Abstract
To foster a circular bioeconomy throughout the management of industrial solid wine residues in the wine industry, this work presents the physicochemical and microbiological dynamics of the composting process with white grape pomace, stalks and wastewater treatment plant sludge from the same winery. Three composting windrows of 41 m3 were constructed with 0, 10 and 20% sludge addition. Physicochemical parameters were assessed following the Test Method for the Examination of Composting and Compost (TMECC), and the diversity and dynamics of the bacterial and fungal communities involved in the composting process were assessed via a high-throughput sequencing metabarcoding approach. The addition of sludge increased the moisture content, bulk density, and pH after six months of turned windrow composting. No effect of sludge addition on the macronutrient composition of the compost was observed. The Shannon‒Wiener index differed among stages and treatments. Bacterial diversity increased over time, while the fungal community appeared to be highly affected by the thermophilic stage, which led to a reduction in diversity that slightly recovered by the end of the process. Furthermore, the sludge exhibited high bacterial diversity but very low fungal diversity. Consequently, the design of on-site biologically based strategies to better manage wine residues can produce soil amendments, improve fertilization, reclaim damaged soils, and ultimately reduce management costs, making composting an economically attractive and sustainable alternative for waste management in the wine industry.
Graphical Abstract

Highlights
-
Physicochemical and microbiological studies of sludge and grape pomace in composting are necessary to foster a circular bioeconomy in the wine industry.
-
Sludge addition improved water retention and bulk density, but no effect on macronutrient composition was observed; nonetheless, an increase in beneficial microorganisms was found.
-
Closing the loop in the management of organic residues via composting in the wine industry will improve economic and environmental performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Composting is one of the most commonly used techniques for valuating organic residues due to its low operational cost and the production of a stable substrate enriched with nutrients, namely, compost. Furthermore, this eco-friendly, low-cost technology can realize the revalorization of organic waste, including sewage sludge, agricultural and forestry residues and animal manures, into organic fertilizers such as compost [1,2,3,4]. Composting is a process of exothermic oxidative microbial degradation consisting of mesophilic (25–45 °C), thermophilic (45–70 °C), late mesophilic and maturation stages [5]. During the first stage, mesophilic microorganisms multiply, quickly metabolizing sugars and simple molecules via exothermic reactions, reaching temperatures close by 45 °C [6]. In this stage, the microbial community changes because of the increase in temperature. Thermophilic microorganisms degrade more complex molecules, such as sugars, fat, and proteins, further increasing the temperature to between 50 and 70 °C, and most pathogenic bacteria and fungi are eliminated [5, 7, 8]. Finally, a cooling phase known as the late mesophilic stage takes place, and the ambient temperature is reached. In this phase, mesophilic microorganisms appear again and degrade molecular products of the thermophilic stage. The process ends in the phase of stabilization or maturation, characterized by rapid decreases in microbial activity and temperature and stabilization of the nutrients and properties of the compost [7, 8].
Since composting is a biological process, different factors affect its development, such as temperature, moisture, carbon:nitrogen (C:N) ratio, pH, aeration, and bulk density [5,6,7]. The optimal physical composting conditions are a pH of 6.5–7.5, a C:N ratio of 25:1–30:1, a moisture content of 45–60%, an oxygen saturation over 5%, and a balanced bulk density and porosity [5,6,7,8]. The carbon (C) content, nitrogen (N) content, C:N ratio, ammonium:nitrate (NH4+:NO3−) ratio, potassium (K) content, and phosphorous (P) content, are among the typically analyzed chemical parameters [5, 8].
The most widely reported microorganisms in composting processes include aerobic bacteria from the phyla Proteobacteria, Bacteroidetes, Firmicutes, Chloroflexi, and Actinobacteria, which produce hydrolases and play key roles in protein and lignin decomposition. Specifically, different beneficial bacterial genera related to the nitrogen cycle (i.e., Nitrosomonas, Nitrospira, Rhizobium), phosphate solubilizers (i.e., Pseudomonas, Aeromonas, Streptomyces), potassium solubilizers (i.e., Enterobacter, Bacillus) and growth promoters (i.e., Kluyvera, Brevundimonas) have been reported to be involved in composting [9, 10]. This bacterial community composition is directly related to temperature, which varies according to the composting stage [5, 6, 11], and these microbes are more commonly found in the mesophilic phase of the composting process.
The most widely reported fungi are also mostly aerobic and are from the phyla Ascomycota and Basidiomycota [5, 8, 11]. Fungi degrade complex molecules and produce powerful ligninolytic, cellulolytic, and pectinolytic agents that also degrade chitin and keratin; moreover, many fungi release water-soluble substances, antibiotics, and dark pigments, which are very important for the humification process [11]. Specifically, different beneficial fungal genera, such as phosphorus solubilizers (i.e., Aspergillus and Trichoderma), phosphate solubilizers (i.e., Aspergillus, Rhizopus, and Talaromyces), biocontrollers (i.e., Gliocadium and Trichoderma), and potassium solubilizers (i.e., Aspergillus), have been identified in the composting process [9, 12]. Changes in these microorganisms during the composting process trigger the transformation, retention, and bioavailability of nitrogen and phosphorus in compost due to the association of these microorganisms with organic phosphorus mineralization, nitrification, and denitrification [13]. A change from this aerobic microbiome to an anaerobic microbiome can produce undesirable effects, such as the generation of bad odors, S2 and H2S from sulfate reduction and the degradation of sulfur-containing compounds, which contaminates the air and reduces the quality of the compost due to sulfur deficiency [14].
