Abstract
Purpose
Routine use of the oncotype DX recurrence score (RS) in patients with early-stage, estrogen receptor-positive, HER2-negative (ER+/HER2−) breast cancer is limited internationally by cost and availability. We created a supervised machine learning model using clinicopathologic variables to predict RS risk category in patients aged over 50 years.
Methods
From January 2012 to December 2018, we identified patients aged over 50 years with T1–2, ER+/HER2−, node-negative tumors. Clinicopathologic data and RS results were randomly split into training and validation cohorts. A random forest model with 500 trees was developed on the training cohort, using age, pathologic tumor size, histology, progesterone receptor (PR) expression, lymphovascular invasion (LVI), and grade as predictors. We predicted risk category (low: RS ≤ 25, high: RS > 25) using the validation cohort.
Results
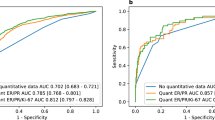
Of the 3880 tumors identified, 1293 tumors comprised the validation cohort in patients of median (IQR) age 62 years (56–68) with median (IQR) tumor size 1.2 cm (0.8–1.7). Most tumors were invasive ductal (80.3%) of low-intermediate grade (80.5%) without LVI (80.9%). PR expression was ≤ 20% in 27.3% of tumors. Specificity for identifying RS ≤ 25 was 96.3% (95% CI 95.0–97.4) and the negative predictive value was 92.9% (95% CI 91.2–94.4). Sensitivity and positive predictive value for predicting RS > 25 was lower (48.3 and 65.1%, respectively).
Conclusion
Our model was highly specific for identifying eligible patients aged over 50 years for whom chemotherapy can be omitted. Following external validation, it may be used to triage patients for RS testing, if predicted to be high risk, in resource-limited settings.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sparano JA, Gray RJ, Makower DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. https://doi.org/10.1056/NEJMoa041588
Harris LN, Ismaila N, McShane LM et al (2016) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 34(10):1134–1150. https://doi.org/10.1200/JCO.2015.65.2289
Andre F, Ismaila N, Henry NL et al (2019) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol 37(22):1956–1964. https://doi.org/10.1200/JCO.19.00945
National Comprehensive Cancer Network (2020) Breast cancer (Version 6.2020). http://www.nccn.org/professionals/physician_gls/breast.pdf. Accessed 21 November 2020
Guth AA, Fineberg S, Fei K, Franco R, Bickell NA (2013) Utilization of oncotype DX in an inner city population: race or place? Int J Breast Cancer 2013:653805. https://doi.org/10.1155/2013/653805
Lund MJ, Mosunjac M, Davis KM et al (2012) 21-Gene recurrence scores: Racial differences in testing, scores, treatment, and outcome. Cancer 118(3):788–796. https://doi.org/10.1002/cncr.26180
Orucevic A, Heidel RE, Bell JL (2016) Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: lessons learned from the 2010 to 2012 national cancer data base analysis. Breast Cancer Res Treat 157(3):427–435. https://doi.org/10.1007/s10549-016-3833-9
Chin-Lenn L, De Boer RH, Segelov E et al (2018) The impact and indications for oncotype DX on adjuvant treatment recommendations when third-party funding is unavailable. Asia Pac J Clin Oncol 14(6):410–416. https://doi.org/10.1111/ajco.13075
Paik S, Tang G, Shak S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734. https://doi.org/10.1200/JCO.2005.04.7985
Allison KH, Kandalaft PL, Sitlani CM, Dintzis SM, Gown AM (2012) Routine pathologic parameters can predict oncotype DX recurrence scores in subsets of ER positive patients: who does not always need testing? Breast Cancer Res Treat 131(2):413–424. https://doi.org/10.1007/s10549-011-1416-3
Cuzick J, Dowsett M, Pineda S et al (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the genomic health recurrence score in early breast cancer. J Clin Oncol 29(32):4273–4278. https://doi.org/10.1200/JCO.2010.31.2835
Klein ME, Dabbs DJ, Shuai Y et al (2013) Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol 26(5):658–664. https://doi.org/10.1038/modpathol.2013.36
Turner BM, Skinner KA, Tang P et al (2015) Use of modified Magee equations and histologic criteria to predict the oncotype DX recurrence score. Mod Pathol 28(7):921–931. https://doi.org/10.1038/modpathol.2015.50
Eaton AA, Pesce CE, Murphy JO et al (2017) Estimating the OncotypeDX score: validation of an inexpensive estimation tool. Breast Cancer Res Treat 161(3):435–441. https://doi.org/10.1007/s10549-016-4069-4
