Abstract
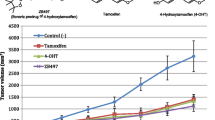
As a prodrug, tamoxifen is activated by the P450 enzyme CYP2D6 that is responsible for converting it to the active metabolites, 4-hydroxytamoxifen and endoxifen. Patients with genetic polymorphisms of CYP2D6 may not receive the full benefit of tamoxifen therapy. There is increasing evidence that poor metabolizer patients have lower plasma concentrations of endoxifen and suffer worse disease outcome, although some clinical studies reported no correlation between CYP2D6 polymorphism and tamoxifen therapy outcome. Endoxifen is currently undergoing clinical trials as a potentially improved and more potent SERM (Selective Estrogen Receptor Modulator) for endocrine therapy that is independent of CYP2D6 status in patients. However, direct administration of endoxifen may present the problem of low bioavailability due to its rapid first-pass metabolism via O-glucuronidation. We have designed and synthesized ZB483, a boronic prodrug of endoxifen suitable for oral administration with greatly enhanced bioavailability by increasing the concentration of endoxifen in mouse blood. Our study demonstrated that ZB483 potently inhibited growth of ER+ breast cancer cells in vitro and was efficiently converted to endoxifen in cell culture media by oxidative deboronation. This metabolic conversion is equally efficient in vivo as indicated in the pharmacokinetic study in mice. Moreover, when administered at the same dose, oral ZB483 afforded a 30- to 40-fold higher plasma level of endoxifen in mice than oral administration of endoxifen. The significantly enhanced bioavailability of endoxifen conferred by the boronic prodrug was further validated in an in vivo efficacy study. ZB483 was demonstrated to be more efficacious than endoxifen in inhibiting xenograft tumor growth in mice at equal dosage but more so at lower dosage. Together, these preclinical studies demonstrate that ZB483 is a promising endocrine therapy agent with markedly enhanced bioavailability in systemic circulation and superior efficacy compared to endoxifen.






Similar content being viewed by others
References
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310(3):1062–1075
Jordan VC (1982) Metabolites of tamoxifen in animals and man: identification, pharmacology and significance. Breast Cancer Res Treat 2:123–138
Fabian C, Tilzer L, Sternson L (1981) Comparative binding affinities of tamoxifen, 4-hydroxytamoxifen, and desmethyltamoxifen for estrogen receptors isolated from human breast carcinoma: correlation with blood levels in patients with metastatic breast cancer. Biopharm Drug Dispos 2:381–390
Patterson JS, Settatree RS, Adam HK et al (1980) Serum concentrations of tamoxifen and major metabolite during long-term nolvadex therapy, correlated with clinical response. Eur J Cancer Suppl 1:89–92
Wakeling AE, Slater SR (1980) Estrogen-receptor binding and biologic activity of tamoxifen and its metabolites. Cancer Treat Rep 64:741–744
Lien EA, Solheim E, Kvinnsland S et al (1988) Identification of4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res 48:2304–2308
Lien EA, Solheim E, Lea OA et al (1989) Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res 49:2175–2183
Lien EA, Solheim E, Ueland PM (1991) Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res 51:4837–4844
Jordan VC, Collins MM, Rowsby L et al (1977) Monohydroxylated metabolite of tamoxifen with potent antiestrogenic activity. J Endocrinol 75:305–316
Hoskins JM, Carey LA, McLeod HL (2009) CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer 9:576–586
Bijl M, van Schaik R, Lammers L et al (2009) The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat 118:125–130
Johnson MD, Zuo H, Lee KH et al (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159
Lim YC, Desta Z, Flockhart DA et al (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478
Lim YC, Li L, Desta Z et al (2006) Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther 318:503–512
Barginear MF, Jaremko M, Peter I et al (2011) Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Ther 90:605–611
Ruddy KJ, Desantis SD, Gelman RS et al (2013) Personalized medicine in breast cancer: tamoxifen, endoxifen, and CYP2D6 in clinical practice. Breast Cancer Res Treat 141:421–427
Jager NG, Rosing H, Schellens JH et al (2014) Tamoxifen dose and serum concentrations of tamoxifen and six of its metabolites in routine clinical outpatient care. Breast Cancer Res Treat 143:477–483
Madlensky L, Natarajan L, Tchu S et al (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 89:718–725
Rae JM, Drury S, Hayes DF et al (2012) ATAC trialists. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst 104:452–460. doi:10.1093/jnci/djs126 Erratum in: J Natl Cancer Inst. 2012 Nov 21;104(22):1772
Hertz DL, McLeod HL, Irvin WJ Jr (2012) Tamoxifen and CYP2D6: a contradiction of data. Oncologist 17:620–630
Province MA, Goetz MP, Brauch H et al (2014) International Tamoxifen Pharmacogenomics Consortium. CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin Pharmacol Ther 95:216–227. doi:10.1038/clpt.2013.186
Wu X, Hawse JR, Subramaniam M et al (2009) The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res 69:1722–1727
Ahmad A, Shahabuddin S, Sheikh S et al (2010) Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects. Clin Pharmacol Ther 88:814–817. doi:10.1038/clpt.2010.196
NCT01273168 (ClinicalTrials.gov): Endoxifen in adults with hormone receptor positive solid tumors
NCT01327781 (ClinicalTrials.gov): Z-Endoxifen hydrochloride in treating patients with metastatic or locally recurrent estrogen receptor-positive breast cancer, ClinicalTrials.gov Identifier: NCT01327781
Zheng Y, Sun D, Sharma AK et al (2007) Elimination of antiestrogenic effects of active tamoxifen metabolites by glucuronidation. Drug Metab Dispos 35:1942–1948
Zhang Y, Huo M, Zhou J et al (2010) PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–314
McClaine RJ, Marshall AM, Wagh PK et al (2010) Ron receptor tyrosine kinase activation confers resistance to tamoxifen in breast cancer cell lines. Neoplasia 12:650–658
Zheng S, Wang G, Jiang Q et al (2013) Boron-based 4-hydroxytamoxifen and endoxifen prodrugs as treatment for de novo tamoxifen resistant breast cancer, PCT/US13/29059
Stearns V, Johnson MD, Rae JM et al (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Beverage JN, Sissung TM, Sion AM et al (2007) CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci 96:2224–2231
Goetz MP, Knox SK, Suman VJ et al (2007) The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101:113–121
Ahmad A, Ali SM, Ahmad MU et al (2010) Orally administered endoxifen is a new therapeutic agent for breast cancer. Breast Cancer Res Treat 122:579–584
Sun D, Sharma AK, Dellinger RW et al (2007) Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab Dispos 35:2006–2014
Acknowledgments
This study was supported by NIH grant 2G12MD007595 and 1U54GM104940.
Conflicts of interest
The authors declare no conflict of interest in this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Changde Zhang and Qiu Zhong have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Zhang, C., Zhong, Q., Zhang, Q. et al. Boronic prodrug of endoxifen as an effective hormone therapy for breast cancer. Breast Cancer Res Treat 152, 283–291 (2015). https://doi.org/10.1007/s10549-015-3461-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3461-9