While microorganisms are needed for the degradation of organic substrates during composting, it is essential to identify pathogenic microorganisms in the final compost product to evaluate potential harmful effects on soil and crops. The pathogenic bacteria Acetobacter aceti (Pasteur) Beijerinck and Acetobacter sp. are responsible for acid rot, and Agrobacterium tumefaciens and Agrobacterium vitis are responsible for crown gall disease [15]. There is a wide range of potentially pathogenic fungi. Some examples include Acremonium alternatum (dying arm disease); Aerobasidium, Alternaria, and Cladosporium (acid rot, cluster rot); Pythium, Phytophthora and Sclerotium rolfsii (root rot); Aspergillus sp. (grape cluster rot); Botryosphaeria, Fusicoccum, Dothiorella and Cytospora (canker); and Botrytis (bunch rot), among others [15, 16].
Winery residues mainly include grape pomace, lees, stalks and sludge from wastewater treatment plant. The composting of winery solid residues is mainly affected by the presence of phytotoxic and antibacterial substances, such as ethanol, organic acids, and phenolic compounds, which hinder microbial development, reducing the agronomic quality of the compost [17,18,19]. Nitrogen supplements such as manure and chemical fertilizers are commonly used to increase the microbial load and nutrient contents and thus enhance the biodegradation rate [9, 20,21,22]. Recent meta-analyses have demonstrated that the use of additives such as zeolite, clinoptildite and biochar can significantly decrease heavy metal bioavailability by at least 40% in the final compost products and increase the total nitrogen content and pH by 16% and 5%, respectively [23]. However, the use of additives and supplements from other industries generates additional expenses for winery companies. Considering that sludge represents approximately 5% of the total residues produced by wineries [17], the composting of grape stalks, pomace and nitrogen-rich sludge has also been used to reduce operational costs [21, 24, 25]. The economic costs of composting stalks and wastewater sludge to produce a sanitized organic amendment for application in vineyards are almost negligible compared to those of other management options [26]. However, it is not common for Chilean companies to compost their own organic residues. Considering the high costs of truck transportation and landfill disposal, as well as the social and environmental impacts, the current management of these wastes by external companies is not a viable solution. Furthermore, the use of compost as a soil amendment can reduce fertilization requirements, restore soil and plant heath, and improve public perception of the company; thereby, composting of winery residues is an economically attractive and sustainable alternative for different wine companies in Chile.
Winery sludge is an alkaline and highly humid substrate with a high nitrogen content and a high number of microorganisms [27], including Proteobacteria, Bacteroidetes, Firmicutes, Nitrospira, Dechloromonas, Arcobacter, Nitrobacter, Planctomycetes, Chloroflexi, as well as several microorganisms that participate in the nitrogen cycle via protein and lignin decomposition [28,29,30]. These phylogenetic groups have also been reported in the early stages of composting, suggesting that sludge could be a suitable supplement in the composting of winery residues and could improve the process conditions, increase the abundance of beneficial microorganisms, and reduce sludge management costs. According to Semitela [31], no thermophilic stage was observed during the composting of winery sludge with grape stalks at the laboratory scale and pilot scale; however, no microbiological analyses were performed. In addition, despite the numerous studies related to the microbial communities involved in composting [11, 13, 30, 32,33,34,35,36,37], no studies have focused on the microbiology of winery sludge composting using grape pomace and stalks. Consequently, this work is focused on the dynamics and physicochemical properties of microbiological communities during the composting of residues from the wine industry, as well as the effect of adding winery sludge as a supplement, with the aim of improving the composting process and fostering a circular economy in the winery sector.
2 Materials and methods
2.1 Raw materials
White grape pomace, stalks, and sludge residues were obtained from Concha y Toro Wine Company (K-650, Pencahue, Maule, Chile, − 35.44505214923765, − 71.81094553551853). Wine residues were collected immediately after the winemaking process. Sludge was obtained from the wastewater treatment plant during the wine production season. The raw material was utilized fresh, and no pretreatment methods were applied before composting. Chemical characterization of the raw sludge and wine residues (WRs) was performed as described in Sect. 2.4 (Table 1).
2.2 Composting process
Three composting piles of 41 m3 each were constructed on site at the winery by mixing 65% residual white grape pomace and 35% stalks by volume. Then, sludge from the wastewater treatment facility of the winery was added as a supplement at 0, 10 and 20% by volume to each of the piles, corresponding to three treatments, T0, T1 and T2, respectively. Each treatment pile had a width of 2 m, a height of 1.5 m and a length of approximately 27 m. The composting system was open, and eight mechanical turnings were applied according to the temperature evolution, with more frequent turning during the thermophilic phase. The moisture content of the piles was kept above 40% by using an irrigation system when needed. Samples were collected following an adapted standard version of Sample Collection and Laboratory Preparation: Field Sampling of Compost Materials [38]. As shown in Fig. 1, five subsamples were randomly taken from the top, middle and bottom layers of each pile, resulting in fifteen subsamples for each treatment, at 0, 50 and 195 days, corresponding to the mesophilic, thermophilic, and stabilization stages, respectively. Therefore, a mixed composite of fifteen 100 g subsamples was prepared as a representative sample of approximately 1500 g for each treatment. For each treatment, 3 replicates were obtained for each of the 3 different stages, resulting in a total of 27 samples, which were stored at 4 °C and − 80 °C for chemical analysis and microbiological analysis, respectively.
2.3 Physicochemical analysis
Temperature, moisture, pH, and bulk density were monitored during the whole composting process. The temperature was monitored two times per week utilizing a bimetal thermometer with a length of 107 cm. The moisture content was estimated by drying a sample at 105 °C and calculating the weight difference [39]. The pH was measured in an aqueous extract from a 1/5 mixture of compost and distilled water at ambient temperature [40]. The bulk density was measured by transferring a sample to a graduated beaker to determine the volume and dry mass for calculation of the ratio [41]. The physicochemical properties, such as bulk density, pH, temperature, electrical conductivity (EEc), pH, organic matter (OM), C content, N content, C:N ratio, ammonium and nitrate (NH4 and NO3) contents, NH4:NO3 ratio, P and K, were also analyzed for each of the different windrows at each stage according to the Test Method for the Examination of Composting and Compost [38]. In summary, EEc was measured in an aqueous extract from a 1/5 mixture of compost and distilled water at ambient temperature [42]; OM was calculated from the values for the combusted solid material and the original oven-dried sample; C was calculated based on the OM fraction [43]; N was obtained by using the classical Kjeldahl procedure [42]; NH4 and NO3 were estimated by colorimetric methods [44]; and P and K were determined using inductively coupled plasma (ICP) [45, 46].
