Abstract
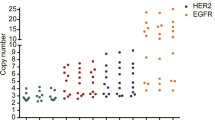
This study was designed to evaluate usefulness of additional fluorescence in situ hybridization (FISH) using other reference genes on chromosome 17 for assessment of HER2 status in invasive breast cancers with increased centromere 17 copy number, and to compare this approach with conventional methods based on the 2007 and 2013 ASCO/CAP guidelines. We performed FISH with probes for SMS, RARA, and TP53 on 253 breast cancers with centromeric probe CEP17 copy number ≥2.6 using tissue microarrays. If one or more gene had a mean copy number <2.6, the largest number for that gene(s) was chosen as an alternative to CEP17 copy number. Of the 243 cases in which re-grading was possible, only 2 had copy numbers ≥2.6 for RARA, SMS, and TP53. Of the 151 breast cancers which were considered HER2 non-amplified by the 2007 ASCO/CAP guidelines using the HER2:CEP17 ratio, 42 (27.8 %) were re-graded as amplified and 33 (21.8 %) as equivocal after FISH using additional reference genes. Of the 101 HER2-non-amplified cases by the 2013 ASCO/CAP guidelines, 2 (2.0 %) were reclassified as amplified and 24 (23.8 %) as equivocal. Of 46 equivocal cases, 35 (76.1 %) were re-graded as amplified. After re-grading, HER2-amplified cases were significantly increased, and the concordance between HER2 FISH and HER2 immunohistochemistry decreased. And some pathologic features of the cases which were designated to have HER2 amplification after additional FISH were not compatible with those of HER2-amplified breast cancers. The use of additional reference genes has been suggested as an option for accurate assessment of HER2 status in breast cancers with increased CEP17 copy number. However, this has limitations in that it can cause over-grading of HER2 status in tumors that lose the new reference genes. Thus, at present, it seems that additional FISH using other reference gene such as SMS, RARA, and TP53 for the cases with increased CEP17 copy number is not suitable for daily practice.




Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY) 235(4785):177–182
Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL (1989) HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 7(8):1120–1128
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical O, College of American P (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. doi:10.1200/JCO.2006.09.2775
Dal Lago L, Durbecq V, Desmedt C, Salgado R, Verjat T, Lespagnard L, Ma Y, Veys I, Di Leo A, Sotiriou C, Piccart M, Larsimont D (2006) Correction for chromosome-17 is critical for the determination of true Her-2/neu gene amplification status in breast cancer. Mol Cancer Ther 5(10):2572–2579. doi:10.1158/1535-7163.MCT-06-0129
Tse CH, Hwang HC, Goldstein LC, Kandalaft PL, Wiley JC, Kussick SJ, Gown AM (2011) Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J Clin Oncol 29(31):4168–4174. doi:10.1200/JCO.2011.36.0107
Vanden Bempt I, Drijkoningen M, De Wolf-Peeters C (2007) The complexity of genotypic alterations underlying HER2-positive breast cancer: an explanation for its clinical heterogeneity. Curr Opin Oncol 19(6):552–557. doi:10.1097/CCO.0b013e3282f0ad8e
Gunn S, Yeh IT, Lytvak I, Tirtorahardjo B, Dzidic N, Zadeh S, Kim J, McCaskill C, Lim L, Gorre M, Mohammed M (2010) Clinical array-based karyotyping of breast cancer with equivocal HER2 status resolves gene copy number and reveals chromosome 17 complexity. BMC Cancer 10:396. doi:10.1186/1471-2407-10-396
Staaf J, Jonsson G, Ringner M, Vallon-Christersson J, Grabau D, Arason A, Gunnarsson H, Agnarsson BA, Malmstrom PO, Johannsson OT, Loman N, Barkardottir RB, Borg A (2010) High-resolution genomic and expression analyses of copy number alterations in HER2-amplified breast cancer. Breast Cancer Res 12(3):R25. doi:10.1186/bcr2568
Wang S, Hossein Saboorian M, Frenkel EP, Haley BB, Siddiqui MT, Gokaslan S, Hynan L, Ashfaq R (2002) Aneusomy 17 in breast cancer: its role in HER-2/neu protein expression and implication for clinical assessment of HER-2/neu status. Mod Pathol 2:137–145. doi:10.1038/modpathol.3880505
Downs-Kelly E, Yoder BJ, Stoler M, Tubbs RR, Skacel M, Grogan T, Roche P, Hicks DG (2005) The influence of polysomy 17 on HER2 gene and protein expression in adenocarcinoma of the breast: a fluorescent in situ hybridization, immunohistochemical, and isotopic mRNA in situ hybridization study. Am J Surg Pathol 29(9):1221–1227
Liu Y, Ma L, Liu D, Yang Z, Yang C, Hu Z, Chen W, Yang Z, Chen S, Zhang Z (2014) Impact of polysomy 17 on HER2 testing of invasive breast cancer patients. Int J Clin Exp Pathol 7(1):163–173
Perez EA, Reinholz MM, Hillman DW, Tenner KS, Schroeder MJ, Davidson NE, Martino S, Sledge GW, Harris LN, Gralow JR, Dueck AC, Ketterling RP, Ingle JN, Lingle WL, Kaufman PA, Visscher DW, Jenkins RB (2010) HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol 28(28):4307–4315. doi:10.1200/JCO.2009.26.2154
Downey L, Livingston RB, Koehler M, Arbushites M, Williams L, Santiago A, Guzman R, Villalobos I, Di Leo A, Press MF (2010) Chromosome 17 polysomy without human epidermal growth factor receptor 2 amplification does not predict response to lapatinib plus paclitaxel compared with paclitaxel in metastatic breast cancer. Clin Cancer Res 16(4):1281–1288. doi:10.1158/1078-0432.CCR-09-1643
