Abstract
Purpose
Cyclin D1 has a central role in cell cycle control and is an important component of estrogen regulation of cell cycle progression. We have previously shown that high cyclin D expression is related to aggressive features of ER-positive but not ER-negative breast cancer. The aims of the present study were to validate this differential ER-related effect and furthermore explore the relationship between cyclin D overexpression and CCND1 gene amplification status in a node-negative breast cancer case–control study.
Methods
Immunohistochemical nuclear expression of cyclin D1 (n = 364) and amplification of the gene CCND1 by fluorescent in situ hybridization (n = 255) was performed on tissue microarray sections from patients with T1-2N0M0 breast cancer. Patients given adjuvant chemotherapy were excluded. The primary event was defined as breast cancer death. Breast cancer-specific survival was analyzed in univariate and multivariable models using conditional logistic regression.
Results
Expression of cyclin D1 above the median (61.7%) in ER breast cancer was associated with an increased risk for breast cancer death (OR 3.2 95% CI 1.5–6.8) also when adjusted for tumor size and grade (OR 3.1). No significant prognostic impact of cyclin D1 expression was found among ER-negative cases. Cyclin D1 overexpression was significantly associated to high expression of the proliferation markers cyclins A (ρ 0.19, p = 0.006) and B (ρ 0.18, p = 0.003) in ER-positive tumors, but not in ER-negative cases. There was a significant association between CCND1 amplification and cyclin D1 expression (p = 0.003), but CCND1 amplification was not statistically significantly prognostic (HR 1.4, 95% CI 0.4–4.4).
Conclusion
We confirmed our previous observation that high cyclin D1 expression is associated to high proliferation and a threefold higher risk of death from breast cancer in ER-positive breast cancer.
Similar content being viewed by others
Introduction
Breast cancer is a heterogeneous disease. Decisions about adjuvant treatment have traditionally been based upon prognostic factors such as age, tumor size, histological grade, proliferation, lymph node involvement, HER2 status, estrogen receptor (ER), progesterone receptor (PgR), and gene expression assays like Oncotype DX [1]. The most commonly used proliferation marker in breast cancer hitherto has been Ki-67, although problems of method standardization cut-offs and reproducibility still remain [2].
Cyclin D1 is a member of the cyclin protein family initiated during G1 and drives the G1/S phase transition. Cyclin D1 binds to CDK4 and CDK6 and induces hyperphosphorylation of Rb, thereby promoting cellular proliferation [3]. Aberrant cyclin D1 expression is common in breast cancer [4]. Cyclin D1 expression has previously been shown to correlate strongly to ER positivity and deregulation of cyclin D1 has been associated with resistance to endocrine therapy in breast cancer cell lines [5, 6], while the role of cyclin D1 overexpression and endocrine resistance in the clinic is still controversial [5, 7–12]. The corresponding gene CCND1 is amplified in approximately 9–30% in breast cancer [10, 13–16]. The importance of improved understanding of cyclin D1 signaling in cancer has recently been underscored due to the introduction of a new class of antineoplastic drugs, the CDK 4/6 inhibitors targeting cell cycle activation by cyclin D1 in breast cancer and other malignant diseases [3].
A large number of previous studies have investigated the prognostic impact of cyclin D1 expression or gene amplification in primary breast cancer [5, 7, 10–14, 16–49].
Most of these studies have used immunohistochemical expression of the cyclin D1 expression [7, 10, 12, 13, 16–22, 25, 26, 28, 29, 31–42, 44, 45, 47–49], some mRNA expression [5, 23, 29, 30, 46], some amplification of the CCND1 gene [10, 11, 13, 14, 16, 24, 27, 43], and several methods [10, 13, 16, 29]. There is still no consensus about which method of assessment of cyclin D1 signaling aberrations is optimal. Studies, which have used several methods of cyclin D1, have, however, shown the results of these to be significantly positively correlated. Seven studies have reported a significant positive association between CCND1 gene amplification and protein expression in addition to the present one [10, 13, 16, 28, 35, 38, 41]. Only one study [11] failed to find this association. A highly significant correlation (ρ 0.43, p < 0.0001) between cyclin D1 mRNA and protein expression has also been reported [29].
The prognostic impact of cyclin D1 amplification or overexpression in unselected breast cancer has been inconsistent; 10 studies reported cyclin D1 expression to be a favorable prognostic marker [19, 25, 28, 32, 37–39, 41, 46, 49], 8 to be unfavorable [13, 14, 18, 26, 33, 34, 42, 44], and 21 studies found no association [7, 11, 12, 16, 17, 20–24, 27, 29–31, 35, 36, 40, 43, 45, 47, 48].
Thirteen studies have studied the impact of cyclin D1 overexpression or gene amplification in ER-positive breast cancer. Six studies [5, 7, 11, 18, 42, 44] reported high cyclin D1 to be associated to higher risk of recurrence or death, while the results were non-significant in 6 [12, 13, 17, 24, 31, 45]. One study, the transATAC study, reported gene amplification to be of adverse prognostic impact, while high expression of nuclear cyclin D1 was favorable [10].
