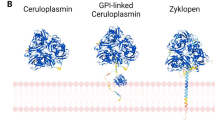
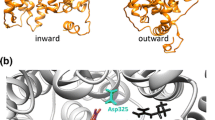
Abstract
Ceruloplasmin (CP) is a mammalian blood plasma ferroxidase. More than 95% of the copper found in plasma is carried by this protein, which is a member of the multicopper oxidase family. Proteins from this group are able to oxidize substrates through the transfer of four electrons to oxygen. The essential role of CP in iron metabolism in humans is particularly evident in the case of loss-of-function mutations in the CP gene resulting in a neurodegenerative syndrome known as aceruloplasminaemia. However, the functions of CP are not limited to the oxidation of ferrous iron to ferric iron, which allows loading of the ferric iron into transferrin and prevents the deleterious reactions of Fenton chemistry. In recent years, a number of novel CP functions have been reported, and many of these functions depend on the ability of CP to form stable complexes with a number of proteins.





Similar content being viewed by others
References
Aaseth J, Haugen M, Forre O (1998) Rheumatoid arthritis and metal compounds—perspectives on the role of oxygen radical detoxification. Analyst 123:3–6
Bakhautdin B, Febbraio M, Goksoy E, delaMotte CA, Gulen MF, Childers EP, Hazen SL, Li X, Fox PL (2013) Protective role of macrophage-derived ceruloplasmin in inflammatory bowel disease. Gut 62:209–219
Banha J, Marques I, Oliveira R, Paixao E, Pereira D, Malho R, Penque D, Costa L (2008) Ceruloplasmin expression by human peripheral blood lymphocytes; a new link between immunity and iron metabolism. Free Rad Biol Med 44:483–492
Bento I, Peixoto C, Zaitsev VN, Lindley PF (2007) Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites. Acta Crystallogr D 63:240–248
Bielli P, Calabrese L (2002) Structure and function relationships in ceruloplasmin: a ‘moonlighting’ protein. Cell Mol Life Sci 59:1413–1427
Brummel-Ziedins KE, Whelihan MF, Gissel M, Mann KG, Rivard GE (2009) Thrombin generation and bleeding in hemophilia A. Hemophilia 15:1118–1125
Chapman AL, Mocatta TJ, Shiva S, Seidel A, Chen B, Khalilova I, Paumann-Page ME, Jameson GN, Winterbourn CC, Kettle AJ (2013) Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J Biol Chem 288:6465–6477
Chen J, Chung DW, Le J, Ling M, Konkle BA, López JA (2013) Normal cleavage of von Willebrand factor by ADAMTS13 in the absence of factor VIII in patients with severe hemophilia A. J Thromb Haemost 11:1769–1772
Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL (2005) Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab 2:309–319
Church WR, Jernigan RL, Toole J, Hewick RM, Knopf J, Knutson GJ, Nesheim ME, Mann KG, Fass DN (1984) Coagulation factors V and VIII and ceruloplasmin constitute a family of structurally related proteins. Proc Natl Acad Sci USA 81:6934–6937
Coffey MJ, Phare SM, Peters-Golden M (2000) Prolonged exposure to lipopolysaccharide inhibits macrophage 5-lipoxygenase metabolism via induction of nitric oxide synthesis. J Immunol 165:3592–3598
Curzon G (1961) Some properties of coupled iron-caeruloplasmin oxidation systems. Biochem J 79:656–663
Curzon G, O’Reilly S (1960) A coupled iron-caeruloplasmin oxidation system. Biochem Biophys Res Commun 2:284–286
De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J (2007) Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J 26:2823–2831
De Filippis V, Vassiliev VB, Beltramini M, Fontana A, Salvato B, Gaitskhoki VS (1996) Evidence for the molten globule state of human apo-ceruloplasmin. Biochim Biophys Acta 1297:119–123
Donley SA, Ilagan BJ, Rim H, Linder MC (2002) Copper transport to mammary gland and milk during lactation in rats. Am J Physiol Endocrinol Metab 283:E667–E675
