Abstract
Purpose
Breast terminal duct lobular units (TDLUs) are the main source of breast cancer (BC) precursors. Higher serum concentrations of hormones and growth factors have been linked to increased TDLU numbers and to elevated BC risk, with variable effects by menopausal status. We assessed associations of circulating factors with breast histology among premenopausal women using artificial intelligence (AI) and preliminarily tested whether parity modifies associations.
Methods
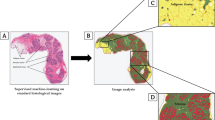
Pathology AI analysis was performed on 316 digital images of H&E-stained sections of normal breast tissues from Komen Tissue Bank donors ages ≤ 45 years to assess 11 quantitative metrics. Associations of circulating factors with AI metrics were assessed using regression analyses, with inclusion of interaction terms to assess effect modification.
Results
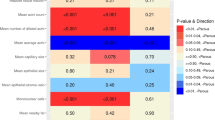
Higher prolactin levels were related to larger TDLU area (p < 0.001) and increased presence of adipose tissue proximate to TDLUs (p < 0.001), with less significant positive associations for acini counts (p = 0.012), dilated acini (p = 0.043), capillary area (p = 0.014), epithelial area (p = 0.007), and mononuclear cell counts (p = 0.017). Testosterone levels were associated with increased TDLU counts (p < 0.001), irrespective of parity, but associations differed by adipose tissue content. AI data for TDLU counts generally agreed with prior visual assessments.
Conclusion
Among premenopausal women, serum hormone levels linked to BC risk were also associated with quantitative features of normal breast tissue. These relationships were suggestively modified by parity status and tissue composition. We conclude that the microanatomic features of normal breast tissue may represent a marker of BC risk.

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request or from the Komen Tissue Bank and Indiana University.
References
Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 55(2):231–273
Wallace TR, Tarullo SE, Crump LS et al (2019) Studies of postpartum mammary gland involution reveal novel pro-metastatic mechanisms. J Cancer Metastasis Treat. https://doi.org/10.20517/2394-4722.2019.01
Jindal S, Gao D, Bell P et al (2014) Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res 16(2):R31. https://doi.org/10.1186/bcr3633
Martinson HA, Jindal S, Durand-Rougely C et al (2015) Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer 136(8):1803–1813. https://doi.org/10.1002/ijc.29181
Schedin P, O’Brien J, Rudolph M et al (2007) Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia 12(1):71–82. https://doi.org/10.1007/s10911-007-9039-3
Lyons TR, O’Brien J, Borges VF et al (2011) Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 17(9):1109–1115. https://doi.org/10.1038/nm.2416
Faupel-Badger JM, Arcaro KF, Balkam JJ et al (2013) Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst 105(3):166–174. https://doi.org/10.1093/jnci/djs505
Milanese TR, Hartmann LC, Sellers TA et al (2006) Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst 98(22):1600–1607. https://doi.org/10.1093/jnci/djj439
Figueroa JD, Pfeiffer RM, Brinton LA et al (2016) Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: a nested case-control study. Breast Cancer Res Treat 159(1):163–172. https://doi.org/10.1007/s10549-016-3908-7
Figueroa JD, Pfeiffer RM, Patel DA et al (2014) Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju286
Kensler KH, Liu EZF, Wetstein SC et al (2020) Automated quantitative measures of terminal duct lobular unit involution and breast cancer risk. Cancer Epidemiol Biomarkers Prev 29(11):2358–2368. https://doi.org/10.1158/1055-9965.EPI-20-0723
Baer HJ, Collins LC, Connolly JL et al (2009) Lobule type and subsequent breast cancer risk: results from the Nurses’ Health Studies. Cancer 115(7):1404–1411. https://doi.org/10.1002/cncr.24167
Santucci-Pereira J, Zeleniuch-Jacquotte A, Afanasyeva Y et al (2019) Genomic signature of parity in the breast of premenopausal women. Breast Cancer Res 21(1):46. https://doi.org/10.1186/s13058-019-1128-x
Nichols HB, Schoemaker MJ, Cai J et al (2019) Breast cancer risk after recent childbirth: a pooled analysis of 15 prospective studies. Ann Intern Med 170(1):22–30. https://doi.org/10.7326/M18-1323
Endogenous H, Breast Cancer Collaborative G, Key TJ et al (2013) Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 14(10):1009–1019. https://doi.org/10.1016/S1470-2045(13)70301-2
Tin Tin S, Reeves GK, Key TJ (2021) Endogenous hormones and risk of invasive breast cancer in pre- and post-menopausal women: findings from the UK Biobank. Br J Cancer 125(1):126–134. https://doi.org/10.1038/s41416-021-01392-z
Key T, Appleby P, Barnes I et al (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94(8):606–616. https://doi.org/10.1093/jnci/94.8.606
O’Leary KA, Rugowski DE, Shea MP et al (2021) Prolactin synergizes with canonical Wnt signals to drive development of ER+ mammary tumors via activation of the Notch pathway. Cancer Lett 503:231–239. https://doi.org/10.1016/j.canlet.2021.01.012
Tworoger SS, Eliassen AH, Sluss P et al (2007) A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol 25(12):1482–1488. https://doi.org/10.1200/JCO.2006.07.6356