Ma Y, Lespagnard L, Durbecq V, Paesmans M, Desmedt C, Gomez-Galdon M, Veys I, Cardoso F, Sotiriou C, Di Leo A, Piccart MJ, Larsimont D (2005) Polysomy 17 in HER-2/neu status elaboration in breast cancer: effect on daily practice. Clin Cancer Res 11(12):4393–4399. doi:10.1158/1078-0432.CCR-04-2256
Vanden Bempt I, Van Loo P, Drijkoningen M, Neven P, Smeets A, Christiaens MR, Paridaens R, De Wolf-Peeters C (2008) Polysomy 17 in breast cancer: clinicopathologic significance and impact on HER-2 testing. J Clin Oncol 26(30):4869–4874. doi:10.1200/jco.2007.13.4296
Vranic S, Teruya B, Repertinger S, Ulmer P, Hagenkord J, Gatalica Z (2011) Assessment of HER2 gene status in breast carcinomas with polysomy of chromosome 17. Cancer 117(1):48–53. doi:10.1002/cncr.25580
Rosenberg CL (2008) Polysomy 17 and HER-2 amplification: true, true, and unrelated. J Clin Oncol 26(30):4856–4858. doi:10.1200/jco.2008.17.2684
Ross JS (2010) Human epidermal growth factor receptor 2 testing in 2010: does chromosome 17 centromere copy number make any difference? J Clin Oncol 28(28):4293–4295. doi:10.1200/JCO.2010.29.6673
Yeh IT, Martin MA, Robetorye RS, Bolla AR, McCaskill C, Shah RK, Gorre ME, Mohammed MS, Gunn SR (2009) Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol 9:1169–1175. doi:10.1038/modpathol.2009.78
Moelans CB, de Weger RA, van Diest PJ (2010) Absence of chromosome 17 polysomy in breast cancer: analysis by CEP17 chromogenic in situ hybridization and multiplex ligation-dependent probe amplification. Breast Cancer Res Treat 120(1):1–7. doi:10.1007/s10549-009-0539-2
Marchio C, Lambros MB, Gugliotta P, Di Cantogno LV, Botta C, Pasini B, Tan DS, Mackay A, Fenwick K, Tamber N, Bussolati G, Ashworth A, Reis-Filho JS, Sapino A (2009) Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol 219(1):16–24. doi:10.1002/path.2574
Troxell ML, Bangs CD, Lawce HJ, Galperin IB, Baiyee D, West RB, Olson SB, Cherry AM (2006) Evaluation of Her-2/neu status in carcinomas with amplified chromosome 17 centromere locus. Am J Clin Pathol 126(5):709–716. doi:10.1309/9EYM-6VE5-8F2Y-CD9F
Varga Z, Tubbs RR, Wang Z, Sun Y, Noske A, Kradolfer D, Bosshard G, Jochum W, Moch H, Ohlschlegel C (2011) Co-amplification of the HER2 gene and chromosome 17 centromere: a potential diagnostic pitfall in HER2 testing in breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-011-1642-8
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical O, College of American P (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clinl Oncol 31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
Broad Institute TCGA Genome Data Analysis Center (2015) SNP6 Copy number analysis (GISTIC2). Broad Institute of MIT and Harvard. doi:10.7908/C1W37V8R
Hanna WM, Ruschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G (2014) HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol 1:4–18. doi:10.1038/modpathol.2013.103
Glynn RW, Miller N, Kerin MJ (2010) 17q12-21—the pursuit of targeted therapy in breast cancer. Cancer Treat Rev 36(3):224–229. doi:10.1016/j.ctrv.2009.12.007
Paroni G, Fratelli M, Gardini G, Bassano C, Flora M, Zanetti A, Guarnaccia V, Ubezio P, Centritto F, Terao M, Garattini E (2012) Synergistic antitumor activity of lapatinib and retinoids on a novel subtype of breast cancer with coamplification of ERBB2 and RARA. Oncogene 31(29):3431–3443. doi:10.1038/onc.2011.506
Dubourg C, Bonnet-Brilhault F, Toutain A, Mignot C, Jacquette A, Dieux A, Gerard M, Beaumont-Epinette MP, Julia S, Isidor B, Rossi M, Odent S, Bendavid C, Barthelemy C, Verloes A, David V (2014) Identification of nine new RAI1-truncating mutations in smith-magenis syndrome patients without 17p11.2 deletions. Mol Syndromol 5(2):57–64. doi:10.1159/000357359
Ping Z, Siegal GP, Almeida JS, Schnitt SJ, Shen D (2014) Mining genome sequencing data to identify the genomic features linked to breast cancer histopathology. J Pathol Inform 5:3. doi:10.4103/2153-3539.126147
Kasiappan R, Shih HJ, Chu KL, Chen WT, Liu HP, Huang SF, Choy CO, Shu CL, Din R, Chu JS, Hsu HL (2009) Loss of p53 and MCT-1 overexpression synergistically promote chromosome instability and tumorigenicity. Mol Cancer Res 7(4):536–548. doi:10.1158/1541-7786.MCR-08-0422
Acknowledgments
This study was supported by a grant from Seoul National University Bundang Hospital, Republic of Korea (02-2014-012) to Park SY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 24 kb)
Online Resource Table S1: Pathologic characteristics of the 35 cases whose HER2 status were upgraded from equivocal (based on the 2013 ASCO/CAP guidelines) to amplified after additional FISH studies
Rights and permissions
About this article
Cite this article
Jang, M.H., Kim, E.J., Kim, H.J. et al. Assessment of HER2 status in invasive breast cancers with increased centromere 17 copy number. Breast Cancer Res Treat 153, 67–77 (2015). https://doi.org/10.1007/s10549-015-3522-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3522-0