Farver O, Bendahl L, Skov LK, Pecht I (1999) Human ceruloplasmin. Intramolecular electron transfer kinetics and equilibration. J Biol Chem 274:26135–26140
Fee JA (1975) Copper proteins. Systems containing the “blue”copper center. Structure and Bonding. Springer, Berlin, pp 1–61
Floris G, Medda R, Padiglia A, Musci G (2000) The physiopathological significance of ceruloplasmin. A possible therapeutic approach. Biochem Pharmacol 60:1735–1741
Fortna RR, Watson HA, Nyquist SE (1999) Glycosyl phosphatidylinositol-anchored ceruloplasmin is expressed by rat Sertoli cells and is concentrated in detergent-insoluble membrane fractions. Biol Reprod 61:1042–1049
Freeman S, Daniel E (1973) Dissociation and reconstitution of human ceruloplasmin. Biochemistry 12:4806–4810
Frieden E (1980) Caeruloplasmin: a multi-functional metalloprotein of vertebrate plasma. Biological Roles of Copper; Ciba Fndn Symp-79. Excerpta Medica, Amsterdam, pp 93–124
Gallwitz M, Enoksson M, Thorpe M, Hellman L (2012) The extended cleavage specificity of human thrombin. PLoS ONE 7:e31756
Gerster JC, Busso N (2003) Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost 1:2510–2515
Griffin SV, Chapman PT, Lianos EA, Lockwood CM (1999) The inhibition of myeloperoxidase by ceruloplasmin can be reversed by anti-myeloperoxidase antibodies. Kidney Int 55:917–925
Guillen C, McInnes IB, Vaughan D, Speekenbrink AB, Brock JH (2000) The effects of local administration of lactoferrin on inflammation in murine autoimmune and infectious arthritis. Arthritis Rheum 43:2073–2080
Harris ZL, Takahashi Y, Miyajima H, Serizawa M, Macgillivray RT, Gitlin JD (1995) Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci USA 92:2539–2543
Harris ZL, Migas MC, Hughes AE, Logan JI, Gitlin JD (1996) Familial dementia due to a frameshift mutation in the caeruloplasmin gene. Q J Med 89:355–359
Harris ZL, Klomp LW, Gitlin JD (1998) Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am J Clin Nutr 67:972S–977S
Harris ZL, Durley AP, Man TK, Gitlin JD (1999) Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 96:10812–10817
Hellman NE, Kono S, Mancini GM, Hoogeboom AJ, De Jong GJ, Gitlin JD (2002) Mechanisms of copper incorporation into human ceruloplasmin. J Biol Chem 277:46632–46638
Holmberg CG (1944) On the presence of a laccase-like enzyme in serum and its relation to the copper in serum. Acta Physiol Scand 8:227–229
Holmberg CG, Laurell CB (1948) Investigations in serum copper II. Acta Chem Scand 2:550–556
Holmberg CG, Laurell CB (1951) Investigations in serum copper III. Acta Chem Scand 5:476–480
Hudson DM, Krisinger MJ, Griffiths TA, MacGillivray RTA (2008) Neither human hephaestin nor ceruloplasmin forms a stable complex with transferrin. J Cell Biochem 103:1849–1855
Iwata T, Kantarci A, Yagi M, Jackson T, Hasturk H, Kurihara H, van Dyke TE (2009) J Periodontol 80:1300–1306
Jennette JC, Falk RJ, Gasim AH (2011) Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens 20:263–270
Jeong SY, David S (2003) Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem 278:2714–27148
Kasper CB, Deutsch HF (1963) Physicochemical studies of human ceruloplasmin. J Biol Chem 238:2325–2337
Kemna EH, Tjalsma H, Willems HL, Swinkels DW (2008) Hepcidin: from discovery to differential diagnosis. Haematologica 93:90–97
Kettle AJ, Winterbourn CC (1997) Myeloperoxidase. A key regulator of neutrophil oxidant production. Redox Rep 3:3–15
Kim IG, Park SY (1998) Requirement of intact human ceruloplasmin for the glutathione-linked peroxidase activity. FEBS Lett 437:293–296
Kingston IB, Kingston BL, Putnam FW (1977) Chemical evidence that proteolytic cleavage causes the heterogeneity present in human ceruloplasmin preparations. Proc Natl Acad Sci USA 74:5377–5381