Six previous studies have analyzed the prognostic impact of cyclin D1 in ER-negative breast cancer. Overexpression was reported to be a favorable factor in two [25, 37], unfavorable in one [45], and of no prognostic impact in three studies [5, 17, 31].
In accordance with these previous inconsistent results, the recent meta-analysis by Xiao-Ling found no overall prognostic effect of cyclin D1 signaling aberrations when ER-positive and ER-negative cases were analyzed together [50]. However, cyclin D1 overexpression was consistently and significantly associated to worse prognosis in ER-positive cases.
We have previously demonstrated that high cyclin D1 expression is linked to increased proliferation in ER-positive breast cancer, while no association was seen in ER-negative disease [17]. Moreover, high cyclin D1 expression was related to shorter metastasis-free survival in patients not receiving adjuvant chemotherapy.
The aim of this study was to further explore the prognostic effect of cyclin D1 aberrations in relation to ER status in a patient material designed for evaluation of prognostic factors in early breast cancer. Furthermore, we wanted to explore the association between the protein expression of cyclin D1 and its corresponding gene, CCND1.
Patients and methods
The source population of the study was a defined cohort of women diagnosed with breast cancer in the Uppsala-Örebro region during 1993–2004 as previously described [51]. The inclusion criteria were tumor size ≤50 mm, no lymph node metastases, and no adjuvant chemotherapy. From the whole cohort of 900 patients, 190 cases and 190 controls (n = 380) were chosen.
Sixteen cases were non-evaluable due to lack of tumor material, leaving 364 samples for Cyclin D1 analyses. Two hundred and fifty-five samples were evaluable for CCND1 amplification analyses (Fig. 1).
The study was approved by the local ethics committee in Uppsala, Sweden.
TMA construction
Paraffin blocks from the patients’ primary tumors were collected. Hematoxylin and eosin sections were reviewed and areas with invasive tumor were selected. Representative areas from each tumor were punched and brought into recipient paraffin blocks to construct TMAs consisting of two cores (diameter 1 mm) of each tumor. 3–4-μm-thick sections were cut from array blocks and transferred to glass slides.
Immunohistochemistry
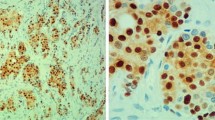
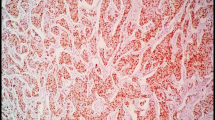
TMA slides were deparaffinized in xylene and rehydrated through a ladder of graded ethanol (absolute ethanol, 95%, 80% and distilled water). For detection of cyclin D1 (RM-9104-S; NeoMarkers), antigen retrieval was performed in a microwave oven for 10 min (750 W) + 15 min (350 W) with the use of a TE (Tris–EDTA pH9 buffer). After antigen retrieval, all TMA slides were processed in an automatic immunohistochemistry staining machine according to standard procedures (Autostainer, Dako, Sweden).
Evaluation of immunoreactivity scores
All cyclin D1 stainings were scored by one investigator (Ahlin C) blinded to all clinical information during scoring. Cells were manually counted in high-power fields. Only unequivocal nuclear staining was accepted. Hot spots were chosen for evaluation, and a minimum of 200 cells per patient were counted. Staining procedure and scoring of ER, PR, Ki-67, cyclin A, cyclin B, cyclin E, and HER2 have been described previously [51–53].
Fluorescence in situ hybridization (FISH) analysis
For two-color FISH analysis, 4-μm-thick TMA sections were cut, mounted on positively charged glass slides (Superfrost™ Plus, Thermo Fisher Scientific) and dried. Sections were then deparaffinized in xylene, dehydrated in absolute ethanol, and subsequently pretreated using a commercial kit (Vysis paraffin pretreatment reagent kit, Abbott molecular) whereby the slides were immersed in pretreatment solution for 30 min at 80 °C followed by incubation in protease solution for 37 min at 37 °C.
FISH analyses were performed using a dual-probe kit containing an orange-labeled CCND1-specific and green-labeled CEP 11 centromere probe (Vysis CCND1/CEP 11 FISH probe kit, Abbott molecular). Hybridization and post-hybridization washes were performed according to the manufacturer’s protocol. Slides were briefly dipped in dH2O after being washed and ProLong® Gold Antifade Mountant with DAPI (Thermo Fisher Scientific) was applied directly.
Gene-specific and centromere copy numbers were estimated by counting ≥20 nuclei in two tissue cores per case at 100x magnitude. A ratio of 1.8 or higher for CCND1/CEP was classified as CCND1 amplification. All FISH analyses were performed by one investigator (Embretsén-Varro E.) blinded to all clinical information.
Statistical analyses
To obtain unbiased estimates of relative risk, controls were selected by incidence density sampling, which involves matching each case to a sample of those who were at risk at the time of the case occurrence.
