s10549-006-9303-z
Cao WM, Gao Y, Yang HJ et al (2016) Novel germline mutations and unclassified variants of BRCA1 and BRCA2 genes in Chinese women with familial breast/ovarian cancer. BMC Cancer 16:64. https://doi.org/10.1186/s12885-016-2107-6
Akcay IM, Celik E, Agaoglu NB et al (2021) Germline pathogenic variant spectrum in 25 cancer susceptibility genes in Turkish breast and colorectal cancer patients and elderly controls. Int J Cancer 148(2):285–295. https://doi.org/10.1002/ijc.33199
Bora E, Caglayan AO, Koc A et al (2022) Evaluation of hereditary/familial breast cancer patients with multigene targeted next generation sequencing panel and MLPA analysis in Turkey. Cancer Genet 262–263:118–133. https://doi.org/10.1016/j.cancergen.2022.02.006
Arslan Ates E, Turkyilmaz A, Alavanda C et al (2022) Multigene panel testing in Turkish hereditary cancer syndrome patients. Medeni Med J 37(2):150–158. https://doi.org/10.4274/MMJ.galenos.2022.22556
Ece Solmaz A, Yeniay L, Gökmen E et al (2021) Clinical contribution of next-generation sequencing multigene panel testing for BRCA negative high-risk patients with breast cancer. Clin Breast Cancer 21(6):e647–e653. https://doi.org/10.1016/j.clbc.2021.04.002
Aktas D, Gultekin M, Kabacam S et al (2010) Identification of point mutations and large rearrangements in the BRCA1 gene in 667 Turkish unselected ovarian cancer patients. Gynecol Oncol 119(1):131–135. https://doi.org/10.1016/j.ygyno.2010.05.018
Maxwell KN, Wubbenhorst B, D’Andrea K et al (2015) Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA 1/2-negative patients with early-onset breast cancer. Genet Med 17(8):630–638. https://doi.org/10.1038/gim.2014.176
Nassar A, Zekri AN, Kamel MM et al (2022) Frequency of pathogenic germline mutations in early and late onset familial breast cancer patients using multi-gene panel sequencing: an Egyptian study. Genes (Basel) 14(1):106. https://doi.org/10.3390/genes14010106
Shin HC, Lee HB, Yoo TK et al (2020) Detection of germline mutations in breast cancer patients with clinical features of hereditary cancer syndrome using a multi-gene panel test. Cancer Res Treat 52(3):697–713. https://doi.org/10.4143/crt.2019.559
Uyisenga JP, Segers K, Lumaka AZ et al (2020) Screening of germline mutations in young Rwandan patients with breast cancers. Mol Genet Genomic Med 8(11):e500. https://doi.org/10.1002/mgg3.1500
Li JY, Jing R, Wei H et al (2019) Germline mutations in 40 cancer susceptibility genes among Chinese patients with high hereditary risk breast cancer. Int J Cancer 144(2):281–289. https://doi.org/10.1002/ijc.31601
Momozawa Y, Iwasaki Y, Parsons MT et al (2018) Germline pathogenic variants of 11 breast cancer genes in 7051 Japanese patients and 11,241 controls. Nat Commun 9(1):4083. https://doi.org/10.1038/s41467-018-06581-8
Tung NM, Robson ME, Ventz S et al (2020) TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38(36):4274–4282. https://doi.org/10.1200/JCO.20.02151
Geyer CE Jr, Garber JE, Gelber RD et al (2022) Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA 1/2 and high-risk, early breast cancer. Ann Oncol 33(12):1250–1268. https://doi.org/10.1016/j.annonc.2022.09.159
Acknowledgements
The authors would like thank our physician assistant Aslınur Moral and nurse practitioner Beren Buyukcolak for their support in the collection of data.
Funding
This retrospective study did not need funding.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by (AI). The first draft of the manuscript was written by (AI; GB) and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Ethical approval of this retrospective study was granted in 2020 by (our university) Ethics Committee (Approval # 01.09.2020/754).
Informed consent
All participants signed a written informed consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Isiklar, A.D., Aliyeva, L., Yesilyurt, A. et al. Frequency of germline pathogenic variants in breast cancer predisposition genes among young Turkish breast cancer patients. Breast Cancer Res Treat 202, 297–304 (2023). https://doi.org/10.1007/s10549-023-07074-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07074-z