Klebanoff SJ (1970) Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science 169:1095–1097
Klomp LWJ, Gitlin JD (1996) Expression of the ceruloplasmin gene in the human retina and brain: implications for a pathogenic model in aceruloplasminemia. Hum Mol Genet 5:1989–1996
Kono S (2013) Aceruloplasminemia: an update. Int Rev Neurobiol 110:125–151
Kono S, Yoshida K, Tomosugi N, Terada T, Hamaya Y, Kanaoka S, Miyajima H (2010) Biological effects of mutant ceruloplasmn on hepcidin-mediated internalization of ferroportin. Biochim Biophys Acta 1802:968–975
Koschinsky ML, Funk WD, van Oost BA, MacGillivray RT (1986) Complete cDNA sequence of human preceruloplasmin. Proc Natl Acad Sci USA 83:5086–5090. https://doi.org/10.1073/pnas.83.14.5086
Kostevich VA, Sokolov AV, Grudinina NA, Zakharova ET, Samygina VR, Vasilyev VB (2015) Interaction of macrophage migration inhibitory factor with ceruloplasmin: role of labile copper ions. Biometals 25:817–826
Linder MC (2010) Nutritional biochemistry of copper, with emphasis on the perinatal period. In: Avigliano L, Rossi L (eds) Biochemical Aspects of Human Nutrition. Research Signpost, Trivandrum, pp 143–179
Linder MC (2016) Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics 8:887–905
LindleyP Card G, Zaitseva I, Zaitsev VN, Reinhammar B, Selin-Lindgren E, Yoshida K (1997) An X-ray structural study of human ceruloplasmin in relation to ferroxidase activity. J Biol Inorg Chem 2:454–463
Logan JI, Harveyson KB, Wisdom GB, Hughes AE, Archbold GP (1994) Hereditary caeruloplasmin deficiency, dementia and diabetes mellitus. Q J Med 87:663–670
Lu Y, Roe JA, Gralla EB, Valentine JS (1993) Metalloprotein ligand redesign: characterization of cooper-cysteinate proteins derived from yeast copper-zinc superoxide dismutase. In: Karlin KD, Tieklar Z (eds) Bioorganic Chemistry of Copper. Chapman & Hall, New York, pp 64–77
Lutsenko S, LeShane ES, Shinde U (2007) Biochemical basis of regulation of human copper-transporting ATPases. Arch Biochem Biophysics 463:134–148. https://doi.org/10.1016/j.abb.2007.04.013
Magdoff-Fairchild B, Lovell FM, Low BW (1969) An X-ray crystallographic study of ceruloplasmin. Determination of molecular weight. J Biol Chem 244:3497–3499
Malenica B, Rudolf M, Kozmar A (2004) Antineutrophil cytoplasmic antibodies (ANCA): diagnostic utility and potential role in the pathogenesis of vasculitis. Acta Dermatovenerol Croat 12(294):313
Marques L, Auriac A, Willemetz A, Banha J, Silva B, Canonne-Hergaux F, Costa L (2012) Immune cells and hepatocytes express glycophosphatidylinositol-anchored ceruloplasmin at their cell surface. Blood Cells Mol Dis 48:110–120
McCombs ML, Bowman BH (1976) Biochemical studies on human ceruloplasmin. Biochim Biophys Acta 434:452–461
McDermott JA, Huber CT, Osaki S, Frieden E (1968) The role of iron in the activity of ceruloplasmin. Biochim Biophys Acta 151:541–544
McKee DJ, Frieden E (1971) Binding of transition metal ions by ceruloplasmin (ferroxidase). Biochemistry 10:3880–3883
Meyer Siegler KL, Iczkowski KA, Vera PL (2006) Macrophage migration inhibitory factor is increased in the urine of patients with urinary tract infection: macrophage migration inhibitory factor-protein complexes in human urine. J Urol 175(1523):1528
Mittal B, Doroudchi MM, Jeong SY, Patel BN, David S (2003) Expression of a membrane-bound form of the ferroxidase ceruloplasmin by leptomeningeal cells. Glia 41:337–346
Miyajima H, Nishimura Y, Sakamoto Mizoguchi K, Shimizu T, Honda N (1987) Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology 37:761–767
Morita H, Ikeda S-I, Yamamoto K, Morita S, Yoshida K, Nomoto S, Kato M, Yanagisawa N (1995) Hereditary ceruloplasmin deficiency with hemosiderosis: a clinicopathological study of a Japanese family. Ann Neurol 37:646–656