The loss of power in comparison with a complete analysis of all cohort members is small since approximately 20% of the entire cohort was chosen as control and all eligible women with an event were included. Conditional logistic regression analysis was performed to estimate the odds ratios (ORs) and confidence interval (CI) using the proportional hazard regression procedure in statistical analysis software (IBM SPSS version 23).
Correlations of Ki 67, cyclin A, cyclin B, cyclin D, and cyclin E to other clinicopathologic parameters were evaluated using Spearman’s correlation test. Cut-off values used for cyclin A, cyclin B, cyclin E, and Ki 67 were defined as the 7th decile as previously described [51–53]. The median (61.7%) was chosen as the cut-off point for cyclin D1, but all analyses were performed also with cyclin D1 as a continuous variable.
Results
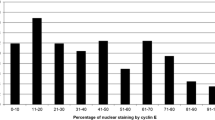
Patient and tumor characteristics according to cyclin D1 status are shown in Table 1. Median cyclin D1 expression was 61.7, 68.7% in ER-positive tumors and 34.3% in ER-negative cases. The association between ER content (percent nuclear staining) and high cyclin D1 was highly significant (Table 2). Cyclin D1 expression was below 1% in 1 of 242 ER-positive cases (0.4%) and in 6 of 115 ER-negative cases (6%). The distributions of cyclin D1 values were different in ER-positive and ER-negative tumors. Although overlapping, cyclin D1 peaked at 80% in ER-positive and less than 5% in ER-negative tumors (Fig. 2).
Correlation between ER, cyclin D1, and proliferation markers including grade
High cyclin D1 expression was significantly associated to PgR positivity and lower grade (Table 1) and ER positivity (Table 2) when ER-positive and ER-negative cancers were tested together. The associations to PgR positivity and low grade were significant only in ER-negative tumors.
A high ER receptor content was significantly associated to low tumor grade, low Ki-67 expression, and low levels of cyclins A, B, and E. In contrast, cyclin D1 showed a positive correlation to ER receptor content (Table 2).
In ER-positive tumors, high cyclin D1 expression showed a significant correlation to high expression of cyclin A and cyclin B. The correlations between cyclin D1 and other proliferation-associated factors were weakly and mostly non-significantly negative in ER-negative tumors (Table 2). The negative correlation between cyclin D1 and cyclin E in ER-negative tumors, however, reached significance (ρ −0.21, p = 0.025).
Prognostic effect of cyclin D1 expression
Cyclin D1 expression was not significantly associated to breast cancer mortality in the study population when ER-positive and ER-negative cases were analyzed together (OR 0.94, 95% CI 0.63–1.4, p = 0.76).
However, in the ER-positive group, high cyclin D1 expression had a significant and strong negative effect on breast cancer mortality in both univariable (OR 3.2) and multivariable (OR 3.1) analyses (Table 3). Cyclin D1 expression remained significantly associated to breast cancer mortality in ER-positive cases also when analyzed as a continuous variable in univariable (p = 0.01) and multivariable (p = 0.03) analyses (data not shown).
The prognostic effect of cyclin D1 expression in ER-positive cases was seen both in patients with (OR 2.0, 95% CI 0.37–10.9, p = 0.4, n = 77) and without (OR 4.0, 95% CI 1.1–14.2, p = 0.03, n = 165) adjuvant endocrine therapy.
CCND1
The median (1.13) and mean (1.24) values of CCND1 copy number were close to 1 in most cases. No tumor had a quotient clearly less than 1. Only five tumors had a value smaller than 1, and the lowest quotient was 0.91. Fourteen tumors (5%) had a quotient higher than or equal to 1.8 and were considered to have amplification of the CCND1 gene. The highest quotient was 4.35.
Correlation of CCND1 amplification and tumor characteristics
There was a statistically significant association between CCND1 amplification and high expression of cyclin D1 (p = 0.003). Mean cyclin D1 expression was 55% in tumors with normal copy number of CCND1 and 78% in the 14 amplified cases. A significant association between CCND1 amplification and cyclin D1 expression was found in both ER-positive (p = 0.003) and ER-negative tumors (p = 0.05). No significant association was found between gene amplification and tumor grade or proliferation markers (Table 4).
Prognostic effect of CCND1 amplification
CCND1 amplification had no significant prognostic impact on breast cancer mortality in all cases together (OR 1.4, 95% CI 0.4–4.4, p value 0.56) or in ER-positive and ER-negative cases separately (data not shown).
Discussion
In a previous study in unselected cases of breast cancer, we found that the prognostic impact of cyclin D expression was dependent on ER receptor status [17]. In ER receptor-positive disease, high cyclin D expression was associated to high proliferation and other markers of tumor aggressiveness, while the opposite was true in ER-negative cases. Moreover, high cyclin D1 was a significant adverse prognostic factor for metastasis-free survival in chemotherapy-naïve patients with ER-positive tumors. The primary aim of the present study was to confirm these findings. Additionally, since amplification of the cyclin D gene CCND1 is one of the reasons for high cyclin D1 expression in breast cancer, we also studied the copy number of CCND1, and its association to cyclin D1 expression and prognosis.