2.4 DNA extraction and sequencing
Each of the samples that had been previously obtained from the piles and stored at − 80 °C was homogenized, and 500 mg was resampled for DNA extraction and subsequent sequencing. Extraction was performed following the protocol of the DNeasy Powersoil Pro kit [47]. Sequencing was performed on the Illumina MiSeq platform; the V3–V4 region of the 16S ribosomal gene was sequenced using the primers 16SF CCTACGGGNGGCWGCAG and 16SR GGACTACHVGGGTATCTAATCC, and the internal transcribed spacer (ITS) gene was amplified using the primers ITS1F CTTGGTCATTTAGAGGAAGTAA and ITS2R GCTGCGTTCTTCATCGATGC. Demultiplexed fastq files were first trimmed to eliminate primers. Then, sequences were cleaned, filtered, trimmed, dereplicated, and merged, and chimeras were removed using the DADA2 pipeline in QIIME V.2 software [48]. Amplicon sequence variants (ASVs) were identified and compared to specialized databases for taxonomic assignment. The SILVA SSU 138 16.12.2019 database [49] was used for bacteria, and the UNITE V10.05.202 database [50] was used for fungi. The samples with the fewest sequences were used to define the rarefaction of each data series. The raw data supporting the findings in this study have been deposited into the National Library of Medicine (US), National Center for Biotechnology Information (NCBI) database under Bioproject ID PRJNA1085751, available at https://www.ncbi.nlm.nih.gov/bioproject/.
2.5 Data processing
Multivariate statistical analysis of the physicochemical and taxonomic data was performed by using Primer6 (Primer E) software. The physicochemical results were standardized, and Euclidean distance matrices were created. Variables with a Pearson’s correlation higher than 0.95 were grouped and represented by one unique variable.
The fourth root was applied to the quantitative taxonomic results to homogenize the data and reduce the dominance effect. Bray‒Curtis similarity matrices were subsequently constructed. The results were tested in triplicate and averaged. Alpha and beta diversity analyses were performed to identify beneficial and pathogenic microorganisms using data collected from the references cited in this manuscript.
The physicochemical and microbiological results were tested by using PERMANOVA with 10,000 permutations and α = 0.05. Kruskal‒Wallis statistical analysis was used with α = 0.05 to test for differences in the number of beneficial and pathogenic microorganisms at the final stage depending on treatment. Canonical analysis of main coordinates (CAP) was used to construct a graphical representation of the biological composition of the samples based on environmental data.
3 Results and discussion
3.1 Physicochemical
Figure 2 shows the temperatures of the different treatments, as well as the ambient temperature throughout the process. The days of irrigation and turning are marked. All the treatments reached temperatures greater than 45 °C in the first week of composting. The temperatures during composting did not show any unusual values, and the trends are comparable to those in similar studies on winery residues [21, 24, 51]. The durations of the phases, especially the thermophilic phase, indicate appropriate decomposition and sanitization [8]. An increase in temperature above 75 °C was not observed, but a progressive decrease occurred after the eighth turning of the material. T0 showed a higher temperature than did T2 and T1 after day 161, which indicated greater persistence of microbial activity due to less maturation. However, all the treatments entered the stabilization phase (< 45 °C) after 185 days. Irrigation and turning of windrows are crucial to regulate humidity and oxygenation, which can influence the development of aerobic microorganisms, the main microorganisms involved in the composting process [52].
The windrows were irrigated periodically to maintain a moisture content greater than 40% during at least the first 140 days of the process. The moisture content of each windrow was recorded throughout the test (Fig. 3A). The treatments started with moisture values between 50 and 60% during the first month. Before irrigation (day 42), since T1 and T2 had higher moisture contents, the presence of sludge seemed to improve the water retention of the windrows during the process. During the last month, no irrigation was performed to accelerate stabilization of the final compost product. In other studies, it has been reported that the moisture of piles can influence microbial activity and reduce the abundance of antibiotic resistance genes (ARGs) [53].
The bulk density (Fig. 3B) reflects the physical structure of a windrow and the pore space it contains. As expected, an increase in bulk density was observed for all treatments, where T2 and T1 exhibited higher values throughout the whole process due to the addition of sludge. Although an increased bulk density means better retention of moisture, higher values negatively affect gas exchange inside the windrows, increasing the number of turnings required. The progressive increase in the bulk density of the treatments is considered normal behavior in composting [6, 8, 54]. It has also been reported that the time at which windrows reach the thermophilic phase is influenced by the bulking agent used and the particle size of the mixture, with higher values shortening the time to the thermophilic phase [55].
The initial pH of the treatments was close to 4.5 (Fig. 3C), and a progressive increase was observed for all treatments. T1 and T2 showed a clear of stabilization, reaching pH 7 on day 108; in contrast, T0 stabilized on day 143. This difference can be explained by the alkaline pH of the sludge and the nitrate content, which induced higher production of ammonia during the thermophilic phase, accelerating the increase in pH and the stability of the compost [24, 25, 51]. Bouhia [56] studied the composting process of olive mill waste sludge and green waste and reported similar changes in pH, with values that started at 5.5 and finally reached 7.9 after 120 days. It has been shown that changes in temperature and pH significantly affect decreases in ARG abundance and pathogen–host interactions (PHIs) in compost with chicken manure, indicating the vital role of these variables in the elimination of pathogenic microorganisms [57].
