Abstract
The rewetting of long-term drained peatlands leads to the development of eutrophic shallow lakes, gradually inhabited by reed communities. These shallow lakes are characterized by significant nutrient and methane emissions. To comprehend the fate of organic compounds from decaying Phragmites australis litter in water and anaerobic soil layers, we conducted a 1.6-year decomposition experiment. The experiment employed bulk and lignin-derived phenol analysis, as well as Fourier-transform infrared spectroscopy. As anticipated, the highest level of decomposition was observed in the surface water body of the shallow lake, while the non-rooted degraded peat exhibited the lowest decay. The bulk mass loss of plant litter decreased with depth from 55 to 27% across the four decomposition environments. Analysis using infrared spectroscopy indicated that the decrease in mass loss was primarily driven by the breakdown of carbohydrates, which constitute a significant portion of plant litter. Interestingly, litter in the rooted degraded peat layer exhibited the highest degree of lignin decay. Furthermore, the study revealed a preferential loss of vanillin phenols and an accumulation of p-hydroxyl phenols. These findings suggest that the increased methane emissions in rewetted fens may be partially attributed to the demethoxylation of vanillin phenols and the subsequent formation of p-hydroxyl phenols. In conclusion, this study provides valuable insights into anaerobic lignin decomposition of plant litter and sheds light on potential mechanisms underlying elevated methane emissions in rewetted peatlands. Furthermore, the study’s findings hold significant implications for both carbon cycling and sequestration within these ecosystems, thereby stimulating further research into the microbial community and its extended effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restoring drained peatlands through rewetting has been proposed as a valid strategy to reduce greenhouse gas (GHG) emissions and restore their unique biodiversity (Emsens et al. 2017; Tiemeyer et al. 2020; Zak and McInnes 2022). In recent years, there has been increasing focus on recreating the carbon and nutrient sink function of these wetlands to reduce their climate impact and mitigate water body eutrophication (Kimmel and Mander 2010; Zak et al. 2010; Jurasinski et al. 2020). However, the restoration efforts often result in the formation of eutrophic shallow lakes colonized by water plants and reed communities due to former intensive land use practices, peat mineralization, and soil subsidence (Jurasinski et al. 2020). The newly formed systems have several major downsides, including high nutrient losses to downstream aquatic ecosystems, elevated methane (CH4) emissions, and slow development of target vegetation over several decades (Kreyling et al. 2021).
Unlike what is reported for pristine peatlands, the decomposition of organic matter in rewetted fens, which leads to elevated GHG emissions, is strongly influenced by an altered hydrological and biogeochemical regime (Antonijević et al. 2023). This regime includes elevated availability of electron acceptors like ferric iron and sulfate, increased nutrient availability, absence of enzyme-inhibiting tannins, and a circum-neutral pH (Brouns et al. 2016; Zak et al. 2019). Respiration measurements conducted on organic soil substrates from rewetted peatlands suggest that highly degraded peat, which has undergone significant aerobic processes due to water level changes, exhibits relatively slow decomposition. However, significant anaerobic CO2 and CH4 production in peat occurs when bioavailable organic matter, such as remaining roots or root exudates, is available (Hahn-Schöfl et al. 2011).
The modern understanding of soil substrate quality challenges the previous belief that lignin moieties contribute to persistent soil carbon in anaerobic soils due to their resistance to degradation (Lankiewicz et al. 2023). It is now widely accepted that soil microbial communities can degrade all types of substrates in almost any soil (Dungait et al. 2012). The preservation of substrates, rather than their conversion to dissolved organic matter (DOM), CO2, or CH4, is associated with other biological, physicochemical, and structural factors, rather than their quality or recalcitrance (Schmidt et al. 2011). The interaction between lignin decomposition products and redox-sensitive iron minerals provides a partial reconciliation between the old and new paradigms (Huang et al. 2019). However, there is currently no consensus on the main mechanisms underlying soil carbon transformation and stabilization in rewetted peatlands (Adamczyk et al. 2020). In rewetted peatlands, highly elevated emissions of CO2 and CH4 indicate the presence of highly active anaerobic decomposition processes (Antonijević et al. 2023). Some of this activity can be attributed to the emergance of particular plant species in these nutrient-rich shallow lakes, notably Ceratpophyllum demersum whose decomposing litter triggers considerable CH4 release (Zak et al. 2015). Recently, it was suggested that ecosystem-specific stabilization mechanisms of organic matter, a critical component in the carbon sequestration function of pristine peatlands, are intricately intertwined with the resurgence of peat-forming plant species (Temmink et al. 2022).
The litterbag technique (Bärlocher 2005) and the analysis of specific biomarkers like lignin phenols have commonly been used to trace decomposition processes. Lignin phenols, derived from lignin, the second most abundant biopolymer known for its chemical stability against microbial degradation (Grgas et al. 2023; Lankiewicz et al. 2023), are frequently employed as vascular plant biomarkers to track organic matter sources in aquatic systems (Opsahl and Benner 1997; Hernes and Benner 2003). They are also used to differentiate plant species, study lignin alteration during litter decomposition, and examine the effect of changing environmental decomposition conditions (Opsahl and Benner 1995; Kalbitz et al. 2006). However, most studies have focused on oxic decomposition conditions, as lignin decay under anoxic conditions was believed to be slow or absent due to the oxygen-dependence of lignin-active enzymes (Lankiewicz et al. 2023). Unlike for aerobic conditions the chemical mechanisms involved in the anaerobic transformation of lignin at the molecular level are still unclear (ibid.). Ratios of phenol sum parameters or specific phenols, which indicate the phenol substitution pattern in the biopolymer, are commonly used as indicators of source or alteration (Hedges and Mann 1979; Ertel and Hedges 1984; Hedges et al. 1988). While some studies have highlighted limitations in the ecological and biogeochemical interpretation of these parameters, they remain widely accepted and utilized (Hernes et al. 2007). Therefore, further information on the chemical behavior of these phenols during transformation processes is desirable to assess the transferability of these ecological interpretations to anoxic systems found in rewetted peatlands. Spectroscopic techniques like Fourier-transform infrared spectroscopy (FTIR) can complement the analysis of structural changes of decomposing leaf litter, as these provide additional information on the molecular alterations of lignin and other biomolecules in litter (Derkacheva and Sukhov 2008).
In this study, we conducted a litterbag experiment during approximately 1.6 years to monitor decomposition in a rewetted fen. Our approach focused on two main aspects. Firstly, we investigated the decomposition of typical plant litter, specifically Phragmites australis, in rewetted fens at different soil depths. Our aim was to understand the efficiency of organic matter decay across the vertical horizon and gain insights into the significance of different stratified layers within a rewetted peatland in terms of litter decomposition and carbon stability. Secondly, we specifically aimed to deepen the understanding of the molecular transformation of lignin during anaerobic decomposition and its impact on porewater lignin formation. To achive this, we conducted chemical analyses specifically targeting lignin decomposition and transformation under anaerobic conditions in the varying biogeochemical regimes of the stratified soil system of a rewetted fen. These chemical analyses include the use of FTIR spectroscopy to analyze leaf litter lignin, al well as the CuO oxidation method to analyze lignin in the leaf litter and porewater along a vertical gradient.