The patients included in this study [51] were selected from the regional cancer registry of Uppsala-Örebro region in order to optimize the analysis of prognostic markers, especially cyclins and other proliferation markers in early breast cancer. Cases given adjuvant chemotherapy were excluded, since there is evidence that adjuvant chemotherapy may interfere with the prognostic effect of proliferation, possibly due to a better effect of chemotherapy in proliferating cells [17, 54].
Cyclin D1 expression was strongly associated to ER positivity in accordance with numerous previous reports [5, 12, 13, 15, 19, 27, 28, 31, 37–41, 43, 45–47, 49, 55–58]. Many cases with high cyclin D1 expression were nevertheless also found in ER-negative tumors. Low expression of cyclin D1 was almost exclusively seen in ER-negative cases.
This study confirms that the prognostic impact of cyclin D1 expression indeed depends on ER status. High expression increased breast cancer mortality in ER-positive cases, while no significant impact was seen in ER-negative cases. In ER-positive cases, cyclin D1 expression was significantly associated to markers of high proliferation including cyclins A and B. No such association was found in ER-negative tumors. On the contrary, like in our previous study [17], there was even an inverse association between the expression of the cyclins D1 and E. An early study by Kenny et al., analyzing cyclin D1 mRNA expression, came to similar conclusions, showing that cyclin D1 mRNA levels were significantly associated to relapse and breast cancer death in ER-positive disease, while no association was found in ER-negative disease [5]. This indicates that high cyclin D1 expression is linked to an activated cell cycle and worse prognosis of breast cancer in ER-positive disease, while cyclin D1 expression does not associate to markers of cell cycle activation or prognosis in ER-negative tumors. Many previous studies focusing on ER-positive breast cancer have found high cyclin D1 expression or gene amplification to be an adverse prognostic sign [5, 7, 11, 18, 42, 44, 50], while the results in ER-negative and in unselected breast cancer patients have been highly inconsistent. For further details, see the Introduction section.
One study, the TransATAC study, has reported results partly at odds with our results and other studies on ER-positive breast cancer [10]. The TransATAC study is a biomarker study on histological samples collected from patients participating in the ATAC trial, which is a randomized trial comparing anastrozole to tamoxifen adjuvant treatment in primary breast cancer. In the TransATAC study, cases with high cyclin D1 expression had a significantly longer time to recurrence and better overall survival in both univariable and multivariable analyses. However, like our study high expression of cyclin D1 was associated to gene amplification of the CCND1 and a high proliferation assessed by Ki-67 IH. Both Ki-67 expression and CCND1 gene amplification were, in contrast to cyclin D1 IH expression, associated to a worse outcome. The reason for these partly internally inconsistent associations and the difference between the results of the TransATAC study and those of our as well as the meta-analysis study are not obvious nor did the authors of the TransATAC publication suggest any explanation. TransATAC is by far the largest published study of cyclin D1 signaling and breast cancer prognosis (n = 1155). Thus, it is improbable that these discrepancies are due to chance alone. Several differences between the TransATAC and the current studies may offer at least a partial explanation. In contrast to our study, all patients received adjuvant endocrine treatment. Although the TransATAC analysis was restricted to ER-positive cases, the definition of ER positivity was based on the Allred score with a cut-off for positivity of 2, which corresponds to as few as 1–10% weakly positive cells [59, 60]. In our studies, we used a cut-off of 10% according to Scandinavian practice and the results of the EBCTCG meta-analysis [61]. Visual inspection of the cumulative recurrence rate curves in the TransATAC study indicated that high recurrence rate in cases with very low cyclin D1 expression (<1%) seems to account for most of the prognostic impact of cyclin D1 expression. In our study, most of the cases with cyclin D1 expression below 1% were ER negative. Thus, one may speculate that one reason for the discrepancy may be different ER receptor classification. Interestingly, the curves depicting the recurrence rate in cases with cyclin D1 expression 1–9%, 10–30%, 30–67%, and >67% in the TransATAC did not show any orderly linear association between prognosis and cyclin D1 expression level. Patients with totally negative cyclin D1 expression had the worst prognosis. However, the second worst prognosis was found in patients having high, 30–67%, cyclin D1 expression. This suggests that the association between cyclin D1 and prognosis in ER-positive breast cancer may not be simply linear. A Swedish study investigating cyclin D1 and benefit of adjuvant tamoxifen may support this interpretation; the benefit of adjuvant tamoxifen was restricted to the group with intermediate cyclin D1 expression, and these patients had the best prognosis while the patients with either very high or low cyclin D1 expression did not benefit and had a worse prognosis after endocrine treatment [12].
In conclusion, despite the partly discordant results of the large transATAC study, most previous analyses of the impact of cyclin D1 expression in ER-positive breast cancer have, like the present one, shown high expression to be a sign of tumor aggressiveness and poor prognosis.