Multivariate analysis revealed significant differences in the physicochemical data depending on the treatment for each of the stages (PERMANOVA, p < 0.05). The addition of sludge significantly affected the physicochemical parameters of the compost in each of the phases. The physicochemical dynamics are described in the following paragraphs.
3.1.1 Mesophilic phase
In the initial mesophilic phase (Table 2), T2 and T1 showed, on average, an electrical conductivity 20% higher than that of T0. The nitrogen content increased by 28% in T2 and 14% in T1 relative to that in T0 due to the higher nitrogen content of the added sludge than the mix of pomace and stalks used for composting. In the study of Bertran [24], a 40% increase in nitrogen content was found when sludge was composted together with stalks, indicating that sludge is an excellent nitrogen source. According to these data, 10% sludge addition would be enough to improve the carbon–nitrogen ratio of the composting material to an optimum level. Thus, T1, with a 10% sludge content, was theoretically the optimal treatment, as shown by its nitrogen content in Table 2. On the other hand, Pinto [51] reported increases in the contents of P and K when using winery sludge. However, this effect was not observed in the treatments in this study. This discrepancy is explained by differences in the composition of the raw composting materials between the two studies: the sludge in this study consisted of solid residues produced by the wastewater treatment plant of a winery, while the raw materials used by Pinto [51] were grape stalks after destemming and grape marc.
3.1.2 Thermophilic phase
T1 and T2 showed, on average, a 20% higher content of nitrogen than T0, which might be related to more complete nitrification [5, 11, 20]. However, less nitrate was observed in the treatments in which sludge was added, but more ammonium was measured in the samples, indicating that fewer nitrate-converting bacteria were present at that time. A 10% lower OM content was observed in T1 and T2 than in T0 (Table 2). This effect may be due to more effective bacterial degradation processes, which were associated with a higher nitrogen content. A decrease in C:N ratio associated with sludge addition in organic residue composting has been previously reported [31, 58]. Specifically, Semitela [31] reported that the C:N ratios of composted substrates decreased significantly in comparison to that of the initial mixture, which consisted of grape stalks and winery waste activated sludge.
The addition of sludge during composting can help produce an environment that facilitates the growth of microorganisms by increasing moisture, nitrate content and the contents of other minerals [5]. Therefore, greater fungal growth was observed in T1 and T2 than in T0 [6, 11]. Furthermore, windrows with sludge showed greater phosphorous mobilization due to the presence of phosphate-solubilizing microorganisms (i.e., Aspergillus and Mycobacterium) [59]. An increase in ammonium content in the samples with sludge was also observed during this phase (Table 2), indicating the enhancement of nitrification processes related to sludge addition and the growth of nitrogen-fixing microorganisms [60].
3.1.3 Stabilization phase
All treatments showed a neutral pH at the stabilization stage (Table 2). EC was 25% higher in T2 than in T0 and T1, which was probably caused by a higher concentration of dissolved salts due to the addition of 20% v/v sludge [5, 11]. The EC of all the final compost samples was below the maximum value of 3 dS/m required for use as a soil amendment [8]. A low final C:N ratio has been reported in different studies on the composting of sludge and pomace stalks, with values in the range of 17 to 20 [55]. The values obtained in this study are consistent with the data published by Carmona [54], with values ranging from 15.5 to 18.2 for the composting of pomace and grape stalks in a 1:1 ratio.
The ammonium and nitrate contents in the final stage were higher in T0 than in T1 and T2, which could be explained by both the enhanced microbial activity of nitrifying microorganisms in previous stages and the major loss of NH3 and nitrate by evaporation when sludge is present [6, 11, 20]. The final phosphorous content was higher in T1 than in T2 and T0 (~ 20% higher), suggesting that 10% is the best proportion of sludge to increase microorganism-mediated phosphorous mobilization [7, 9, 11].
3.2 Dynamics of the microbial communities
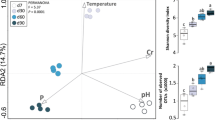
Figure 4 shows the CAP results for the relationship between the composition of the microbial communities (bacteria–fungi) in the different treatments and phases of composting and physicochemical parameters.
Canonic analysis of the main components (CAP). A Bacterial communities during the composting process. B Fungal communities during the composting process. EC electrical conductivity, OC organic carbon, C:N carbon:nitrogen ratio, AN ammonium:nitrate ratio, TN total nitrogen, A ammonium, P phosphorus, K potassium
PERMANOVA revealed significant differences in the bacterial and fungal communities in different stages and different treatments (p < 0.05). Figure 4A shows that the bacterial communities were clearly differentiated in the mesophilic stage, which is mainly attributable to the different sludge contents in the treatments. In the thermophilic stage, lower differentiation was observed among the treatments, which was related to increases in conductivity and NH3+:NO3 ratio. In the stabilization stage, there were fewer differences in bacterial community composition. In this stage, the C:N ratio reached its lowest value due to the degradation of carbon in organic matter, and the phosphorous content increased in all the treatments, especially in T1. Similar to bacteria, the composition of the fungal communities was significantly different in the three stages of the composting process. In the mesophilic stage, the fungal communities were less heterogeneous than the bacterial communities. In the thermophilic stage, the richness of the fungal communities decreased with differences among the treatments, which were related to the greater C:N ratio in T0 and the lower ammonium content than those in the sludge-added treatments. In the stabilization stage, the fungal communities showed substantial differences among treatments, mostly due to the increase in phosphorous and decrease in organic carbon in T1 and T2.
As shown in the CAP results in Fig. 4, the bacterial and fungal dynamics varied considerably during the composting process. While the bacterial community composition was affected by sludge addition and tended to homogenize at the end of the composting process, the fungal community was initially homogeneous and tended to differentiate at the end.