While significant advancements have been made in our understanding of lignin decomposition, driven primarily by improved insights into the pivotal role of fungi (Lankiewicz et al. 2023), as well as bacteria and their associated enzymes (Raji et al. 2021; Grgas et al. 2023), a controversial debate still persists regarding the extent and importance of specific factors, such as nitrogen and metals, in various environmental contexts (Huang et al. 2023; Peng et al 2023; Yi et al. 2023). A recent work in natural wet and dry fen peatlands showed substantial organic matter losses in the acrotelm due to the degradation of specific hemicellulose structures (Serk et al. 2022). Unlike in natural peatlands, there exists a significant knowledge gap concerning the decay of organic matter in rewetted fen peatlands, particularly in the context of lignin decomposition. Our hypothesis suggests the conventional expectation that lignin decomposition would predominantly take place in the aerobic water layer, with some limited decomposition occurring in the newly formed “high-reactive” mud layer, while decomposition in the anaerobic soil layers below would be minimal.
Methods and materials
Study site
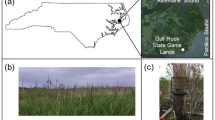
The decomposition experiment took place in a fen peatland located in Zarnekow, Dargun, Mecklenburg-Western Pomerania, northeast Germany, within the River Peene valley (53° 52′ 33.0″ N, 12° 53′ 18.5″ E). The study area experiences a moderately continental temperate climate, with an average annual air temperature of 9.2 °C and precipitation of 583 mm (1991–2020, Teterow, German Meteorological Service, DWD). The site has been drained since the eighteenth century and utilized as intensive grassland from the 1960s, leading to peat mineralization and subsequent soil shrinkage of up to 1 m. Consequently, a typical vertical degradation gradient formed, with highly decomposed peat at the soil surface up to ~ 0.3 m depth and less decomposed peat extending to a depth of 10 m (Zak and Gelbrecht 2007). Following the rewetting process in winter 2004/2005, a eutrophic shallow lake with an average water depth of 0.5 m was formed above the highly degraded peat layer. The initial colonization of the lake consisted of submerged and floating macrophytes, with subsequent establishment of reed communities in the following years (Antonijević et al. 2023). Anoxic conditions in the waterlogged soils were permanently reinstated shortly after rewetting, except for two dry phases in 2010 and 2012. Former studies observed a significant increase in nutrient and methane mobilization, reaching levels up to 100 times higher than in natural counterparts (Zak et al. 2010; Antonijević et al. 2023). Due to incomplete decomposition of above-ground biomass from hydrophytes and helophytes, a highly reactive detritus mud layer formed, sequestering approximately 50 g C m−2 year−1 (Cabezas et al. 2014). This detritus-mud layer primarily contributes to high methane and carbon dioxide emissions (Han-Schöfl et al. 2011). In contrast, the rooted degraded peat layer exhibited significantly lower gaseous carbon fluxes but demonstrated the highest mobilization of DOM, as well as elevated rates of phosphorus and ammonium mobilization (Zak and Gelbrecht 2007). The less decomposed peat layer, on the other hand, exhibited generally low mobilization of carbon and nutrients.
Experimental setup
The experimental setup followed the methodology of Schipper and Reddy (1995) with slight modifications. In March 2015, we buried three dialysis samplers in the soil of the rewetted fen Zarnekow, each comprising 14 chambers with a volume of 50 mL, spaced approximately 10 m apart. The chambers were filled with ultrapure water and isolated from the environment using a 0.2 µm polysulfon membrane (Pall Corporation, USA). The positioning of the chambers ensured that the upper two chambers were exposed to the surface water, while the remaining 12 chambers were located at different soil depth (see Fig. 1).
Schema of the study site system: stratified shallow lake as a newly formed ecosystem after rewetting (right) of a drained peatland (middle) compared to the former undrained peatland. The schematic representation also shows the experimental setup. Leaf litter was placed in every second chamber of the dialysis sampler, while empty chambers were utilized to monitor background pore water characteristics
This arrangement allowed the study of decomposition across a range of depths, from the aerobic surface waterbody of the shallow lake to the anaerobic highly degraded peat layer beneath the former rhizosphere, reaching a soil depth of approximately 40 cm. To investigate the decomposition of plant litter, 1.5 g pre-leached and freeze-dried P. australis leaves were placed in every second chamber of the dialysis sampler. Additionally, 100 µL of a natural inoculum, obtained from mixing 50 mL of water, 15 g detritus mud, and 15 g highly degraded peat from the same site, were added to each chamber. While we acknowledge that decomposing litter in anaerobic sediments typically consists of root material, we intentionally used P. australis leaf litter throughout all depths to enable a more consistent assessment of compartmental dependence on litter decomposition. This approach facilitated the comparison of the local biogeochemistry’s impact on decomposition, as different plant parts, such as leaves and rhizomes, exhibit variations in their macromolecular composition and subsequent decomposition processes (Reuter et al. 2020).
Sample collection
For practical reasons, we divided the sampling of three dialysis samplers with incubated plant litter into three consecutive sampling occasions, occurring at approximately 581, 588, and 595 days into the litter decomposition period. Each time, one dialysis sampler was excavated and immediately transferred into a 520 L glove bag (AtmosbagQR, Sigma Aldrich, Germany) that has been thoroughly flushed with argon to obtain an oxygen-free atmosphere. Pore waters from each chamber were sampled with 50 mL syringes, transferred to 100 mL Schlenk tubes, ensuring they were maintained under an inert atmosphere during transport. All samples were stored on ice and transported immediately to the laboratory. Surface waters, located approximately 15 cm below the water table, were collected using 1 L glass bottles (Schott, Germany). All water samples were filtered within 24 h through 0.2 µm PTFE membrane filter (Omnipore, Merck, Germany). Water samples from anoxic environments were filtered under nitrogen (N2) atmosphere in a glovebox (LABstar, mbraun, Germany), and the samples were kept under an inert atmosphere until analysis. Subsamples for dissolved organic carbon (DOC) analysis were additionally passed through a chelating filter to prevent iron precipitation in the water samples. P. australis leaves that had been incorporated in the dialysis samplers were freeze-dried, non-leaf organic matter was carefully removed, and weighed to determine mass loss. Finally, all leaves were ground to a fine powder with a vibrating cup mill (Pulverisette 9, Fritsch, Germany).