A few previous studies have suggested that high cyclin D1 expression may be associated to tamoxifen resistance [7, 42], which might partly explain the negative impact of cyclin D1 in ER-positive breast cancer. We therefore tested the prognostic impact of cyclin D1 expression separately in patients treated or not with adjuvant tamoxifen, but found no indication that the prognostic effect was restricted to adjuvant tamoxifen use; on the contrary, the impact of cyclin D1 expression was even stronger in the group that has not received hormonal treatment. This issue is still controversial, since some studies have found cyclin D1 overexpression to be associated to tamoxifen resistance [5, 7, 8, 12], some to improved effect of endocrine treatment [7–9, 42], and some studies found no association [10, 11]. Although we cannot exclude the possibility that part of the negative impact of cyclin D1 overexpression might be explained by endocrine treatment resistance, the significant association between high cyclin D1 expression and aggressive biological features of the tumor at diagnosis indicates that adjuvant treatment resistance cannot be the only explanation.
The proportion of patients with amplification of CCND1 in the present study was low (5%), in accordance with previous large studies (n = 613, 738, 1155) where between 8.7 and 10% of cases have been found to have amplification [10, 13, 14]. Other smaller studies (n = 93, 117) have published higher frequencies of CCND1 amplification, 24.4 and 30%, respectively [15, 16]. Amplification of CCND1 thus seems to explain only a small part of cases with high cyclin D1 expression. We did not find CCND1 amplification prognostic possibly due to statistical power, since the number of amplified cases was low and confidence levels were wide (1.4, 95% CI 0.4–4.4). Seven previous studies have tested the prognostic impact of CCND1 amplification, of which two reported a significant association between amplification and worse prognosis [10, 14], one reported a significant association in ER-positive cases only [13], while five studies failed to find a significant association [11, 16, 24, 27, 43].
Our results support previous studies on the close link between ER signaling and cyclin D1 in cell cycle activation [62]. Moreover, a recent publication [63] demonstrated that cyclin D1 overexpression increased stem cell-like behavior and migration in ER-positive breast cancer cell lines, while the opposite was true in ER-negative cells [17], indicating that the effect of cyclin D1 expression in ER-positive and ER-negative breast cancer may be fundamentally different. Inhibition of the cyclin D kinases CDK4/6 by palbociclib had selective antitumor efficacy in ER-positive cell lines, while non-luminal/basal subtypes were resistant [64].
Our results may have implications for drugs targeting cyclin D1. The first phase III studies on the CDK4/inhibitors palbociclib [65, 66] or ribociclib [67] have shown impressive results in ER-positive breast cancer combined with letrozole [66, 67] or fulvestrant [65]. We have found only little data concerning the effect of CDK4/6 inhibition in ER-negative breast cancers; a phase II study of palbociclib monotherapy in advanced heavily pretreated breast cancer recruited four cases with triple-negative disease, none of which experienced a response [68].
In conclusion, this study showed that high expression of cyclin D1 is associated to cell cycle activation and poor prognosis in ER-positive tumors only. High cyclin D1 expression was significantly linked to the expression of ER and gene amplification of CCND1, although only a small proportion of cases overexpressing cyclin D1 could be attributed to gene amplification. Although a substantial proportion of ER-negative tumors also express high levels of cyclin D1, the biological role of cyclin D1 signaling in tumors lacking ER, if any, remains to be explored.
References
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F, Committee EG (2015) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v8–30. doi:10.1093/annonc/mdv298
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM, Gown AM, Symmans WF, Piper T, Mehl E, Enos RA, Hayes DF, Dowsett M, Nielsen TO, International Ki67 in Breast Cancer Working Group of the Breast International G, North American Breast Cancer G (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906. doi:10.1093/jnci/djt306
O’Leary B, Finn RS, Turner NC (2016) Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 13(7):417–430. doi:10.1038/nrclinonc.2016.26
Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J (1994) Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 57(3):353–361
Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, Sutherland RL, Robertson JF (1999) Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res 5(8):2069–2076
Arnold A, Papanikolaou A (2005) Cyclin D1 in breast cancer pathogenesis. J Clin Oncol 23(18):4215–4224. doi:10.1200/JCO.2005.05.064
Stendahl M, Kronblad A, Ryden L, Emdin S, Bengtsson NO, Landberg G (2004) Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer 90(10):1942–1948. doi:10.1038/sj.bjc.6601831
Jirstrom K, Stendahl M, Ryden L, Kronblad A, Bendahl PO, Stal O, Landberg G (2005) Adverse effect of adjuvant tamoxifen in premenopausal breast cancer with cyclin D1 gene amplification. Cancer Res 65(17):8009–8016. doi:10.1158/0008-5472.CAN-05-0746
Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim YD, Kim HY (2003) Cyclin D1 expression and patient outcome after tamoxifen therapy in estrogen receptor positive metastatic breast cancer. Oncol Rep 10(1):141–144
Lundgren K, Brown M, Pineda S, Cuzick J, Salter J, Zabaglo L, Howell A, Dowsett M, Landberg G, Ai Trans (2012) Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res 14(2):R57. doi:10.1186/bcr3161
Bostner J, Ahnstrom Waltersson M, Fornander T, Skoog L, Nordenskjold B, Stal O (2007) Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene 26(49):6997–7005. doi:10.1038/sj.onc.1210506
Ahnstrom M, Nordenskjold B, Rutqvist LE, Skoog L, Stal O (2005) Role of cyclin D1 in ErbB2-positive breast cancer and tamoxifen resistance. Breast Cancer Res Treat 91(2):145–151. doi:10.1007/s10549-004-6457-4
Elsheikh S, Green AR, Aleskandarany MA, Grainge M, Paish CE, Lambros MB, Reis-Filho JS, Ellis IO (2008) CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Cancer Res Treat 109(2):325–335. doi:10.1007/s10549-007-9659-8
Rodriguez C, Hughes-Davies L, Valles H, Orsetti B, Cuny M, Ursule L, Kouzarides T, Theillet C (2004) Amplification of the BRCA2 pathway gene EMSY in sporadic breast cancer is related to negative outcome. Clin Cancer Res 10(17):5785–5791. doi:10.1158/1078-0432.CCR-03-0410
Roy PG, Pratt N, Purdie CA, Baker L, Ashfield A, Quinlan P, Thompson AM (2010) High CCND1 amplification identifies a group of poor prognosis women with estrogen receptor positive breast cancer. Int J Cancer 127(2):355–360. doi:10.1002/ijc.25034
Takano Y, Takenaka H, Kato Y, Masuda M, Mikami T, Saegusa M, Okayasu I (1999) Cyclin D1 overexpression in invasive breast cancers: correlation with cyclin-dependent kinase 4 and oestrogen receptor overexpression, and lack of correlation with mitotic activity. J Cancer Res Clin Oncol 125(8–9):505–512
Aaltonen K, Amini RM, Landberg G, Eerola H, Aittomaki K, Heikkila P, Nevanlinna H, Blomqvist C (2009) Cyclin D1 expression is associated with poor prognostic features in estrogen receptor positive breast cancer. Breast Cancer Res Treat 113(1):75–82. doi:10.1007/s10549-008-9908-5
Beca F, Pereira M, Cameselle-Teijeiro JF, Martins D, Schmitt F (2015) Altered PPP2R2A and Cyclin D1 expression defines a subgroup of aggressive luminal-like breast cancer. BMC Cancer 15:285. doi:10.1186/s12885-015-1266-1
Bilalovic N, Vranic S, Basic H, Tatarevic A, Selak I (2005) Immunohistochemical evaluation of cyclin D1 in breast cancer. Croat Med J 46(3):382–388
Bonnefoi H, Diebold-Berger S, Therasse P, Hamilton A, van de Vijver M, MacGrogan G, Shepherd L, Amaral N, Duval C, Drijkoningen R, Larsimont D, Piccart M (2003) Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: are there molecular markers in the primary tumour that predict for 5-year clinical outcome? Ann Oncol 14(3):406–413
Bukholm IR, Bukholm G, Nesland JM (2001) Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int J Cancer 93(2):283–287
Chen S, Chen CM, Yu KD, Yang WT, Shao ZM (2012) A prognostic model to predict outcome of patients failing to achieve pathological complete response after anthracycline-containing neoadjuvant chemotherapy for breast cancer. J Surg Oncol 105(6):577–585. doi:10.1002/jso.22140
Cheng CW, Liu YF, Yu JC, Wang HW, Ding SL, Hsiung CN, Hsu HM, Shieh JC, Wu PE, Shen CY (2012) Prognostic significance of cyclin D1, beta-catenin, and MTA1 in patients with invasive ductal carcinoma of the breast. Ann Surg Oncol 19(13):4129–4139. doi:10.1245/s10434-012-2541-x
Choschzick M, Heilenkotter U, Lebeau A, Jaenicke F, Terracciano L, Bokemeyer C, Sauter G, Simon R (2010) MDM2 amplification is an independent prognostic feature of node-negative, estrogen receptor-positive early-stage breast cancer. Cancer Biomark 8(2):53–60. doi:10.3233/DMA-2011-0806
Gillett C, Smith P, Gregory W, Richards M, Millis R, Peters G, Barnes D (1996) Cyclin D1 and prognosis in human breast cancer. Int J Cancer 69(2):92–99. doi:10.1002/(SICI)1097-0215(19960422)69:2<92:AID-IJC4>3.0.CO;2-Q