The Shannon‒Wiener index values in Table 3 reveal significant differences between stages and treatments. Bacterial diversity increased over time, while the fungal community appeared to be highly affected by the thermophilic stage, leading to a reduction in diversity that slightly recovered by the end of the process. Furthermore, the sludge exhibited high bacterial diversity but very low fungal diversity. When used as a composting supplement, sludge mainly provides a bacterial community; this community tends to homogenize in the thermophilic stage due to the environmental conditions (i.e., high temperatures) that regulate the growth of the different bacterial groups [8]. This finding is in accordance with Liu et al. [13], who stated that the physicochemical changes during different composting stages had a higher impact on the bacterial community composition than did differences in the composting materials. On the other hand, fungi seemed to be less affected; fungal communities are highly sensitive to environmental conditions, which resulted in noticeable changes in fungal community composition during composting.
The main bacterial phyla present in all stages and treatments were Actinobacteria, Proteobacteria, Firmicutes and Chloroflexi. A similar composition was found by Bouhia [61] when composting olive mill wastewater sludge, except for Chloroflexi. The main fungal phyla found in all stages and treatments were Ascomycota, Rozellomycota and Basidiomycota, which are the same as those reported by Antunes and Tortosa [33, 34]. This shows that the composition of microbial communities involved in composting is independent of the type of initial organic material utilized.
3.2.1 Beneficial microorganisms
Figure 5A, B show the composition of the microbial communities in the stabilization stage as well as the roles of the identified microbes as beneficial or pathogenic microorganisms in vineyards. The principal beneficial fungal genera found in all the samples were Aspergillus and Talaromyces, where Aspergillus was the most abundant genus in all the treatments, especially in T1 and T2, showing the effect of sludge addition. Aspergillus is a common fungal genus found in compost that participates in processes of mineral solubilization, such as phosphorus and potassium solubilization [12]. Furthermore, this genus has been reported to produce secondary metabolites such as gibberellic acid, indoleacetic acid, and siderophores [9]. The genus Talaromyces has been shown to have positive effects on plant growth. It was found to be an endophyte in plants and has been reported to be a phosphate solubilizer and a promising biocontrol agent against phytopathogenic fungi [62]. The beneficial bacterial genera present in the sludge were Streptomyces, Mycobacterium, Mesorhizobium, Gordonia, Rhodococcus, Brevundimonas, and Azospirillum. Representatives of the genus Rhodobacter were also observed in the sludge but were not found during composting, suggesting that the conditions of the composting process were not optimal for their growth. Therefore, the beneficial genera Streptomyces, Mycobacterium, and Mesorhizobium, which are involved in phosphate solubilization, and Brevundimonas, which participates in nitrogen fixation and promotes plant growth [36, 63], were the most abundant in all the treatments.
At the genus level, the second most abundant bacteria in T0 was Streptomyces; however, sludge addition affected the abundance of this genus, which was most abundant in T1 and T2. Mycobacterium and Mesorhizobium were found in all the treatments. These genera are commonly observed in soil and have been extensively studied for their roles in promoting plant growth via several mechanisms, such as by producing siderophores, phytohormones, cellulases, lipases, proteases and chitinases; enhancing nutrient availability; stimulating root growth [59]; fixing nitrogen [64]; solubilizing phosphate; and protecting plants from pathogens [60, 65].
3.2.2 Pathogenic microorganisms
In the final stage, Acetobacter, Penicillium, Cladosporium, and Alternaria were observed. These genera have been reported as possible causes of acid rot in fruit [66, 67]. The genera Agrobacterium and Acremonium can cause crown gall disease and stem decline, respectively [66]. Both Agrobacterium and Acremonium have the most likely negative effects on vines since they can directly affect wood, thus causing ecological and economic issues for vineyards, reducing production and causing plant death [68]. However, their abundance was very low in every treatment, and together they represented less than 1% of the communities.
Acremonium was found only in T1 and T2, although this genus was not present in the sludge. This suggests that adding sludge produced the appropriate conditions for its appearance. The genera Penicillium, Alternaria, Agrobacterium, and Acetobacter were part of the sludge microbiota but were also present in T0 regardless of sludge addition; therefore, their presence was not directly related to the addition of sludge. However, Cladosporium was absent in the sludge, and among the treatments, it was present only in T0. Sludge addition resulted in significant differences between the bacteria in T0 and T1, and the addition of 10% sludge in T1 resulted in a lower number of pathogenic bacteria. This can be explained by the high temperatures (75 °C) reached during the thermophilic phase, which eliminated pathogenic bacteria [8].
4 Conclusions
In this work it was studied the physicochemical and microbiological dynamics and products of a grape pomace composting process involving the addition of industrial waste sludge. This study aimed to evaluate the efficiency of alternative resources in minimizing environmental pollution and fomenting a circular bioeconomy in the wine industry. The addition of sludge to the composting process improved water retention and bulk density with no effect on the macronutrient composition of the final product. The microbial communities exhibited highly dynamic compositions during the different stages of the process. Sludge promoted bacterial diversity at the first stage; furthermore, after the thermophilic stage, communities tended to homogenize, and at the end of the composting process, a high abundance of beneficial microorganisms and low abundance of potentially pathogenic microorganisms were found in all the treatments, independent of the addition of sludge. Considering that it is technically possible to compost residual grape pomace stalks with supplemented sludge, composting winery residues could be an economically attractive and sustainable alternative for wine companies. The next step is to determine the effect of compost on soil quality, water use and plant growth in industrial vineyards while considering the economic and environmental implications of incorporating this technology into the current industry. Finally, this methodology can be applied to future research on the addition of other industrial residues, further contributing to the realization of a circular economy and sustainability.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. If any raw data files are needed in another format, they are available from the corresponding author upon reasonable request.
References
Martínez-Blanco J, Lazcano C, Christensen TH, Muñoz P, Rieradevall J, Møller J, Antón A, Boldrin A, Martínez-Blanco J, Rieradevall J, Lazcano C, Christensen TH, Møller J, Boldrin A, Muñoz P, Antón A, Sustain Dev A. Compost benefits for agriculture evaluated by life cycle assessment. A review. Agron Sustain Dev. 2013;33(4): 721732. https://doi.org/10.1007/s13593-013-0148-7.