Sample analysis
Molecular composition of organic matter by FTIR
The molecular composition of leaf litter was analyzed by FTIR spectroscopy. Potassium bromide (KBr) pellets with a diameter of 13 mm and a thickness of approximately 1 mm were prepared. The pellets were made by mixing 300 mg oven dried KBr (for IR spectroscopy, MERCK) and 1 mg leaf litter sample in ball mill for 45 s. The mixture was then compressed into pellets using a hydraulic press with a pressure of 10 tons for several minutes under vacuum. All KBr pellets were measured immediately after preparation. The infrared spectra were collected by using an IRTracer-100 spectrometer (Shimadzu) equipped with a DLATGS detector. FTIR spectra were recorded in the absorbance mode between 400 and 4000 cm−1 at a spectral resolution of 4 cm−1. Each spectrum was the result of the co-addition of 200 scans. Before each sample, a background spectrum was recorded using a pure KBr pellet. The spectra were corrected for atmospheric CO2, baseline-corrected, and multiplied by a factor of 100 using the Labsolution IR software (Shimadzu Corp, Germany). To enhance peak determination accuracy, the second derivative spectra were generated using the Savitzky-Golay algorithm with 13 convolution points. All absorbance spectra were vector-normalized in the region 800 to 1900 cm−1 using Microsoft Excel. To represent the absolute losses of specific biopolymers after decomposition, the spectra were multiplied by the percentage of remaining organic carbon. The presented spectra represent the mean of all litter samples from a specific layer. For semiquantitative analysis of specific FTIR bands, especially of the aromatic signal at 1514 cm−1, a peak fitting routine was applied to the spectra in the range 1850 and 1200 cm−1, following the method described by Reuter et al. (2020) using the Peak analyzer software of OriginPro 8.5.0 SRI. The band positions were determined from the second derivative spectra and fixed for all fitting routines. Voigt-shaped bands with fixed peak widths were used for the fitting, with the software optimizing only the band height of each FTIR signal. The aromatic substructures of the lignin biopolymer contribute to several distinct bands in the FTIR spectrum (Faix 1991). The most prominent lignin band is the aromatic skeletal vibration at 1514 cm−1 which is commonly used to quantify leaf lignin content (Duboc et al. 2012). The intensity of the 1514 cm−1 band is here utilized as a second semiquantitative measure for lignin in the leaf litter and is referred to as lignin(FTIR). Unlike the CuO oxidation method (see below), FTIR spectroscopy can detect and quantify the remaining lignin biopolymer within the litter, even after molecular modifications have occurred. This contrasts with the CuO oxidation method, where phenols may not be detectable using mass spectrometry if the lignin biopolymer has undergone molecular alterations due to microbial decomposition activity, such as demethoxylation, condensation reactions, or other changes in molecular moieties (Ertel and Hedges 1984).
Lignin analysis by the CuO oxidation method
To avoid any carbon contamination, all glassware was heated to 450 °C for 4 h before use. Water was obtained from an Arium Pro Ultrapure Water System from Sartorius (Göttingen, Germany). The lignin analysis was performed after Reuter et al. (2017). In brief, the analysis of porewater lignin was performed directly on 15 mL water sample which was oxidized in a microwave digestion system (Microprep A, MLS GmbH, Germany) after addition of 1.76 mL NaOH (50%) and 300 mg CuO, 150 mg (NH4)2Fe(SO4)2⋅6H2O, and 10 mg glucose. For leaf litter analysis, the method was slightly modified. 5 mg finely ground leaf litter was transferred to the reaction vessel and 15 mL ultrapure water was added in addition to the other reagents. Lignin phenols were solid phase extracted with OasisQR HLB extraction cartridges (60 mg, 3 mL, Waters). In this study, we consider the following lignin-derived phenols: p-hydroxybenzaldehyde, p-hydroxybenzophenone and p-hydroxybenzoic acid (P-phenols); vanillin, acetovanillone, and vanillic acid (V-phenols); syringaldehyde, acetosyringone, and syringic acid (S-phenols), p-coumaric acid and ferulic acid (C-phenols). The quantification of these phenols was performed using a 7000C GC triplequad system from Agilent (Palo Alto, CA, USA) equipped with a DB-5 ms Ultra Inert capillary column (60 m × 0.25 mm × 0.25 µm) in multiple reaction monitoring (MRM) mode.
Due to the varying concepts associated with the term lignin (Hatfield and Fukushima 2005), we define lignin and related terms as follows. The lignin macromolecule refers to the unmodified biopolymer with an amorphous structure. Individual lignin monomers are released from the lignin macromolecule upon CuO-oxidation (Thévenot et al. 2010). We estimate the amount of lignin in the sample by considering the sum of the V- and S-phenols, which we term lignin(CuO) or Λ6. Lignin decomposition, observed as lignin(CuO), may involve structural modifications of the macromolecule, which can be detected through alterations in the ratios between monomers released during CuO oxidation, or quantitative changes that can be detected by considering the total amount of released monomers. The molar ratios between the phenols, i.e., P/V, S/V and C/V, are used as indicators of lignin decomposition.
Statistical analysis
All statistical tests were performed with RStudio (Version 1.1.383, RStudio, Inc.). To test the difference of parameters between initial versus decomposed leaves and pore water background chambers versus pore water leaf-containing chambers the Mann–Whitney-U test (Wilcoxon rank sum test in R) was performed. To examine layer dependency, the Kruskal–Wallis rank sum test, followed by pairwise comparisons using the Wilcoxon rank sum test, was performed. Linear correlations were assessed using the linear regression model in R.
Results
Bulk changes of leaf litter
The mass loss of leaf litter after 581–595 days of decomposition varied across different layers, ranging from 54% in the water body, where aerobic decomposition conditions prevail, to 26% in the deepest anaerobic sediment layer (Table 1). There was a strong linear relationship between litter mass loss and depth (R2 = 0.91, p < 0.001), indicating a decline in mass loss with increasing depth.
The C/N values of the decomposed leaves exhibited a higher dependency on the layer (p < 0.01) rather than a linear correlation with depth (R2 = 0.71, p < 0.001). The litter C/N values ranged from 14.4 ± 0.9 in the surface water to 23.4 ± 1.8 in the highly degraded peat layer. This trend was primarily driven by variations in litter N content. While litter C content remained relatively constant across all samples, there was an increase in litter N content from the deepest litter to the litter decomposed in the surface water (Table 1). Furthermore, compared to the initial leaves (C/N = 32.6 ± 1.5), all leaves showed a relative enrichment of litter N (p < 0.001).
The percentage loss of organic carbon also exhibited a significant dependency on the decomposition environment (p < 0.01), decreasing from the upper to the deepest layer. However, there was no significant difference between the former rhizosphere and the degraded peat. As the loss of organic carbon is partly derived from the mass loss data and changes in carbon content, this correlation was expected. The loss of organic carbon, however, showed a weaker correlation with depth (R2 = 0.72, p < 0.001) compared to mass loss, but revealed a higher dependency on the layer.