Guo LL, Gao P, Wu YG, Jian WC, Hao CY, Li H, Lin XY (2007) Alteration of cyclin D1 in Chinese patients with breast carcinoma and its correlation with Ki-67, pRb, and p53. Arch Med Res 38(8):846–852. doi:10.1016/j.arcmed.2007.06.004
Husdal A, Bukholm G, Bukholm IR (2006) The prognostic value and overexpression of cyclin A is correlated with gene amplification of both cyclin A and cyclin E in breast cancer patient. Cell Oncol 28(3):107–116
Hwang TS, Han HS, Hong YC, Lee HJ, Paik NS (2003) Prognostic value of combined analysis of cyclin D1 and estrogen receptor status in breast cancer patients. Pathol Int 53(2):74–80
Jacquemier J, Charafe-Jauffret E, Monville F, Esterni B, Extra JM, Houvenaeghel G, Xerri L, Bertucci F, Birnbaum D (2009) Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Res 11(2):R23. doi:10.1186/bcr2249
Kreike B, Hart G, Bartelink H, van de Vijver MJ (2010) Analysis of breast cancer related gene expression using natural splines and the Cox proportional hazard model to identify prognostic associations. Breast Cancer Res Treat 122(3):711–720. doi:10.1007/s10549-009-0588-6
Lee A, Park WC, Yim HW, Lee MA, Park G, Lee KY (2007) Expression of c-erbB2, cyclin D1 and estrogen receptor and their clinical implications in the invasive ductal carcinoma of the breast. Jpn J Clin Oncol 37(9):708–714. doi:10.1093/jjco/hym082
Lim SC (2003) Role of COX-2, VEGF and cyclin D1 in mammary infiltrating duct carcinoma. Oncol Rep 10(5):1241–1249
Lin H, Huang JF, Qiu JR, Zhang HL, Tang XJ, Li H, Wang CJ, Wang ZC, Feng ZQ, Zhu J (2013) Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp Mol Pathol 94(1):73–78. doi:10.1016/j.yexmp.2012.08.004
Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC (2000) Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97(8):4262–4266. doi:10.1073/pnas.060025397
Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmaker R, Peterse J (1996) A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer 73(6):728–734
Millar EK, Dean JL, McNeil CM, O’Toole SA, Henshall SM, Tran T, Lin J, Quong A, Comstock CE, Witkiewicz A, Musgrove EA, Rui H, Lemarchand L, Setiawan VW, Haiman CA, Knudsen KE, Sutherland RL, Knudsen ES (2009) Cyclin D1b protein expression in breast cancer is independent of cyclin D1a and associated with poor disease outcome. Oncogene 28(15):1812–1820. doi:10.1038/onc.2009.13
Mylona E, Tzelepis K, Theohari I, Giannopoulou I, Papadimitriou C, Nakopoulou L (2013) Cyclin D1 in invasive breast carcinoma: favourable prognostic significance in unselected patients and within subgroups with an aggressive phenotype. Histopathology 62(3):472–480. doi:10.1111/his.12013
Pelosio P, Barbareschi M, Bonoldi E, Marchetti A, Verderio P, Caffo O, Bevilacqua P, Boracchi P, Buttitta F, Barbazza R, Dalla Palma P, Gasparini G (1996) Clinical significance of cyclin D1 expression in patients with node-positive breast carcinoma treated with adjuvant therapy. Ann Oncol 7(7):695–703
Peurala E, Koivunen P, Haapasaari KM, Bloigu R, Jukkola-Vuorinen A (2013) The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res 15(1):R5. doi:10.1186/bcr3376
Reed W, Florems VA, Holm R, Hannisdal E, Nesland JM (1999) Elevated levels of p27, p21 and cyclin D1 correlate with positive oestrogen and progesterone receptor status in node-negative breast carcinoma patients. Virchows Arch 435(2):116–124
Reis-Filho JS, Savage K, Lambros MB, James M, Steele D, Jones RL, Dowsett M (2006) Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol 19(7):999–1009. doi:10.1038/modpathol.3800621
Rudas M, Lehnert M, Huynh A, Jakesz R, Singer C, Lax S, Schippinger W, Dietze O, Greil R, Stiglbauer W, Kwasny W, Grill R, Stierer M, Gnant MF, Filipits M, Austrian B, Colorectal Cancer Study G (2008) Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res 14(6):1767–1774. doi:10.1158/1078-0432.CCR-07-4122
Seshadri R, Lee CS, Hui R, McCaul K, Horsfall DJ, Sutherland RL (1996) Cyclin DI amplification is not associated with reduced overall survival in primary breast cancer but may predict early relapse in patients with features of good prognosis. Clin Cancer Res 2(7):1177–1184
Tobin NP, Lundgren KL, Conway C, Anagnostaki L, Costello S, Landberg G (2012) Automated image analysis of cyclin D1 protein expression in invasive lobular breast carcinoma provides independent prognostic information. Hum Pathol 43(11):2053–2061. doi:10.1016/j.humpath.2012.02.015
Umekita Y, Ohi Y, Sagara Y, Yoshida H (2002) Overexpression of cyclin D1 predicts for poor prognosis in estrogen receptor-negative breast cancer patients. Int J Cancer 98(3):415–418
Utsumi T, Yoshimura N, Maruta M, Takeuchi S, Ando J, Mizoguchi Y, Harada N (2000) Correlation of cyclin D1 MRNA levels with clinico-pathological parameters and clinical outcome in human breast carcinomas. Int J Cancer 89(1):39–43