Bouhia Y, Hafidi M, Ouhdouch Y, Lyamlouli K. Olive mill waste sludge: from permanent pollution to a highly beneficial organic biofertilizer: a critical review and future perspectives. In: Ecotoxicology and environmental safety, vol. 259. Orlando: Academic Press; 2023. https://doi.org/10.1016/j.ecoenv.2023.114997.
Al-Alawi M, Szegi T, El Fels L, Hafidi M, Simon B, Gulyas M. Green waste composting under GORE(R) cover membrane at industrial scale: physico-chemical properties and spectroscopic assessment. Int J Recycl Org Waste Agric. 2019;8:385–97. https://doi.org/10.1007/s40093-019-00311-w.
Al-Alawi M, El Fels L, Benjreid R, Szegi T, Hafidi M, Simon B, Gulyas M. Evaluation of the performance of encapsulated lifting system composting technology with a GORE(R) cover membrane: physico-chemical properties and spectroscopic analysis. Environ Eng Res. 2020;25(3):299–308. https://doi.org/10.4491/eer.2019.061.
Day M, Shaw K. Chapter 2: Biological, chemical, and physical processes of composting. In: Stoffella PJ, Kahn BA, editors. Compost utilization in horticultural cropping systems, vol. 1. 1st ed. Boca Raton: CRC Press; 2001. p. 1–34. https://doi.org/10.1201/9781420026221.ch2.
Casco JM, Herrero RM. Compostaje. 4th ed. Madrid: Ediciones Mundi-Prensa; 2008.
Bueno P, Díaz J, Cabrera F. Capítulo 4. Factores que afectan al proceso de Compostaje. In: Compostaje. 2012.
Román P, Martínez MM, Pantoja A. Manual Compostaje FAO, 2nd edition. FAO; 2013. http://www.fao.org/documents/card/en/c/0230bef3-2008-49a0-a45e-ff5370895219/.
Sánchez ÓJ, Ospina DA, Montoya S. Compost supplementation with nutrients and microorganisms in composting process. In: Waste management, vol. 69. New York: Pergamon Press; 2017. p. 136–53. https://doi.org/10.1016/j.wasman.2017.08.012.
Scattareggia JP. Aislamiento y selección de Bacterias Solubilizadoras de Fósforo de un suelo cultivado con tomate para industria (Solanum lycopersicum L.). Mendoza: Universidad Nacional de Cuyo; 2016.
Insam H, de Bertoldi M. Chapter 3 Microbiology of the composting process. In: Waste management series, vol. 8. Amsterdam: Elsevier; 2007. p. 25–48.
Beltrán Pineda ME. La solubilización de fosfatos como estrategia microbiana para promover el crecimiento vegetal. Corpoica Cienc Tecnol Agropecu. 2014;15(1):101–13.
Liu X, Rong X, Yang J, Li H, Hu W, Yang Y, Jiang G, Xiao R, Deng X, Xie G, Luo G, Zhang J. Community succession of microbial populations related to C-N–P–S biological transformations regulates product maturity during cow-manure-driven composting. Bioresour Technol. 2023;369(December 2022): 128493. https://doi.org/10.1016/j.biortech.2022.128493.
Chen L, Li W, Zhao Y, Zhou Y, Zhang S, Meng L. Effects of compound bacterial agent on gaseous emissions and compost maturity during sewage sludge composting. J Clean Prod. 2022;366(July): 133015. https://doi.org/10.1016/j.jclepro.2022.133015.
Servicio Agrícola y Ganadero. Compendio de bacterias y hongos de frutales y vides en Chile (Acuña R, editor; First). División Protección Agrícola y Forestal. Subdepartamento de Vigilancia y Control Oficial Agrícola; 2010.
Hepp C, Reyes C, Naguil A, Monsalve M. Siembra de otoño en cereales para pastoreo y conservación de forraje en la zona intermedia de Aysén (Patagonia). Instituto de Investigaciones Agropecuarias; 2021.
Bharathiraja B, Iyyappan J, Jayamuthunagai J, Kumar RP, Sirohi R, Gnansounou E, Pandey A. Critical review on bioconversion of winery residues into value-added products. Ind Crops Prod. 2020;158(January): 112954. https://doi.org/10.1016/j.indcrop.2020.112954.
Burg P, Vítěz T, Turan J, Burgová J. Evaluation of grape pomace composting process. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis. 2014;62(5):875–81. https://doi.org/10.11118/actaun201462050875.
Zacharof M-P. Grape winery waste as feedstock for bioconversions: applying the biorefinery concept. Waste Biomass Valorization. 2017;8:1122. https://doi.org/10.1007/s12649-016-9674-2.
Bustamante MA, Paredes C, Marhuenda-Egea FC, Pérez-Espinosa A, Bernal MP, Moral R. Co-composting of distillery residues with animal manures: carbon and nitrogen transformations in the evaluation of compost stability. Chemosphere. 2008. https://doi.org/10.1016/j.chemosphere.2008.03.030.
Bustamante MA, Paredes C, Morales J, Mayoral AM, Moral R. Study of the composting process of winery and distillery residues using multivariate techniques. Bioresour Technol. 2009;100(20):4766–72. https://doi.org/10.1016/j.biortech.2009.04.033.
Martínez Salgado MM, Ortega Blu R, Janssens M, Fincheira P. Grape pomace compost as a source of organic matter: evolution of quality parameters to evaluate maturity and stability. J Clean Prod. 2019;216:56–63. https://doi.org/10.1016/J.JCLEPRO.2019.01.156.
Abdellah YAY, Shi ZJ, Luo YS, Hou WT, Yang X, Wang RL. Effects of different additives and aerobic composting factors on heavy metal bioavailability reduction and compost parameters: a meta-analysis. In: Environmental pollution, vol. 307. Amsterdam: Elsevier Ltd; 2022. https://doi.org/10.1016/j.envpol.2022.119549.