FTIR spectroscopy of leaf litter
The FTIR spectra of leaf litter provide insights into the breakdown of the different biopolymers, which contribute to the observed mass loss of the litter (Fig. 2). The specific FTIR peaks were identified after Kačuráková et al. (1994), Pandey (1999), and Pandey and Pitman (2003): The broad band observed at 3400 cm−1 corresponds to the strong hydrogen-bonded O–H stretch of carbohydrates. The peaks at 2920 cm−1 and 2851 cm−1 represent the antisymmetric and symmetric C–H stretching of methylene groups, which are associated with organic lipids. The sharp band at 1738 cm−1 is attributed to aliphatic ester groups, mainly present as unconjugated C=O in xylans (hemicellulose). The prominent amide I band of proteins appears at 1657 cm−1, and the amide II band is located at 1547 cm−1. The bands at 1591 cm−1, 1601 cm−1, and 1514 cm−1 are assigned to the aromatic skeletal vibrations. The aromatic band at 1514 cm−1 is easily quantifiable and serves as a measure of litter lignin content. The C–H deformation bands of lignin and carbohydrates are observed at 1466 cm−1 and 1424 cm−1, respectively, while the symmetric C–H deformation of cellulose and hemicellulose is located at 1375 cm−1. The peak at 1317 cm−1 is associated with the CH2 wagging and the C–H vibration in cellulose, as well as the C1–O vibration of syringyl derivatives. The band at 1236 cm−1 is linked to the syringyl ring and C–O stretch in lignin and xylans. The antisymmetric C–O–C vibration in cellulose and hemicellulose appears at 1163 cm−1, marking the beginning of the region dominated by strong carbohydrates signals. The peak heights of the 1100 cm−1 band, 1055 cm−1 band, and 1065 cm−1 band serve as a semiquantitative marker for the litter carbohydrate content. The peak at 1107 cm−1 is related to the antisymmetric ring stretch of glucose, as well as the aromatic skeletal and C–O stretch. The bands at 1055 cm−1 and 1034 cm−1 are associated with the C–O stretches of hemicellulose and cellulose. The peak at 989 cm−1, appearing as a shoulder, corresponds to the C–OH stretching in hemicellulose, and the band at 897 cm−1 is attributed to the C-H deformation in cellulose.
FTIR spectra of initial and decomposed Phragmites australis leaves. Spectra represent the mean of replicates and had undergo vector normalization, followed by multiplication with the percentage of remaining organic carbon in the sample (see Appendix for the min/max normalized FTIR spectra in Fig. 5)
Due to the linear relationship between the concentration of a biopolymer and the intensity of the resulting FTIR band, it is possible to normalize the FTIR spectra to organic carbon loss. Therefore, decreases in band intensity between different samples in Fig. 2 represent the percentage loss of the underlying biopolymer due to decomposition. This normalization procedure allowed us to determine the percentual loss of lignin(FTIR), as well as carbohydrate and protein losses. Interestingly, despite variations in mass loss, all decomposed leaf litters exhibited a relatively similar amount of proteins, as indicated by the strong spectral overlap of all spectra in the region where the amidic bands arise (1657 cm−1 and 1547 cm−1 in Fig. 2). This finding is consistent with bulk N measurements, which revealed that 108 ± 11% of the initially present N remained in the leaf litter after decomposition across all soil layers (Table 1). It is worth noting that former litter decomposition studies have demonstrated a highly linear relationship between litter N and litter protein content (Tremblay and Benner 2005). In line with this, a strong linear correlation (R2 = 0.95, p < 0.001) between the area of the amide II band and the bulk N content of all leaf litter samples is observed in this study.
Similarly, the lignin band at 1514 cm−1 only deviates minorly for all decomposed litters indicating only minor differences in the loss of lignin(FTIR) over depth. The losses of lignin(FTIR) are statistically significant (p < 0.01) but do not exhibit a dependency on soil layer or depth and account for 9.6 ± 5.9% of leaf litter over all depth (Table 2). In contrast, much stronger deviations are however seen in the carbohydrate region. The intensity decrease of the carbohydrate bands shows a highly significant correlation with the mass loss (R2 = 0.92, p < 0.001), suggesting that the lower mass losses of litter with increasing soil depth primarily result from varying degrees of carbohydrate degradation over the about 1.6 years decomposition period.
CuO lignin analyses of leaf litter
The CuO oxidation of leaf litter revealed a decrease in lignin(CuO) concentration due to decomposition. The initial undecomposed litter had a lignin(CuO) concentration from 5.60 mg C/100 mg C. After 581–595 days, the lignin(CuO) concentrations in the decomposed litter ranged from 4.52 mg C/100 mg C to 2.35 mg/100 mg C (Table 3). The percentual lignin(CuO) losses exceeded mass losses for all litters from all depth, ranging from 45.7% to 69%. This indicates that the percentual loss of lignin(CuO) was greater than the overall mass loss during decomposition. Interestingly, the pattern of lignin(CuO) decomposition differed from carbohydrate decomposition and overall mass loss. The highest decrease in lignin(CuO) as a fraction of the remaining litter tissue was observed for litter decomposed in the former rhizosphere. After decomposition, the lignin content dropped to 2.35 mg lignin-C/100 mg litter-C, resulting in a 69% loss of initial litter-lignin while experiencing only a 32.2% overall mass loss. Similar lignin(CuO) losses were observed for litters decomposed in the surface water of the rewetted fen, with an overall lignin(CuO) loss of 67.0% at an overall mass loss of 54.6%. In the detritus mud and highly degraded peat, leaf litters lost about 45% lignin(CuO) at mass losses of 43.1% and 26.8%, respectively. Regarding the single lignin phenol families, all P-, V- and S-phenols decreased during decomposition. However, the C-phenols exhibited a different trend. The concentrations of C-phenols did not decrease during decomposition, but remained relatively stable for all decomposed litters, with overall values of 3.25 mg-C/100 mg litter-C to 2.71 mg-C/100 mg litter-C (Table 3). This suggests that the C-phenols were less susceptible to decomposition compared to the core-lignin biopolymer.
Lignin phenols in dissolved organic matter
The DOC concentrations in the rewetted fen Zarnekow showed an increase with depth, ranging from 21 mg/L in the surface water to 217 mg/L in the highly degraded peat (Table 4).
Lignin phenols were detected in all water samples. The concentrations of vanillyl and syringyl phenols, which serve as an estimate for dissolved lignin (∑6), were relatively consistent, ranging narrowly from 47.4 to 41.4 µg/L in the surface water, detritus mud, and former rhizosphere. However, in the highly degraded peat, the concentrations of these phenols were substantially higher at 163 µg/L. When normalized to DOC, dissolved lignin concentrations showed a decreasing trend with depth, ranging from 367 µg/100 mg DOC in the surface water to 62 µg/100 mg DOC in the highly degraded peat. It is worth noting that the observed high lignin yield of 367 µg/100 mg DOC in the surface water is probably a result of low DOC concentration in the surface water. Interestingly, among all phenol families, the P-phenols were the most abundant in all water samples, with concentrations ranging from 46.1 to 252 µg/L.