Ingoldsby H, Webber M, Wall D, Scarrott C, Newell J, Callagy G (2013) Prediction of oncotype DX and TAILORx risk categories using histopathological and immunohistochemical markers by classification and regression tree (CART) analysis. Breast 22(5):879–886. https://doi.org/10.1016/j.breast.2013.04.008
Gage MM, Rosman M, Mylander WC et al (2015) A validated model for identifying patients unlikely to benefit from the 21-gene recurrence score assay. Clin Breast Cancer 15(6):467–472. https://doi.org/10.1016/j.clbc.2015.04.006
Kim HS, Umbricht CB, Illei PB et al (2016) Optimizing the use of gene expression profiling in early-stage breast cancer. J Clin Oncol 34(36):4390–4397. https://doi.org/10.1200/JCO.2016.67.7195
Orucevic A, Bell JL, McNabb AP, Heidel RE (2017) Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat 163(1):51–61. https://doi.org/10.1007/s10549-017-4170-3
Orucevic A, Bell JL, King M, McNabb AP, Heidel RE (2019) Nomogram update based on TAILORx clinical trial results—oncotype DX breast cancer recurrence score can be predicted using clinicopathologic data. Breast 46:116–125. https://doi.org/10.1016/j.breast.2019.05.006
Kim I, Choi HJ, Ryu JM et al (2019) A predictive model for high/low risk group according to oncotype DX recurrence score using machine learning. Eur J Surg Oncol 45(2):134–140. https://doi.org/10.1016/j.ejso.2018.09.011
Early Breast Cancer Trialists Collaborative Group (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
Fisher B, Dignam J, Wolmark N et al (1997) Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst 89(22):1673–1682. https://doi.org/10.1093/jnci/89.22.1673
Williams AD, Reyes SA, Arlow RL, Tchou J, De La Cruz LM (2018) Is age trumping genetic profiling in clinical practice? Relationship of chemotherapy recommendation and oncotype DX recurrence score in patients aged < 50 years versus ≥ 50 years, and trends over time. Ann Surg Oncol 25(10):2875–2883. https://doi.org/10.1245/s10434-018-6600-9
Polley MY, Leung SC, McShane LM et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906. https://doi.org/10.1093/jnci/djt306
Pan H, Gray R, Braybrooke J et al (2017) 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377(19):1836–1846. https://doi.org/10.1056/NEJMoa1701830
Pesce C, Kuchta K, Erdogan B, Wang C, Yao K, El-Tamer M (2018) Predicting oncotype DX scores using clinicopathologic features: a report from the national cancer database. J Clin Oncol 36(15_suppl):551. https://doi.org/10.1200/JCO.2018.36.15_suppl.551
Hou Y, Zynger DL, Li X, Li Z (2017) Comparison of oncotype DX with modified Magee equation recurrence scores in low-grade invasive carcinoma of breast. Am J Clin Pathol 148(2):167–172. https://doi.org/10.1093/ajcp/aqx059
Acs G, Esposito NN, Kiluk J, Loftus L, Laronga C (2012) A mitotically active, cellular tumor stroma and/or inflammatory cells associated with tumor cells may contribute to intermediate or high oncotype DX Recurrence Scores in low-grade invasive breast carcinomas. Mod Pathol 25(4):556–566. https://doi.org/10.1038/modpathol.2011.194
Kalinsky K, Barlow WE, Meric-Bernstam F, et al (2020) First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET)+/- chemotherapy (CT) in patients (pts) with 1–3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer (BC) with recurrence score (RS) < 25: SWOG S1007 (RxPonder). Presented at 2020 SABCS, virtual, Abstract GS1-00, 8–12 December 2020
Acknowledgements
The authors thank Summer Koop, MSc, editor and Michael Mcgregor, MA, MFA, editor at Memorial Sloan Kettering Cancer Center, for editing the manuscript.
Funding
The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Monica Morrow has received honoraria from Roche. All other authors have no conflict of interest disclosures to report. This study was presented in poster format at the American Society of Clinical Oncology Annual Meeting in 2020. The findings presented in this manuscript have not been published elsewhere.
Ethical approval
This study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Informed consent
Informed consent was waived by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pawloski, K.R., Gonen, M., Wen, H.Y. et al. Supervised machine learning model to predict oncotype DX risk category in patients over age 50. Breast Cancer Res Treat 191, 423–430 (2022). https://doi.org/10.1007/s10549-021-06443-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06443-w