Bertran E, Sort X, Soliva M, Trillas I. Composting winery waste: sludges and grape stalks. Bioresour Technol. 2004;95(2):203–8. https://doi.org/10.1016/j.biortech.2003.07.012.
Bustamante MA, Paredes C, Moral R, Moreno-Caselles J, Pérez-Murcia MD, Pérez-Espinosa A, Bernal MP. Co-composting of distillery and winery residues with sewage sludge. Water Sci Technol. 2007;56(2):187–92. https://doi.org/10.2166/wst.2007.488.
Ruggieri L, Cadena E, Martínez-Blanco J, Gasol C, Rieradevall J, Gabarrell X, Gea T, Sort X, Sánchez A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J Clean Prod. 2009;17:830–8. https://doi.org/10.1016/j.jclepro.2008.12.005.
Pascual JA, Morales AB, Ayuso LM, Segura P, Ros M. Characterisation of sludge produced by the agri-food industry and recycling options for its agricultural uses in a typical Mediterranean area, the Segura River basin (Spain). Waste Manag. 2018;82:118–28. https://doi.org/10.1016/j.wasman.2018.10.020.
Gao P, Xu W, Sontag P, Li X, Xue G, Liu T, Sun W. Correlating microbial community compositions with environmental factors in activated sludge from four full-scale municipal wastewater treatment plants in Shanghai, China. Appl Microbiol Biotechnol. 2016;100(10):4663–73. https://doi.org/10.1007/s00253-016-7307-0.
Kallistova AI, Pimenov NV, Kozlov MN, Nikolaev IA, Dorofeev AG, Aseeva VG, Grachev VA, Men’ko EV, Berestovskaia II, Nozhevnikova AN, Kevbrina MV. Microbial composition of the activated sludges of the Moscow wastewater treatment plants. Mikrobiologiia. 2014;83(5):615–25.
Neklyudov AD, Fedotov GN, Ivankin A. Intensification of composting processes by aerobic microorganisms: a review. Appl Biochem Microbiol. 2008;44:6–18.
Semitela S, Pirra A, Braga FG. Impact of mesophilic co-composting conditions on the quality of substrates produced from winery waste activated sludge and grape stalks: lab-scale and pilot-scale studies. Bioresour Technol. 2019;289: 121622. https://doi.org/10.1016/j.biortech.2019.121622.
Santos M, Diánez F, del Valle MG, Tello JC. Grape marc compost: microbial studies and suppression of soil-borne mycosis in vegetable seedlings. World J Microbiol Biotechnol. 2008;24:1493–505.
Antunes LP, Martins LF, Pereira RV, Thomas AM, Barbosa D, Lemos LN, Silva GMM, Moura LMS, Epamino GWC, Digiampietri LA, Lombardi KC, Ramos PL, Quaggio RB, De Oliveira JCF, Pascon RC, Da Cruz JB, Da Silva AM, Setubal JC. Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics. Sci Rep. 2016;6(December):1–13. https://doi.org/10.1038/srep38915.
Tortosa G, Castellano-Hinojosa A, Correa-Galeote D, Bedmar EJ. Evolution of bacterial diversity during two-phase olive mill waste (“alperujo”) composting by 16S rRNA gene pyrosequencing. Bioresour Technol. 2017;224:101–11. https://doi.org/10.1016/j.biortech.2016.11.098.
Viel A, Stellin F, Carlot M, Nadai C, Concheri G, Stevanato P, Squartini A, Corich V, Giacomini A. Characteristics of compost obtained from winemaking byproducts. Waste Biomass Valorization. 2018;9(11):2021–9. https://doi.org/10.1007/s12649-017-0160-2.
Sun Y, Men M, Xu B, Meng Q, Bello A, Xu X, Huang X. Assessing key microbial communities determining nitrogen transformation in composting of cow manure using illumina high-throughput sequencing. Waste Manag. 2019;92:59–67. https://doi.org/10.1016/j.wasman.2019.05.007.
Martins GL, de Souza AJ, Mendes LW, Gontijo JB, Rodrigues MM, Coscione AR, Oliveira FC, Regitano JB. Physicochemical and bacterial changes during composting of vegetable and animal-derived agro-industrial wastes. Bioresour Technol. 2023;376(November 2022):1–9. https://doi.org/10.1016/j.biortech.2023.128842.
TMECC. Test methods for the examination of composting and compost, USDA and U.S. Composting Council; 2002.
TMECC Method 03.09. Total solids and moisture. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
TMECC Method 04.11. Electrometric pH determinations for compost. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
TMECC Method 03.03. Bulk density. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
TMECC Method 04.02. Nitrogen. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
TMECC Method 05.07-A. Loss on ignition organic matter method. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
TMECC Method 04.10. Electrometrical conductivity for compost. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
TMECC Method 04.03-A. Total phosphorus. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
TMECC Method 04.04-A. Total potassium. In: Test methods for the examination of composing and compost. New York: The United States Composting Council; 2002.
Qiagen. Qiagen DNeasy PowerSoil Pro Kits. Qiagen. 2022. https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/microbial-dna/dneasy-powersoil-pro-kit.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. https://doi.org/10.1038/s41587-019-0209-9.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41(D1):D590–6. https://doi.org/10.1093/nar/gks1219.
Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019;47(D1):D259–64. https://doi.org/10.1093/nar/gky1022.
Pinto R, Brito LM, Gonçalves F, Mourao I, Torres L, Coutinho J. Recycling wastes from Douro wine industry by composting. Acta Hortic. 2021;1305:285–92. https://doi.org/10.17660/ActaHortic.2021.1305.39.
Azim K, Soudi B, Boukhari S, Perissol C, Roussos S, Thami Alami I. Composting parameters and compost quality: a literature review. Org Agric. 2018;8(2):141–58. https://doi.org/10.1007/s13165-017-0180-z.