Dissolved organic carbon and dissolved lignin were also analyzed in the water samples collected from the leaf-containing chambers of the dialysis sampler. These samples will be termed litter-DOM as DOM characteristics might be affected from the proximity to the decomposing litter. By comparing the background-DOM and litter-DOM, the impact of decomposing leaf litter on the surrounding DOM characteristics, such as DOC and porewater lignin, can be observed. It is important to note that the changes in leaf litter composition discussed earlier are cumulative, as they result from approximately 1.6 years of decomposition. In contrast, DOM characteristics provide only a snapshot, as processes like diffusion, DOM formation, and mineralization can alter the DOM composition within a few days. The DOC concentration, as well as dissolved lignin concentrations, were significantly elevated in the leaf-containing chambers in the surface water, detritus mud, and former rhizosphere (p < 0.01). However, no statistical difference in dissolved lignin due to decomposing litter was observed in the highly degraded layer. While the amount of lignin phenols increased in the vicinity of decomposing litter, this trend disappeared when dissolved lignin was normalized to DOC (Fig. 3a). Only the yield of C-phenols showed a significant increase in all soil layers (p < 0.001, Fig. 3b).
Discussion
The observed linear decrease in mass loss with increasing soil depth in the rewetted peatland aligns with findings from other studies conducted in various ecosystems, such as forest and grassland floors, as well as wetland soils (Hemminga et al. 1988; Gill and Burke 2002). The depth-dependent patterns observed in the decomposition rates of different biopolymers underscore the significance of environmental factors, microbial activity, and the unique composition of soil layers in shaping decomposition processes in rewetted peatlands. Specifically, this trend can be attributed to oxygen availability, where the surface water body with greater oxygen levels promotes higher decomposition rates, while lower anoxic layers exhibit slower decomposition rates due to limited availability of electron acceptors and nutrients (Zak et al. 2019). The importance of specific biopolymer losses, such as carbohydrates and lignin, will be discussed in detail in the following sections.
Bulk decomposition in different soils of a rewetted fen
The use of infrared spectroscopy provides valuable insights into the composition of decomposed litter. Our findings indicate that variations in mass loss are primarily caused by the degradation of carbohydrates, presumably hemicellulose (Serk et al. 2022), which constitute a significant portion of plant litter. This observation highlights the importance of considering specific biopolymer losses when assessing decomposition, as relying solely on mass loss data may overlook the underlying changes in the litter composition (ibid.). Interestingly, the total nitrogen content in the decomposed litter did not follow the same trend as mass loss or carbohydrate degradation (Table 1). Instead, it exhibited dependence on the soil layer. The nitrogen content can serve as an indicator of microbial biomass (Qu et al. 2022), suggesting that the increase in microbial activity within the litter is influenced by the physical and chemical characteristics of the soil layer (Emsens et al. 2020; Weil et al. 2020). This indicates that the rate of carbohydrate degradation in the litter follows a distinct, depth-dependent pattern, while microbial activity and nutrient dynamics also play a role in shaping the decomposition process. It is crucial to acknowledge that the decomposition of lignin, as demonstrated in this study, exhibited a different pattern compared to carbohydrate degradation. The lignin losses observed through CuO oxidation showed a substantial decrease, indicating significant decomposition of lignin during the 1.6 years period. This highlights the complex nature of decomposition and the differential decomposition rates of different biopolymers within leaf litter.
Leaf lignin decomposition and internal modification
Despite the modern belief that microbial communities are capable of decomposing various natural biopolymers, there is still a consensus that lignin undergoes limited decomposition in anaerobic wetland soils (Dungait et al. 2012). This consensus is justified by the observation that microorganisms require oxygen to decompose the aromatic substructures of organic matter. Therefore, it is expected that lignin, being a highly aromatic biopolymer, would accumulate in anaerobic soil layers (Serk et al. 2022). In this study, two contrasting methods were applied to analyze lignin decomposition. Consistent with the consensus of limited lignin decomposition in anaerobic environments, FTIR spectroscopy revealed that the total amount of aromatic compounds in the leaf litter, referred to as lignin(FTIR), only exhibited minor decreases during decomposition, ranging from 6.4 to 11.8% in all anaerobic soils. This indicates that lignin(FTIR) decomposition occurs to a significantly lesser extent compared to carbohydrate decomposition, which exhibited losses ranging from 24.9 to 43%. Furthermore, it is noteworthy that lignin(FTIR) decomposition in the anaerobic soil layer occurs to a lesser extent compared to the litter decomposed in the surface water, where 16.9% lignin was lost from the litter. These findings highlight the relative resistance of lignin to degradation in anaerobic conditions, emphasizing its persistence in organic matter.
The analysis of lignin using CuO oxidation and mass spectrometry provided contrasting results compared to FTIR spectroscopy. Lignin(CuO) losses were found to be considerably higher than carbohydrate losses, ranging from 45.7 to 69%. Although the lignin biopolymer is still predominantly present in the leaf litter, the CuO analysis suggests that internal molecular modifications resulting from microbial decomposition render certain molecular subunits undetectable. These modifications, such as demethylation or demethoxylation of lignin side chains, have been extensively documented in the literature and are known to contribute to methane production (Conrad 2020). Notably, a significant depletion of lignin was observed in the former rhizosphere, with exceptionally high lignin(CuO) losses of 69%. These losses exceeded carbohydrate losses in the litter by more than twofold, even though carbohydrates are generally considered to be more easily decomposable. One possible explanation for these trends is the presence of a microbial community like anaerobic fungi and bacteria which are specifically adapted to lignin decomposition (Grgas et al. 2023; Lankiewicz et al. 2023), as the organic matter in the former rhizosphere is predominantly composed of lignin-rich senescent roots. This hypothesis finds support in the functional breadth (FB) and home-field advantage (HFA) hypotheses (Fanin et al. 2016). The FB hypothesis suggests that microbial communities in recalcitrant litter environments possess a wider functional ability to decompose a broader range of litter species. The HFA hypothesis, on the other hand, proposes that decomposer communities may be specialized towards the litter they most frequently encounter (Hunt et al. 1988). It was demonstrated that lignin generally exhibits greater FB and HFA effects compared to more bioavailable saccharides, albeit with a delay of 100 days (Fanin et al. 2016).