Moshkov KA, Lakatos S, Hajdu J, Zavodszky P, Neifakh SA (1979) Proteolysis of human ceruloplasmin. Some peptide bonds are particularly susceptible to proteolytic attack. Eur J Biochem 94:127–131
Mostad EJ, Prohaska JR (2011) Glycophosphatidylinositol-linked ceruloplasmin is expressed in multiple rodent organs and is lower following dietary copper deficiency. Exp Biol Med 236:298–308
Mukhopadhyay CK, Mazumder B, Lindley PF, Fox PL (1997) Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc Natl Acad Sci USA 94:11546–11551
Naughton DP, Knappitt J, Fairburn K, Gaffney K, Blake DR, Grootveld M (1995) Detection and investigation of the molecular nature of low-molecular-mass copper ions in isolated rheumatoid knee-joint synovial fluid. FEBS Lett 361:167–172
Ortel TL, Takahashi N, Putnam FW (1984) Structural model of human ceruloplasmin based on internal triplication, hydrophilic/hydrophobic character, and secondary structure of domains. Proc Natl Acad Sci USA 81:4761–4765
Osaki S (1966) Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin). J Biol Chem 241:5053–5059
Osaki S, Johnson DA (1969) Mobilization of liver iron by ferroxidase (ceruloplasmin). J Biol Chem 244:5757–5768
Osaki S, Walaas O (1968) Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase. III. Effects of deuterium and temperature on the enzymic oxidation of ferrous ion. Arch Biochem Biophys 125:918–925
Osaki S, Johnson DA, Frieden E (1966) The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem 241:2746–2751
Osaki S, Johnson DA, Frieden E (1971) The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem 246:3018–3023
Panasenko OM, Chekanov AV, Vlasova II, Sokolov AV, Ageeva KV, Pulina MO, Cherkalina OS, Vasilyev VB (2008) A study of the effect of ceruloplasmin and lactoferrin on the chlorination activity of leukocytic myeloperoxidase using the chemiluminescence method. Biofizika 53:573–581
Panasenko OM, Gorudko IV, Sokolov AV (2013) Hypochlorous acid as a precursor of free radicals in living systems. Biochemistry (Moscow) 78:1466–1489
Park YS, Suzuki K, Mumby S, Taniguchi N, Gutteridge JM (2000) Antioxidant binding of caeruloplasmin to myeloperoxidase. Myeloperoxidase is inhibited, but oxidase, peroxidase and immunoreactive properties of caeruloplasmin remain intact. Free Radic Res 33:261–265
Patel BN, David S (1997) A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem 272:20185–20190
Patel BN, Dunn RJ, David S (2000) Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem 275:4305–4310
Pemberton S, Lindley P, Zaitzev V, Card G, Tuddenham EGD, Kemball-Clark G (1997) A molecular model for the triplicated A domains of human factor VIII based on the crustal structure of human ceruloplasmin. Blood 89:2413–2421
Poillon WN, Bearn AG (1966) The molecular structure of human ceruloplasmin: evidence for subunits. Biochim Biophys Acta 127:407–427
Polishchuk R, Di Pentima A, Lippincott-Scwartz J (2004) Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol 6:297–307
Poulik MD (1962) Electrophoretic and immunological studies on structural subunits of human ceruloplasmin. Nature 194:842–844
Poulik MD (1968) Heterogeneity and structure of human ceruloplasmin. Ann N Y Acad Sci 151:476–501
Prozorovski VN, Rashkovetski LG, Vasiliev VB, Shavlovski MM, Neifakh SA (1982) Evidence that human ceruloplasmin molecule consists of homologous parts. Int J Pept Prot Res 19:40–53
Pulina MO, Zakharova ET, Solovyov KV, Bass MG, Sokolov AV, Shavlovski MM, Vasilyev VB (2002) Studies of lactoferrin-ceruloplasmin complex. Biochem Cell Biol 80:35–39
Qi W, Jiajie J, Shuangying H, Meng Z, Kuanyu L, Tong Q (2016) Iron together with lipid downregulates protein levels of ceruloplasmin in macrophages associated with rapid foam cell formation. J Atheroscler Thromb 23:1201–1211