Abdellah YAY, Luo YS, Sun SS, Yang X, Ji HY, Wang RL. Phytochemical and underlying mechanism of Mikania micrantha Kunth on antibiotic resistance genes, and pathogenic microbes during chicken manure composting. Bioresour Technol. 2023;367: 128241. https://doi.org/10.1016/j.biortech.2022.128241.
Carmona E, Moreno MT, Avilés M, Ordovás J. Compostaje de residuos de la industria vinícola y su uso como sustrato para el cultivo sin suelo de plantas ornamentales. Span J Agric Res. 2012;10(2):482–91. https://doi.org/10.5424/sjar/2012102-320-11.
Morales AB, Bustamante MA, Marhuenda-Egea FC, Moral R, Ros M, Pascual JA. Agri-food sludge management using different co-composting strategies: study of the added value of the composts obtained. J Clean Prod. 2016;121:186–97. https://doi.org/10.1016/j.jclepro.2016.02.012.
Bouhia Y, Hafidi M, Ouhdouch Y, Zeroual Y, Lyamlouli K. Organo-mineral fertilization based on olive waste sludge compost and various phosphate sources improves phosphorus agronomic efficiency, Zea mays agro-physiological traits, and water availability. Agronomy. 2023. https://doi.org/10.3390/agronomy.
Abdellah YAY, Chen HY, Sun SS, Yang X, Luo YS, Bello A, Mohamed TA, Ren RJ, Li WT, Ahmed RM, Wang RL. Evaluating the impact of the humic acid amendment on antibiotic resistance genes reduction and product quality during swine manure composting. J Environ Chem Eng. 2023;11: 110412. https://doi.org/10.1016/j.jece.2023.110412.
Fernández FJ, Sánchez-Arias V, Rodríguez L, Villaseñor J. Feasibility of composting combinations of sewage sludge, olive mill waste and winery waste in a rotary drum reactor. Waste Manag. 2010;30(10):1948–56. https://doi.org/10.1016/j.wasman.2010.04.007.
Gangwar M, Saini P, Nikhanj P, Kaur S. Plant growth-promoting microbes (PGPM) as potential microbial bio-agents for eco-friendly agriculture. In: Advances in soil microbiology: recent trends and future prospects. Singapore: Springer; 2017. p. 37–55. https://doi.org/10.1007/978-981-10-7380-9_3.
Pinto C, Gomes AC. Vitis vinifera microbiome: from basic research to technological development. Biocontrol. 2016;61(3):243–56. https://doi.org/10.1007/s10526-016-9725-4.
Bouhia Y, Hafidi M, Ouhdouch Y, Soulaimani A, Zeroual Y, Lyamlouli K. Microbial intervention improves pollutant removal and semi-liquid organo-mineral fertilizer production from olive mill wastewater sludge and rock phosphate. J Environ Manag. 2024;354: 120317. https://doi.org/10.1016/j.jenvman.2024.120317.
Kulišová M, Vrublevskaya M, Lovecká P, Vrchotová B, Stránská M, Kolařík M, Kolouchová I. Fungal endophytes of Vitis vinifera—plant growth promotion factors. Agriculture. 2021;11(12):1250. https://doi.org/10.3390/agriculture11121250.
Naqqash T, Imran A, Hameed S, Shahid M, Majeed A, Iqbal J, Hanif MK, Ejaz S, Malik KA. First report of diazotrophic Brevundimonas spp. as growth enhancer and root colonizer of potato. Sci Rep. 2020;10(1):12893. https://doi.org/10.1038/s41598-020-69782-6.
Verma JP, Yadav J, Tiwari KN, Kumar A. Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol Eng. 2013;51:282–6. https://doi.org/10.1016/j.ecoleng.2012.12.022.
Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney RK. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz J Microbiol. 2016;47(1):85–95. https://doi.org/10.1016/j.bjm.2015.11.030.
Acuña R. Compendio De Bacterias Y Hongos De Frutales Y Vides En Chile. 2010.
Cortés A, Oliveira LFS, Ferrari V, Taffarel SR, Feijoo G, Moreira MT. Environmental assessment of viticulture waste valorisation through composting as a biofertilisation strategy for cereal and fruit crops. Environ Pollut. 2020;264:1–8. https://doi.org/10.1016/j.envpol.2020.114794.
Díaz GA, Auger J, Besoain X, Bordeu E, Latorre BA. Prevalence and pathogenicity of fungi associated with grapevine trunk diseases in Chilean vineyards. Ciencia e Investigación Agraria. 2013;40(2):327–39. https://doi.org/10.4067/S0718-16202013000200008.
Funding
This work was supported by the Production Promotion Corporation of the Government of Chile (Corporación de Fomento de la Producción, CORFO) through the “Proyecto CORFO PI3486, Desarrollo y validación de tecnologías para la composta y su efecto sobre el suelo, la viña y el vino”.
Author information
Authors and Affiliations
Contributions
Alex Echeverría-Vega: methodology, investigation, visualization, writing—original draft, writing—review and editing. Almendra Espinoza-Mondaca: methodology, investigation, writing—original draft. Eduardo Arqueros-Sanhueza: conceptualization, methodology, visualization, writing—original draft. Denisse Mellado-Quintanilla: methodology, investigation, visualization, writing—review and editing. Rosa Roa-Roco: conceptualization, methodology, supervision, project administration. Alvaro González: funding acquisition, resources. Rodrigo Morales-Vera: conceptualization, methodology, investigation, visualization, writing—original draft writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Echeverría-Vega, A., Espinoza-Mondaca, A., Arqueros-Sanhueza, E. et al. Management of industrial wine residues: physicochemical, bacterial and fungal dynamics during composting processes. Discov Appl Sci 6, 354 (2024). https://doi.org/10.1007/s42452-024-06047-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06047-1