Previous studies using 13C CPMAS NMR and the van Soest procedure have reported decreasing CuO lignin concentrations during decomposition, accompanied by increasing concentrations of aromatic compounds (Chabbi and Rumpel 2004; Kalbitz et al. 2006). These findings could be interpreted as internal lignin modifications. One possible process that may contribute to these observations is the humification of leaves. Phenols containing methoxyl groups are known to serve as precursors for ring condensation reactions (Stevenson 1994). In our study, we observed a specific loss of vanillin phenols in leaf litter without a corresponding increase in porewater lignin vanillin phenols. This suggests that internal modifications of vanillin phenols may occur, leading to the formation of condensed aromatic rings or other successor products. Several studies have found that de-methoxylation is the first step during anaerobic degradation of lignin followed by ring cleavage and fermentation into methane and carbon dioxide (Khan and Ahring 2019). Consequently, it is plausible to suggest that the elevated methane emissions observed in the rewetted study site Zarnekow (Antonijević et al. 2023) may be partially attributed to the loss or demethoxylation of vanillin phenols and their further cleavage or reorganization of lignin-lignin linkages by anaerobic fungi or bacteria (Lankiewicz et al. 2023). A recent study demonstrated a significant increase in the abundance and diversity of methylotrophic methanogens in rewetted fen peatlands, like the site under investigation (Weil et al. 2023). It can be expected that certain anaerobic organisms can deconstruct unpretreated, naturally occurring lignin in anaerobic environments throughout the biosphere (Lankiewicz et al. 2023).
Lignin transformation
Lignin phenol sum parameters (P, V, S, C) are commonly used as source indicators in the analysis of soil and sediment samples to investigate terrestrial plant sources (Thévenot et al. 2010). These parameters can distinguish between vascular and non-vascular plants based on the presence of V-phenols, as well as between angiosperms and gymnosperms based on the presence of S-phenols. Additionally, different tissue types, such as non-woody and woody tissues, can be distinguished based on the presence of C-phenols (Hedges and Mann 1979). In the case of P. australis leaf litter, the observed concentrations of S- and C-phenols align with these trends, indicating the expected presence of grass-type lignin.
In addition to their use as source indicators, changes in the concentrations of lignin phenol families during leaf litter decomposition experiments can provide insights into the selective modifications or reactivity of individual phenols within the lignin polymer. Two distinct trends were observed in the changes of lignin phenol families during leaf litter decomposition experiments, with the former rhizosphere environment exhibiting the most pronounced effects. The first trend involves the selective loss of V-phenols. According to a conceptual model for the transformation of solid litter lignin to porewater lignin, extracellular enzymes are released during decomposition, resulting in the release of smaller lignin polymers that can be further processed in the dissolved phase (Strakova et al. 2011). This model suggests that each phenol monomer is lost at a constant ratio. The selective loss of V-phenols within the leaf litter, which does not correspond to an increase in V-phenols in porewater lignin and furthermore no transformation to P-phenols via demethoxylation, suggests that a different process may be occurring, such as alterations or transformations of V-phenols within the leaf tissue. These modifications may render the V-phenols undetectable upon CuO oxidation. The exact nature of these alterations and the mechanisms involved require further investigation. The second trend relates to the distinct behavior of C-phenols compared to other phenol families. C-phenols exhibited a different decomposition pattern, indicating their unique reactivity. It is widely reported that C-phenols, which are covalently linked to hemicellulose through lignin-carbohydrate complexes, show higher reactivity and preferential loss during decomposition compared to other phenol families (Opsahl and Benner 1995; Chabbi and Rumpel 2004). It is worth noting that the presence of suberins, which have a similar chemical composition to C-phenols, can pose limitations when using C-phenols as exclusive lignin indicators. Opsahl and Benner (1995) suggested using Λ6 instead of Λ8 to estimate the absolute lignin content. However, in herbaceous tissues, C-phenols account for approximately 30% of the lignin content (Hedges and Mann 1979), indicating their importance in understanding decomposition patterns. In our study, the decomposed leaf litter exhibited increased percentages of C-phenols in the remaining lignin in all layers from 34.4 ± 0.9% in the initial leaf litter up to for example 53.7 ± 2.2% in the former rhizosphere litter (Fig. 4), suggesting that under anaerobic conditions, the decomposition of C-phenols may be inhibited. This trend was also observed in porewater lignin in the presence of leaf litter, supporting the notion of accumulation due to inhibition of mineralization and alteration. Thus, C-phenols serve as a more sensitive indicator of active decomposition processes occurring in the immediate vicinity (microsite) compared to overall lignin indicators or elevated DOC concentrations. These trends in the selective modifications and reactivity of lignin phenol families provide valuable insights into the complex dynamics of leaf litter decomposition and the fate of lignin during this process. Further research is needed to elucidate the specific mechanisms responsible for these trends and their implications for carbon and nutrient cycling in wetland ecosystems.
Conclusions
Our findings provide valuable insights into the decomposition processes within rewetted peatlands. Firstly, we observed that the primary contributor to mass loss in decomposing litter was the breakdown of carbohydrates, emphasizing their pivotal role in the overall organic matter loss within the litter. Secondly, our analysis of lignin, utilizing CuO oxidation and mass spectrometry, yielded results distinct from those obtained through FTIR spectroscopy. While FTIR spectroscopy indicated a relatively minor loss of lignin, our findings suggest that lignin is more likely subjected to internal modifications within the litter, rather than being lost to the environment. Furthermore, the decomposition of lignin exhibited unique patterns, notably the selective loss of V-phenols and the stability of C-phenols. These observations underscore the complexity of lignin decomposition and hint at the presence of specific microbial communities or enzymatic processes that selectively modify or stabilize different phenol groups within the lignin polymer. Intriguingly, the rooted degraded soil layer displayed unexpectedly high levels of lignin decomposition, possibly due to the presence of methylotrophic lignin-decomposing microorganisms adapted to water-saturated anaerobic conditions. The exact relationship between this observed high lignin decomposition, past drainage activities, and the presence of elevated levels of nutrients and terminal electron acceptors warrants further investigation. Moreover, the duration of this decomposition trend following rewetting remains uncertain. The results of this study suggest that the removal of the degraded rooted soil layer may temporarily enhance carbon storage in rewetted organic soils. However, on the other hand, it could lead to a lower net carbon sequestration due to reduced biomass production after the removal of nutrient-rich topsoil. Therefore, a careful evaluation is necessary when considering the benefits of topsoil removal for carbon sequestration.