Reilly CA, Sorlie M, Aust SD (1998) Evidence for a protein–protein complex during iron loading into ferritin by ceruloplasmin. Arch Biochem Biophys 354:165–171
Royle NJ, Irwin DM, Koschinsky ML, MacGillivray RT, Hamerton JL (1987) Human genes encoding prothrombin and ceruloplasmin map to 11p11-q12 and 3q21-24, respectively. Somatic Cell Mol Genet 13:285–292
Rydén L (1971) Evidence for proteolytic fragments in commercial samples of human ceruloplasmin. FEBS Lett 18:321–325
Rydén L (1972) Single-chain structure of human ceruloplasmin. Eur J Biochem 26:380–386
Rydén L (1982) Model of the active site in the blue oxidases based on the ceruloplasmin-plastocyanin homology. Proc Natl Acad Sci USA 79:6767–6771
Sabatucci A, Vachette P, Vasilyev VB, Beltramini M, Sokolov A, Pulina M, Salvato B, Angelucci CB, Maccarrone M, Cozzani I, Dainese E (2007) Structural characterization of the ceruloplasmin:lactoferrin complex in solution. J Mol Biol 371:1038–1046
Samokyszyn VM, Miller DM, Reif DW, Aust SD (1989) Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J Biol Chem 264:21–26
Samygina VR, Sokolov AV, Pulina MO, Bartunik H, Vasilyev VB (2008) X-ray diffraction study of highly purified human ceruloplasmin. Crystallogr Rep 53:655–662
Samygina VR, Sokolov AV, Bourenkov G, Petoukhov MV, Pulina MO, Zakharova ET, Vasilyev VB, Bartunik H, Svergun DI (2013) Ceruloplasmin: macromolecular assemblies with iron-containing acute phase proteins. PLoS ONE 8:1–12
Samygina VR, Sokolov AV, Bourenkov G, Schneider TR, Anashkin VA, Kozlov SO, Kolmakov NN, Vasilyev VB (2017) Rat ceruloplasmin: new labile copper binding site and zinc/copper mosaic. Metallomics 9:1828–1838
Sang QA (1995) Specific proteolysis of ceruloplasmin by leukocyte elastase. Biochem Mol Biol Int 37:573–581
Sato M, Gitlin JD (1991) Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J Biol Chem 266:5128–5134
Sawatzki G (1987) The role of iron binding proteins in bacterial infections. In: Winkelmann G, van der Helm D, Neilands JB (eds) Iron transport in microbes, plants and animals. VCH Veragsgesellschaft, Weinheim, pp 448–477
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376:1094–1108
Segelmark M, Persson B, Hellmark T, Wieslander J (1997) Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin Exp Immunol 108:167–174
Shen BW, Spiegel PC, Chang C-H, Huh J-W, Lee J-C, Kim J, Kim Y-H, Stoddard BL (2008) The tertiary structure and domain organization of coagulation factor VIII. Blood 111:1240–1247
Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT (2006) Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol 2:486–493
Shokeir MHK (1973) The molecular structure of human ceruloplasmin: a proposed model. Clin Biochem 6:9–14
Simons K, Bearn AG (1969) Isolation and partial characterization of the polypeptide chains of human ceruloplasmin. Biochim Biophys Acta 175:260–270
So AK, Varisco PA, Kemkes-Matthes B, Herkenne-Morard C, Chobaz-Peclat V, Gerster JC, Busso N (2003) Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost 1:2510–2515
Sokolov AV, Zakharova ET, Shavlovski MM, Vasilyev VB (2005a) Isolation of stable human ceruloplasmin and its interaction with salmon protamine. Russ J Bioorg Chem 31:238–248
Sokolov AV, Pulina MO, Zakharova ET, Shavlovski MM, Vasilyev VB (2005b) Effect of lactoferrin on the ferroxidase activity of ceruloplasmin. Biochemistry (Moscow) 70:1015–1019
Sokolov AV, Pulina MO, Zakharova ET, Susorova AS, Runova OL, Kolodkin NI, Vasilyev VB (2006) Identification and isolation from breast milk of ceruloplasmin–lactoferrin complex. Biochemistry (Moscow) 71(160):166