van Diest PJ, Michalides RJ, Jannink L, van der Valk P, Peterse HL, de Jong JS, Meijer CJ, Baak JP (1997) Cyclin D1 expression in invasive breast cancer. Correlations and prognostic value. Am J Pathol 150(2):705–711
Wachter DL, Fasching PA, Haeberle L, Schulz-Wendtland R, Dimmler A, Koscheck T, Renner SP, Lux MP, Beckmann MW, Hartmann A, Rauh C, Schrauder MG (2013) Prognostic molecular markers and neoadjuvant therapy response in anthracycline-treated breast cancer patients. Arch Gynecol Obstet 287(2):337–344. doi:10.1007/s00404-012-2534-9
Yang C, Nan K, Zhang Y, Chen Y, Qin S (2016) High expression of cyclin D1 is correlated with the expression of estrogen receptor and good prognosis in breast cancer. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 32(1):84–87
Xu XL, Chen SZ, Chen W, Zheng WH, Xia XH, Yang HJ, Li B, Mao WM (2013) The impact of cyclin D1 overexpression on the prognosis of ER-positive breast cancers: a meta-analysis. Breast Cancer Res Treat 139(2):329–339. doi:10.1007/s10549-013-2563-5
Ahlin C, Zhou W, Holmqvist M, Holmberg L, Nilsson C, Jirstrom K, Blomqvist C, Amini RM, Fjallskog ML (2009) Cyclin A is a proliferative marker with good prognostic value in node-negative breast cancer. Cancer Epidemiol Biomark Prev 18(9):2501–2506. doi:10.1158/1055-9965.EPI-09-0169
Nimeus-Malmstrom E, Koliadi A, Ahlin C, Holmqvist M, Holmberg L, Amini RM, Jirstrom K, Warnberg F, Blomqvist C, Ferno M, Fjallskog ML (2010) Cyclin B1 is a prognostic proliferation marker with a high reproducibility in a population-based lymph node negative breast cancer cohort. Int J Cancer 127(4):961–967
Lundgren C, Ahlin C, Holmberg L, Amini RM, Fjallskog ML, Blomqvist C (2014) Cyclin E1 is a strong prognostic marker for death from lymph node negative breast cancer. A population-based case-control study. Acta Oncol. doi:10.3109/0284186X.2014.965274
Stal O, Skoog L, Rutqvist LE, Carstensen JM, Wingren S, Sullivan S, Andersson AC, Dufmats M, Nordenskjold B (1994) S-phase fraction and survival benefit from adjuvant chemotherapy or radiotherapy of breast cancer. Br J Cancer 70(6):1258–1262
Bostrom P, Soderstrom M, Palokangas T, Vahlberg T, Collan Y, Carpen O, Hirsimaki P (2009) Analysis of cyclins A, B1, D1 and E in breast cancer in relation to tumour grade and other prognostic factors. BMC Res Notes 2:140. doi:10.1186/1756-0500-2-140
Huang W, Nie W, Zhang W, Wang Y, Zhu A, Guan X (2016) The expression status of TRX, AR, and cyclin D1 correlates with clinicopathological characteristics and ER status in breast cancer. Onco Targets Ther 9:4377–4385. doi:10.2147/OTT.S94703
Mohammadizadeh F, Hani M, Ranaee M, Bagheri M (2013) Role of cyclin D1 in breast carcinoma. J Res Med Sci 18(12):1021–1025
Ravikumar G, Ananthamurthy A (2014) Cyclin D1 expression in ductal carcinoma of the breast and its correlation with other prognostic parameters. J Cancer Res Ther 10(3):671–675. doi:10.4103/0973-1482.138135
Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, Bishop H, Ellis I, Larsimont D, Sasano H, Carder P, Cussac AL, Knox F, Speirs V, Forbes J, Buzdar A (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol 26(7):1059–1065. doi:10.1200/JCO.2007.12.9437
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17(5):1474–1481
Early Breast Cancer Trialists’ Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
Butt AJ, McNeil CM, Musgrove EA, Sutherland RL (2005) Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Relat Cancer 12(Suppl 1):S47–59. doi:10.1677/erc.1.00993
Lamb R, Lehn S, Rogerson L, Clarke RB, Landberg G (2013) Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle 12(15):2384–2394. doi:10.4161/cc.25403
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11(5):R77. doi:10.1186/bcr2419
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439. doi:10.1016/S1470-2045(15)00613-0
Turner NC, Huang Bartlett C, Cristofanilli M (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(17):1672–1673. doi:10.1056/NEJMc1510345
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, Andre F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748. doi:10.1056/NEJMoa1609709
DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, Lal P, Feldman M, Zhang P, Colameco C, Lewis D, Langer M, Goodman N, Domchek S, Gogineni K, Rosen M, Fox K, O’Dwyer P (2015) CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 21(5):995–1001. doi:10.1158/1078-0432.CCR-14-2258
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahlin, C., Lundgren, C., Embretsén-Varro, E. et al. High expression of cyclin D1 is associated to high proliferation rate and increased risk of mortality in women with ER-positive but not in ER-negative breast cancers. Breast Cancer Res Treat 164, 667–678 (2017). https://doi.org/10.1007/s10549-017-4294-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4294-5