Data availability
All data, materials and software application supporting our published claims comply with field standards. Other datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Adamczyk B, Heinonsalo J, Simon J (2020) Mechanisms of carbon sequestration in highly organic ecosystems—importance of chemical ecology. Chem Open 9:464
Antonijević D, Hoffmann M, Prochnow A et al (2023) The unexpected long period of elevated CH4 emissions from an inundated fen meadow ended only with the occurrence of cattail (Typha latifolia). Glob Chang Biol. https://doi.org/10.1111/gcb.16713
Bärlocher F (2005) Leaf mass loss estimated by litter bag technique. In: Graça MA, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition. Springer, Dordrecht. https://doi.org/10.1007/1-4020-3466-0_6
Brouns K, Keuskamp JA, Potkamp G, Verhoeven JTA, Hefting MM (2016) Peat origin and land use effects on microbial activity, respiration dynamics and exo-enzyme activities in drained peat soils in the Netherlands. Soil Biol Biochem 95:144–155. https://doi.org/10.1016/j.soilbio.2015.11.018
Cabezas A, Pallasch M, Schoenfelder I, Gelbrecht J, Zak D (2014) Carbon, nitrogen, and phosphorus accumulation in novel ecosystems: shallow lakes in degraded fen areas. Ecol Eng 66:63–71. https://doi.org/10.1016/j.ecoleng.2013.10.037
Chabbi A, Rumpel C (2004) Decomposition of plant tissue submerged in an extremely acid mining lake sediment: phenolic CuO-oxidation products and solid-state 13C NMR spectroscopy. Soil Biol Biochem 36:1161–1169. https://doi.org/10.1016/j.soilbio.2004.02.026´
Conrad R (2020) Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: a mini review. Pedosphere 30:25–39. https://doi.org/10.1016/S1002-0160(18)60052-9
Derkacheva O, Sukhov D (2008) Investigation of lignins by FTIR spectroscopy. Macromol Symp 265:61–68. https://doi.org/10.1002/masy.200850507
Duboc O, Zehetner F, Djukic I, Tatzber M, Berger TW, Gerzabek MH (2012) Decomposition of European beech and Black pine foliar litter along an Alpine elevation gradient: mass loss and molecular characteristics. Geoderma 189–190:522–531. https://doi.org/10.1016/j.geoderma.2012.06.018
Dungait JAJ, Hopkin DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol 18:1781–1796. https://doi.org/10.1111/j.1365-2486.2012.02665.x
Emsens W-J, Aggenbach CJS, Smolders AJP, Zak D, van Diggelen R (2017) Restoration of endangered fen communities: the ambiguity of iron–phosphorus binding and phosphorus limitation. J Appl Ecol 54:1755–1764. https://doi.org/10.1111/1365-2664.12915
Emsens WJ, van Diggelen R, Aggenbach CJS et al (2020) Recovery of fen peatland microbiomes and predicted functional profiles after rewetting. ISME J 14:1701–1712. https://doi.org/10.1038/s41396-020-0639-x
Ertel JR, Hedges JI (1984) The lignin component of humic substances: distribution among soil and sedimentary humic, fulvic, and base-insoluble fractions. Geochim Cosmochim Acta 48:2065–2074. https://doi.org/10.1016/0016-7037(84)90387-9
Faix O (1991) Classification of lignins from different botanical origins by FT-IR. Spectroscopy 45:21–28. https://doi.org/10.1515/hfsg.1991.45.s1.21
Fanin N, Fromin N, Bertrand I (2016) Functional breadth and home-field advantage generate functional differences among soil microbial decomposers. Ecology 97:1023–1037. https://doi.org/10.1890/15-1263.1
Gill RA, Burke IC (2002) Influence of soil depth on the decomposition of Bouteloua gracilis roots in the shortgrass steppe. Plant Soil 241:233–242. https://doi.org/10.1023/A:1016146805542
Grgas D, Rukavina M, Bešlo D, Štefanac T, Crnek V, Šikić T, Habuda-Stanić M, Landeka Dragičević T (2023) The bacterial degradation of lignin—a review. Water 15(7):1272. https://doi.org/10.3390/w15071272
Hahn-Schöfl M, Zak D, Minke M et al (2011) Organic sediment formed during inundation of a degraded fen grassland emits large fluxes of CH4 and CO2. Biogeosciences 8:1539–1550. https://doi.org/10.5194/bg-8-1539-2011
Hatfield R, Fukushima RS (2005) Can Lignin Be Accurately Measured? Crop Sci 45:832–839. https://doi.org/10.2135/cropsci2004.0238
Hedges JI, Mann DC (1979) The characterization of plant tissues by their lignin oxidation products. Geochim Cosmochim Acta 43:1803–1807. https://doi.org/10.1016/0016-7037(79)90028-0
Hedges JI, Blanchette RA, Weliky K, Devol AH (1988) Effects of fungal degradation on the CuO oxidation products of lignin: A controlled laboratory study. Geochim Cosmochim Acta 52:2717–2726. https://doi.org/10.1016/0016-7037(88)90040-3
Hemminga MA, Kok C, De Munck W (1988) Decomposition of Spartina anglica roots and rhizomes in a salt marsh of the Westerschelde estuary. Mar Ecol Prog Ser 48:175–184. https://doi.org/10.3354/meps048175
Hernes PJ, Benner R (2003) Photochemical and microbial degradation of dissolved lignin phenols: Implications for the fate of terrigenous dissolved organic matter in marine environments. J Geophys Res 108:3291. https://doi.org/10.1029/2002JC001421
Hernes PJ, Robinson AC, Aufdenkampe AK (2007) Fractionation of lignin during leaching and sorption and implications for organic matter “freshness”. Geophys Res Lett 34:L17401. https://doi.org/10.1029/2007GL031017
Huang W, Hammel KE, Hao J, Thompson A, Timokhin VI, Hall SJ (2019) Enrichment of lignin-derived carbon in mineral-associated soil organic matter. Environ Sci Technol 53:7522–7531. https://doi.org/10.1021/acs.est.9b01834
Huang W, Yu W, Yi B et al (2023) Contrasting geochemical and fungal controls on decomposition of lignin and soil carbon at continental scale. Nat Commun 14:2227. https://doi.org/10.1038/s41467-023-37862-6
Hunt HW, Ingham ER, Coleman DC, Elliott ET, Reid CPP (1988) Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine forest. Ecology 69:1009–1016. https://doi.org/10.2307/1941256
Jurasinski G, Ahmad S, Anadon-Rosell A et al (2020) From understanding to sustainable use of peatlands: the WETSCAPES approach. Soil Syst 4:14. https://doi.org/10.3390/soilsystems4010014
Kačuráková M, Ebringerová A, Hirsch J, Hromádková Z (1994) Infrared study of arabinoxylans. J Sci Food Agric 66:423–427. https://doi.org/10.1002/jsfa.2740660323
Kalbitz K, Kaiser K, Bargholz J, Dardenne P (2006) Lignin degradation controls the production of dissolved organic matter in decomposing foliar litter. Eur J Soil Sci 57:504–516. https://doi.org/10.1111/j.1365-2389.2006.00797.x
Khan MU, Ahring BK (2019) Lignin degradation under anaerobic digestion: influence of lignin modifications—a review. Biomass Bioenerg 128:105325. https://doi.org/10.1016/j.biombioe.2019.105325
Kimmel K, Mander U (2010) Ecosystem services of peatlands: implications for restoration. Prog Phys Geog 3:491–514. https://doi.org/10.1177/0309133310365595
Kreyling J, Tanneberger F, Jansen F et al (2021) Rewetting does not return drained fen peatlands to their old selves. Nature Commun 12:5693. https://doi.org/10.1038/s41467-021-25619-y
