Abstract
Purpose
Body composition parameters including low muscle mass, muscle attenuation (which reflects muscle quality) and adipose tissue measurements have emerged as prognostic factors in cancer patients. However, knowledge regarding the possibility of excessive muscle loss during specific systemic therapies is unknown. We describe the changes in body composition and muscle attenuation (MA) during taxane- and anthracycline-based regimens and its association with overall survival (OS) in metastatic breast cancer patients.
Methods
The lumbar skeletal muscle index (LSMI) was used as marker of muscle mass. LSMI, MA, subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT) and intramuscular adipose tissue (IMAT) were measured before and after first-line treatment with paclitaxel (n = 73) or 5-fluorouracil-doxorubicin-cyclophosphamide (FAC) (n = 25) using CT-images. Determinants of the change of LSMI and MA were analyzed using multiple linear regression. OS was assessed using Cox proportional hazard models.
Results
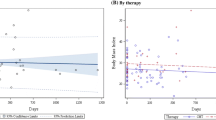
MA significantly decreased during paclitaxel treatment (− 0.9 HU, p = 0.03). LSMI (p = 0.40), SAT (p = 0.75), VAT (p = 0.84) and IMAT (p = 0.10) remained stable. No significant alterations in body composition parameters during FAC-treatment were observed. Previous (neo-)adjuvant chemotherapy contributed to larger loss of MA during the current treatment. Body composition changes during chemotherapy were not associated with OS.
Conclusions
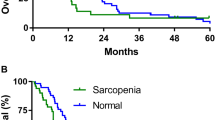
MA decreased during treatment with paclitaxel, while muscle mass was stable. Body composition changes are not associated with survival in the absence of progressive disease.



Similar content being viewed by others
References
Fielding RA, Vellas B, Evans WJ et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12:249–256
Goodpaster BH, Carlson CL, Visser M et al (1985) Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol 2001(90):2157–2165
Aubrey J, Esfandiari N, Baracos VE et al (2014) Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol 210:489–497
Kitamura I, Koda M, Otsuka R et al (2014) Six-year longitudinal changes in body composition of middle-aged and elderly Japanese: age and sex differences in appendicular skeletal muscle mass. Geriatr Gerontol Int 14:354–361
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Kazemi-Bajestani SM, Mazurak VC, Baracos V (2016) Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 54:2–10
Cooper AB, Slack R, Fogelman D et al (2015) Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 22:2416–2423
Rier HN, Jager A, Sleijfer S et al (2016) Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast 31:9–15
Antoun S, Lanoy E, Iacovelli R et al (2013) Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 119:3377–3384
Hayashi N, Ando Y, Gyawali B et al (2016) Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep 35:1727–1731
Dalal S, Hui D, Bidaut L et al (2012) Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manage 44:181–191
Miyamoto Y, Baba Y, Sakamoto Y et al (2015) Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS ONE 10:e0129742
Rutten IJ, van Dijk DP, Kruitwagen RF et al (2016) Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle 7:458–466
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA et al (2016) Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34:1339–1344
Cooper AB, Slack R, Fogelman D et al (2015) Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 22:2416–2423
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Shachar SS, Williams GR, Muss HB et al (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67
Ghersi D, Willson ML, Chan MM et al (2015) Taxane-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev 6:003366
Chiu N, Chiu L, Chow R et al (2016) Taxane-induced arthralgia and myalgia: a literature review. J Oncol Pharm Pract 23:56–57
Al-Majid S, Waters H (2008) The biological mechanisms of cancer-related skeletal muscle wasting: the role of progressive resistance exercise. Biol Res Nurs 10:7–20
Shen W, Punyanitya M, Wang Z et al (1985) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004(97):2333–2338
Baracos VE, Reiman T, Mourtzakis M et al (2010) Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 91:1133S–1137S
Rollins KE, Tewari N, Ackner A et al (2016) The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr 35:1103–1109
Akahori T, Sho M, Kinoshita S et al (2015) Prognostic significance of muscle attenuation in pancreatic cancer patients treated with neoadjuvant chemoradiotherapy. World J Surg 39:2975–2982
Short KR, Nygren J, Bigelow ML et al (2004) Effect of short-term prednisone use on blood flow, muscle protein metabolism, and function. J Clin Endocrinol Metab 89:6198–6207
Louard RJ, Bhushan R, Gelfand RA et al (1994) Glucocorticoids antagonize insulin’s antiproteolytic action on skeletal muscle in humans. J Clin Endocrinol Metab 79:278–284
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Fujiwara N, Nakagawa H, Kudo Y et al (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63:131–140
Tamandl D, Paireder M, Asari R et al (2016) Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 26:1359–1367
Stene GB, Helbostad JL, Amundsen T et al (2015) Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol 54:340–348
Yip C, Goh V, Davies A et al (2014) Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 24:998–1005
Stephens NA, Skipworth RJ, Macdonald AJ et al (2011) Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J Cachexia Sarcopenia Muscle 2:111–117
Prado CM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13:3264–3268
Acknowledgements
We thank Suze Roodenburg-Kooij for identifying the L3-level at all CT-images. We thank Daan Blinde for his participation in identifying patients eligible for inclusion using the radiology-registration in the Albert Schweitzer Hospital. We thank the ORAS foundation (Oncological Research Albert Schweitzer hospital) and the Leerhuis of the Albert Schweitzer hospital, Dordrecht, the Netherlands for the foundation of this study.
Funding
Funded by the ORAS foundation (Oncological Research Albert Schweitzer hospital) and the Leerhuis of the Albert Schweitzer hospital, Dordrecht, the Netherlands. The foundation had no involvement in the conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2017_4574_MOESM1_ESM.tif
Kaplan–Meier curve for overall survival between included and excluded patients. The excluded patients undergoing palliative chemotherapy but without abdominal CT-imaging before and/or after treatment were included in this analysis. Median OS for the included patients was 33 months and for the excluded patients 23 months (log-rank p = 0.012). Supplementary material 1 (TIFF 78 kb)
Rights and permissions
About this article
Cite this article
Rier, H.N., Jager, A., Sleijfer, S. et al. Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 168, 95–105 (2018). https://doi.org/10.1007/s10549-017-4574-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4574-0