Sokolov AV, Pulina MO, Ageeva KV, Ayrapetov MI, Berlov MN, Volgin GN, Markov AG, Yablonsky PK, Kolodkin NI, Zakharova ET, Vasilyev VB (2007a) Interaction of ceruloplasmin, lactoferrin, and myeloperoxidase. Biochemistry (Moscow) 72:409–415
Sokolov AV, Pulina MO, Ageeva KV, Runova OL, Zakharova ET, Vasilyev VB (2007b) Identification of leukocyte cationic proteins that interact with ceruloplasmin. Biochemistry (Moscow) 2:872–877
Sokolov AV, Ageeva KV, Pulina MO, Cherkalina OS, Samygina VR, Vlasova II, Panasenko OM, Zakharova ET, Vasilyev VB (2008) Ceruloplasmin and myeloperoxidase in complex affect the enzymatic properties of each other. Free Radic Res 42:989–998
Sokolov AV, Pulina MO, Ageeva KV, Tcherkalina OS, Zakharova ET, Vasilyev VB (2009a) Identification of complexes formed by ceruloplasmin with matrix metalloproteinases 2 and 12. Biochemistry (Moscow) 74:1388–1392
Sokolov AV, Ageeva KV, Pulina MO, Zakharova ET, Vasilyev VB (2009b) Effect of lactoferrin on oxidative features of ceruloplasmin. Biometals 22:521–529
Sokolov AV, Prozorovskii VN, Vasilyev VB (2009c) Study of interaction of ceruloplasmin, lactoferrin, and myeloperoxidase by photon correlation spectroscopy. Biochemistry (Moscow) 74:1225–1227
Sokolov AV, Golenkina EA, Kostevich VA, Vasilyev VB, Sud’yina GF (2010) Interaction of ceruloplasmin and 5-lipoxygenase. Biochemistry (Moscow) 75:1464–1469
Sokolov AV, Kostevich VA, Romanico DN, Zakharova ET, Vasilyev VB (2012) Two-stage method for purification of ceruloplasmin based on its interaction with neomycin. Biochemistry (Mosc) 77:631–838
Sokolov AV, Kostevich VA, Runova OL, Gorudko IV, Vasilyev VB, Cherenkevich SN, Panasenko OM (2014) Proatherogenic modification of LDL by surface-bound myeloperoxidase. Chem Phys Lipids 180:72–80
Sokolov AV, Acquasaliente L, Kostevich VA, Frasson R, Zakharova ET, Pontarollo G, Vasilyev VB, De Filippis V (2015a) Thrombin inhibits the anti-myeloperoxidase and ferroxidase functions of ceruloplasmin: relevance in rheumatoid arthritis. Free Radic Biol Med 86:279–294
Sokolov AV, Kostevich VA, Zakharova ET, Samygina VR, Panasenko OM, Vasilyev VB (2015b) Interaction of ceruloplasmin with eosinophil peroxidase as compared to its interplay with myeloperoxidase: reciprocal effect on enzymatic properties. Free Radic Res 49:800–811
Stoj C, Kosman DJ (2003) Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: implication for function. FEBS Lett 554:422–426
Suarez-Almazor ME, Spooner C, Belseck E (2000) Penicillamine for treating rheumatoid arthritis. Cochrane Database Syst Rev 4:CD001460
Swain JA, Darley-Usmar V, Gutteridge JM (1994) Peroxynitrite releases copper from caeruloplasmin: implications for atherosclerosis. FEBS Lett 342:49–52
Takahashi N, Ortel TL, Putnam FW (1984) Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proc Natl Acad Sci USA 81:390–394
Takahashi Y, Miyajima S, Shirabe S, Nagataki S, Suenaga A, Gitlin JD (1996) Characterization of a nonsense mutation in the ceruloplasmin gene resulting in diabetes and neurodegenerative disease. Hum Mol Genet 5:81–84
Tams JW, Johnsen AH, Fahrenkrug J (1999) Identification of pituitary adenylate cyclase-activating polypeptide1-38-binding factor in human plasma, as ceruloplasmin. Biochem J 341:271–276
Taylor JC, Oey L (1982) Ceruloplasmin. Plasma inhibitor of the oxidative inactivation of 1-protease inhibitor. Am Rev Respir Dis 126:476–482
Terada K, Kawarada Y, Miura N, Yasui O, Koyama K, Sugiyama T (1995) Copper incorporation into ceruloplasmin in rat livers. Biochim Biophys Acta 1270:58–62
Thomas T, Schreiber G, Jaworowski A (1989) Developmental patterns of gene expression of secreted proteins in brain and choroid plexus. Dev Biol 134:38–47