Lankiewicz TS, Choudhary H, Gao Y et al (2023) Lignin deconstruction by anaerobic fungi. Nat Microbiol 8:596–610. https://doi.org/10.1038/s41564-023-01336-8
Opsahl S, Benner R (1995) Early diagenesis of vascular plant tissues: Lignin and cutin decomposition and biogeochemical implications. Geochim Cosmochim Acta 59:4889–4904. https://doi.org/10.1016/0016-7037(95)00348-7
Opsahl S, Benner R (1997) Distribution and cycling of terrigenous dissolved organic matter in the ocean. Nature 386:480–482. https://doi.org/10.1038/386480a0
Pandey KK (1999) A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J Appl Polym Sci 71:1969–1975. https://doi.org/10.1002/(SICI)1097-4628(19990321)71:12%3c1969::AID-APP6%3e3.0.CO;2-D
Pandey KK, Pitman AJ (2003) FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int Biodeterior Biodegrad 52:151–160
Peng Q, Lin L, Tu Q, Wang X, Zhou Y, Chen J, Jiao N, Zhou J (2023) Unraveling the roles of coastal bacterial consortia in degradation of various lignocellulosic substrates. mSystems 8(4):e0128322. https://doi.org/10.1128/msystems.01283-22
Qu W, Xie B, Hua H, Bohrer G, Penuelas J, Wu C, Han G (2022) Long-term nitrogen enrichment accelerates soil respiration by boosting microbial biomass in coastal wetlands. Soil Biol Biochem 175:108864. https://doi.org/10.1016/j.soilbio.2022.108864
Raji O, Arnling Bååth J, Vuong TV, Larsbrink J, Olsson L, Master ER (2021) The coordinated action of glucuronoyl esterase and α-glucuronidase promotes the disassembly of lignin–carbohydrate complexes. FEBS Lett 595:351–359. https://doi.org/10.1002/1873-3468.14019
Reuter H, Gensel J, Elvert M, Zak D (2017) Direct analysis of lignin phenols in freshwater dissolved organic matter. Anal Chem 89(24):13449–13457. https://doi.org/10.1021/acs.analchem.7b03729
Reuter H, Gensel J, Elvert M, Zak D (2020) Evidence for preferential protein depolymerization in wetland soils in response to external nitrogen availability provided by a novel FTIR routine. Biogeosciences 17:499–514. https://doi.org/10.5194/bg-17-499-2020
Schipper LA, Reddy KR (1995) In situ determination of detrital breakdown in wetland soil-floodwater profile. Soil Sci Soc Am J 59:565–568. https://doi.org/10.2136/sssaj1995.03615995005900020042x
Schmidt M, Torn M, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. https://doi.org/10.1038/nature10386
Serk H, Nilsson MB, Figueira J, Krüger JP, Leifeld J, Alewell C, Schleucher J (2022) Organochemical characterization of peat reveals decomposition of specific hemicellulose structures as the main cause of organic matter loss in the acrotelm. Environ Sci Technol 56:17410–17419. https://doi.org/10.1021/acs.est.2c03513
Stevenson FJ (1994) Humus chemistry genesis, composition, reactions. Wiley, New York, p 496
Strakova P, Niemi RM, Freeman C et al (2011) Litter type affects the activity of aerobic decomposers in a boreal peatland more than site nutrient and water table regimes. Biogeosciences 8:2741–2755. https://doi.org/10.5194/BG-8-2741-2011
Temmink RJM, Lamers LPM, Angelini C et al (2022) Recovering wetland biogeomorphic feedbacks to restore the world’s biotic carbonhotspots. Science 376:eabn1479. https://doi.org/10.1126/science.abn1479
Thévenot M, Dignac M, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42:1200–1211
Tiemeyer B, Freibauer A, Borraz EA et al (2020) A new methodology for organic soils in national greenhouse gas inventories: data synthesis, derivation and application. Ecol Indic 109:105838. https://doi.org/10.1016/j.ecolind.2019.105838
Tremblay L, Benner R (2005) Microbial contributions to N-immobilization and organic matter preservation in decaying plant detritus. Geochim Cosmochim Acta 70:133–146. https://doi.org/10.1016/j.gca.2005.08.024
Weil M, Wang H, Bengtsson M et al (2020) Long-term rewetting of three formerly drained peatlands drives congruent compositional changes in pro- and eukaryotic soil microbiomes through environmental filtering. Microorganisms 8(4):550. https://doi.org/10.3390/microorganisms8040550
Weil M, Wang H, Zak D, Urich T (2023) Spatial and temporal niche separation of methanomassiliicoccales phylotypes in temperate fens. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiad049
Yi B, Lu C, Huang W, Yu W, Yang J, Howe A, Weintraub-Leff SR, Hall SJ (2023) Resolving the influence of lignin on soil organic matter decomposition with mechanistic models and continental-scale data. Glob Chang Biol 29:5968–5980. https://doi.org/10.1111/gcb.16875
Zak D, Gelbrecht J (2007) The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 85:141–151. https://doi.org/10.1007/s10533-007-9122-2
Zak D, McInnes RJ (2022) A call for refining the peatland restoration strategy in Europe. J Appl Ecol 59(11):2698–2704. https://doi.org/10.1111/1365-2664.14261
Zak D, Wagner C, Payer B, Augustin J, Gelbrecht J (2010) Phosphorus mobilization in rewetted fens: the effect of altered peat properties and implications for their restoration. Ecol Appl 20:1336–1349. https://doi.org/10.1890/08-2053.1
Zak D, Reuter H, Augustin J, Shatwell T, Barth M, Gelbrecht J, McInnes RJ (2015) Changes of the CO2 and CH4 production potential of rewetted fens in the perspective of temporal vegetation shifts. Biogeosciences 12:2455–2468. https://doi.org/10.5194/bg-12-2455-2015
Zak D, Roth C, Unger V, Goldhammer T, Fenner N, Freeman C, Jurasinski G (2019) Unraveling the importance of polyphenols for microbial carbon mineralization in rewetted riparian peatlands. Front Environ Sci 7:147. https://doi.org/10.3389/fenvs.2019.00147
Acknowledgements
The concept for this paper was developed at the workshop titled “Peatlands for climate change mitigation in agriculture” that took place in Aarhus, Denmark, on 4–5 October 2022, and which was sponsored by the Organisation for Economic Co-operation and Development (OECD) Co-operative Research Programme: Sustainable Agricultural and Food Systems. The opinions expressed and arguments employed in this publication are the sole responsibility of the authors and do not necessarily reflect those of the OECD or of the governments of its member countries.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research has been supported by the Deutsche Forschungsgemeinschaft (Grant no. ZA 742/2-1).
Author information
Authors and Affiliations
Contributions
JR: Investigation, Methodology, Writing—original draft, Writing—review & editing. HR: Conceptualization, Methodology, Data curation, Writing—review & editing. DZ: Conceptualization, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Klaus Butterbach-Bahl
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Fig. 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reuter, J., Reuter, H. & Zak, D. Decomposition of lignin and carbohydrates in a rewetted peatland: a comparative analysis of surface water and anaerobic soil layers. Biogeochemistry 167, 545–561 (2024). https://doi.org/10.1007/s10533-023-01102-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-023-01102-2