Gabrielson M, Ubhayasekera K, Ek B et al (2018) Inclusion of Plasma prolactin levels in current risk prediction models of premenopausal and postmenopausal breast cancer. JNCI Cancer Spectr 2(4):pky055. https://doi.org/10.1093/jncics/pky055
Tworoger SS, Eliassen AH, Zhang X et al (2013) A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res 73(15):4810–4819. https://doi.org/10.1158/0008-5472.CAN-13-0665
Endogenous H, Breast Cancer Collaborative G, Key TJ et al (2010) Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol 11(6):530–542. https://doi.org/10.1016/S1470-2045(10)70095-4
Khodr ZG, Sherman ME, Pfeiffer RM et al (2014) Circulating sex hormones and terminal duct lobular unit involution of the normal breast. Cancer Epidemiol Biomarkers Prev 23(12):2765–2773. https://doi.org/10.1158/1055-9965.EPI-14-0667
Oh H, Pfeiffer RM, Falk RT et al (2018) Serum insulin-like growth factor (IGF)-I and IGF binding protein-3 in relation to terminal duct lobular unit involution of the normal breast in Caucasian and African American women: the Susan G. Komen. Tissue Bank Int J Cancer 143(3):496–507. https://doi.org/10.1002/ijc.31333
Eliassen AH, Tworoger SS, Hankinson SE (2007) Reproductive factors and family history of breast cancer in relation to plasma prolactin levels in premenopausal and postmenopausal women. Int J Cancer 120(7):1536–1541. https://doi.org/10.1002/ijc.22482
Hankinson SE, Eliassen AH (2010) Circulating sex steroids and breast cancer risk in premenopausal women. Horm Cancer 1(1):2–10. https://doi.org/10.1007/s12672-009-0003-0
Trabert B, Sherman ME, Kannan N et al (2020) Progesterone and Breast Cancer. Endocr Rev. https://doi.org/10.1210/endrev/bnz001
Stanczyk FZ, Mathews BW, Sherman ME (2015) Relationships of sex steroid hormone levels in benign and cancerous breast tissue and blood: a critical appraisal of current science. Steroids 99(Pt A):91–102. https://doi.org/10.1016/j.steroids.2014.12.011
de Bel TLG, Ogony J, Stallings-Mann M, Carter JM, Hilton T, Radisky DC, Vierkant RA, Broderick B, Hoskin TL, Winham SJ, Frost MH, Visscher DW, Allers T, Degnim AC, Sherman ME, van der Lakk JAWM (2022) Automated quantification of levels of breast terminal duct lobular (TDLU) involution using deep learning. NPJ Breast Cancer 8:13
Sherman ME, Figueroa JD, Henry JE et al (2012) The Susan G. Komen for the Cure Tissue Bank at the IU Simon Cancer Center: a unique resource for defining the “molecular histology” of the breast. Cancer Prev Res (Phila) 5(4):528–535. https://doi.org/10.1158/1940-6207.CAPR-11-0234
Wang M, Wu X, Chai F et al (2016) Plasma prolactin and breast cancer risk: a meta- analysis. Sci Rep 6:25998. https://doi.org/10.1038/srep25998
Bernichtein S, Touraine P, Goffin V (2010) New concepts in prolactin biology. J Endocrinol 206(1):1–11. https://doi.org/10.1677/JOE-10-0069
Goffin V (2017) Prolactin receptor targeting in breast and prostate cancers: new insights into an old challenge. Pharmacol Ther 179:111–126. https://doi.org/10.1016/j.pharmthera.2017.05.009
Haricharan S, Dong J, Hein S et al (2013) Mechanism and preclinical prevention of increased breast cancer risk caused by pregnancy. Elife 2:e00996. https://doi.org/10.7554/eLife.00996
Secreto G, Girombelli A, Krogh V (2019) Androgen excess in breast cancer development: implications for prevention and treatment. Endocr Relat Cancer 26(2):R81–R94. https://doi.org/10.1530/ERC-18-0429
Morris PG, Hudis CA, Giri D et al (2011) Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 4(7):1021–1029. https://doi.org/10.1158/1940-6207.CAPR-11-0110
Mullooly M, Yang HP, Falk RT et al (2017) Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res 19(1):8. https://doi.org/10.1186/s13058-016-0791-4
Carter JM, Hoskin TL, Pena MA et al (2018) Macrophagic “crown-like structures” are associated with an increased risk of breast cancer in benign breast disease. Cancer Prev Res (Phila) 11(2):113–119. https://doi.org/10.1158/1940-6207.CAPR-17-0245
Chen M, Yang Y, Xu K et al (2020) androgen receptor in breast cancer: from bench to bedside. Front Endocrinol (Lausanne) 11:573. https://doi.org/10.3389/fendo.2020.00573
Oh H, Khodr ZG, Sherman ME et al (2016) Relation of serum estrogen metabolites with terminal duct lobular unit involution among women undergoing diagnostic image-guided breast biopsy. Horm Cancer 7(5–6):305–315. https://doi.org/10.1007/s12672-016-0265-2
Falk RT, Brinton LA, Dorgan JF et al (2013) Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res 15(2):R34. https://doi.org/10.1186/bcr3416
van der Laak J, Litjens G, Ciompi F (2021) Deep learning in histopathology: the path to the clinic. Nat Med 27(5):775–784. https://doi.org/10.1038/s41591-021-01343-4
Funding
The authors thank donors to the Komen Tissue Bank. This work is supported in part by the Mayo Clinic Cancer Center P30CA15083-45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sherman, M.E., de Bel, T., Heckman, M.G. et al. Serum hormone levels and normal breast histology among premenopausal women. Breast Cancer Res Treat 194, 149–158 (2022). https://doi.org/10.1007/s10549-022-06600-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06600-9