Vachette P, Dainese E, Vasilyev VB, Di Muro P, Beltramini M, Svergun DI, De Filippis V, Salvato B (2002) A key structural role for active site type 3 copper ions in human ceruloplasmin. J Biol Chem 277:40823–40831
Van Eden ME, Aust SD (2000) Intact human ceruloplasmin is required for the incorporation of iron into human ferritin. Arch Biochem Biophys 381:119–126
Varfolomeeva EY, Semenova EV, Sokolov AV, Aplin KD, Timofeeva KE, Vasilyev VB, Filatov MV (2016) Ceruloplasmin decreases respiratory burst reaction during pregnancy. Free Radical Res 50:909–919
Vasilyev VB (2010) Interactions of caeruloplasmin with other proteins participating in inflammation. Bioch Soc Transact 38:947–951
Vasilyev VB, Kachurin AM, Soroka NV (1988) Dismutation of superoxide radicals by ceruloplasmin—details of the mechanism. Biokhimiya 53:2051–2058
Vassiliev VB, Kachurin AM, Rocco G-P, Beltramini M, Salvato B, Gaitskhoki VS (1997) Copper depletion/repletion of human ceruloplasmin is followed by the changes in its spectral features and functional properties. J Inorg Biochem 65:167–174
Vassiliev V, Harris ZL, Zatta P (2005) Ceruloplasmin in neurodegenerative diseases. Brain Res Rev 49:633–640
Walker FJ, Fay PJ (1990) Characterization of an interaction between protein C and ceruloplasmin. J Biol Chem 265:1834–1836
Ward DM, Kaplan J (2012) Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 1823:426–1433
Wooten L, Shulze R, Lancey R, Lietzow M, Linder MC (1996) Ceruloplasmin is found in milk and amniotic fluid and may have a nutritional role. J Nutr Biochem 7:632–639
Yang FM, Friedrichs WE, Cupples RL, Bonifacio MJ, Sanford JA, Horton WA, Bowman BH (1990) Human ceruloplasmin, tissue-specific expression of transcripts produced by alternative splicing. J Biol Chem 265:10780–10785
Yang S, Hua Y, Nakamura T, Keep RF, Xi G (2006) Up-regulation of brain ceruloplasmin in thrombin preconditioning. Acta Neurochir Suppl (Wien) 96:203–206
Yoshida K, Furihata K, Takeda S, Nakamura A, Yamamoto K, Morita H, Hiyamuta S, Ikeda S, Shimizu N, Yanagisawa N (1995) A mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. Nat Genet 9:267–272
Young SN, Curzon G (1972) A method for obtaining linear reciprocal plots with caeruloplasmin and its application in a study of the kinetic parameters of caeruloplasmin substrates. Biochem J 129:273–283
Zagryazhskaya AN, Lindner SC, Grishina ZV, Galkina SI, Steinhilber D, Sud’ina GF (2010) Nitric oxide mediates distinct effects of various LPS chemotypes on phagocytosis and leukotriene synthesis in human neutrophils. Int J Biochem Cell Biol 42:921–931
Zaitsev VN, Zaitseva I, Papiz M, Lindley P (1999) An X-ray crystallographc study of the binding sites of the azide inhibitor and organic substrates to ceruloplasmin, a multicopper oxidase in the plasma. J Biol Inorg Chem 4:579–587
Zaitseva I, Zaitsev V, Card G, Moshkov K, Bax B, Ralph A, Lindley P (1996) The X-ray structure of human serum ceruloplasmin at 3.1 Å: nature of the copper centres. J Biol Inorg Chem 1:15–23
Zakharova ET, Shavlovski MM, Bass MG, Gridasova AA, Pulina MO, De Filippis V, Beltramini M, Di Muro P, Salvato B, Fontana A, Vasilyev VB, Gaitskhoki VS (2000) Interaction of lactoferrin with ceruloplasmin. Arch Biochem Biophys 374:222–228
Acknowledgements
The author is grateful to Dr. Alexey Sokolov (Institute of Experimental Medicine, Saint-Petersburg) and Dr. Valeria Samygina (Institute of Crystallography, Moscow) for help and fruitful consultations.
Funding
This work was supported by Grant 18-015-00241 from the Russian Foundation for Basic Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vasilyev, V.B. Looking for a partner: ceruloplasmin in protein–protein interactions. Biometals 32, 195–210 (2019). https://doi.org/10.1007/s10534-019-00189-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-019-00189-1