Abstract
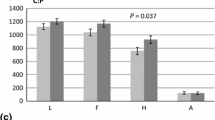
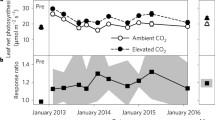
With their dominant share in global plant biomass carbon (C), forests and their responses to atmospheric CO2 enrichment are key to the global C balance. In this free air CO2 enrichment (FACE) study, we assessed respiratory losses from stems and soil, and fine root growth of ca. 110-year-old Picea abies growing in a near-natural forest in NW Switzerland. We anticipated a stimulation of all three variables in response to a ca. 150 ppm higher CO2 concentration in the tree canopies. During the first 2.5 years of the experiment, stem CO2 efflux (R stem) remained unresponsive to CO2 enrichment. This indicates that there is no enhancement of metabolic activity in phloem and xylem of these mature trees. Soil CO2 efflux (R soil) beneath trees experiencing elevated CO2 (eCO2) showed a slight but significant reduction compared to R soil under control trees. High CO2 trees did not increase their fine root biomass in in-growth cores after 20 months under FACE relative to the fine root fractions collected in undisturbed soil. Tree growth (stem radial increment, not shown here) remained completely unchanged although earlier experiments showed largest responses (if any) during the early years after a step increase in atmospheric CO2 concentration. The data presented here suggest C saturation of the study trees at the current close to 400 ppm CO2 ambient concentrations. Together with the high local atmospheric N-deposition rates (ca. 20 kg N ha−1 a−1), our findings imply that factors other that C and N supply appear to constrain growth and metabolism of these mature P. abies trees under eCO2.





Similar content being viewed by others
References
Acosta M, Pokorný R, Janouš D, Marek MV (2010) Stem respiration of Norway spruce trees under elevated CO2 concentration. Biol Plant 54:773–776
Allen AS, Andrews JA, Finzi AC, Matamala R, Richter DD, Schlesinger WH (2000) Effects of free-air CO2 enrichment (FACE) on belowground processes in a Pinus taeda forest. Ecol Appl 10:437–448
Andrews JA, Harrison KG, Matamala R, Schlesinger WH (1999) Separation of root respiration from total soil respiration using carbon-13 labeling during free-air carbon dioxide enrichment (FACE). Soil Sci Soc Am J 63:1429–1435
Bader MKF, Körner C (2010) No overall stimulation of soil respiration under mature deciduous forest trees after 7 years of CO2 enrichment. Glob Change Biol 16:2830–2843
Bader MKF, Hiltbrunner E, Körner C (2009) Fine root responses of mature deciduous forest trees to free air carbon dioxide enrichment (FACE). Funct Ecol 23:913–921
Bader MKF, Siegwolf RTW, Körner C (2010) Sustained enhancement of photosynthesis in mature deciduous forest trees after 8 years of free air CO2 enrichment. Planta 232:1115–1125
Bader MKF, Leuzinger S, Keel SG, Siegwolf RTW, Hagedorn F, Schleppi P, Körner C (2013) Central European hardwood trees in a high-CO2 future: synthesis of an 8-year forest canopy CO2 enrichment project. J Ecol 101:1509–1519
Bernhardt ES, Barber JJ, Pippen JS, Taneva L, Andrews JA, Schlesinger WH (2006) Long-term effects of free air CO2 enrichment (FACE) on soil respiration. Biogeochemistry 77:91–116
Bhupinderpal-Singh NA, Ottosson-Löfvenius M, Högberg MN, Mellander P-E, Högberg P (2003) Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell Environ 26:1287–1296
Blankinship JC, Niklaus PA, Hungate BA (2011) A meta-analysis of responses of soil biota to global change. Oecologia 165:553–565
Bloemen J, McGuire MA, Aubrey DP, Teskey RO, Steppe K (2013) Transport of root-respired CO2 via the transpiration stream affects aboveground carbon assimilation and CO2 efflux in trees. New Phytol 197:555–565
Carey EV, DeLucia EH, Ball JT (1996) Stem maintenance and construction respiration in pinus ponderosa grown in different concentrations of atmospheric CO2. Tree Physiol 16:125–130
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. P Natl Acad Sci USA 104:4990–4995
Comstedt D, Boström B, Marshall JD, Holm A, Slaney M, Linder S, Ekblad A (2006) Effects of elevated atmospheric carbon dioxide and temperature on soil respiration in a boreal forest using δ13C as a labeling tool. Ecosystems 9:1266–1277
Curtis PS, Wang XZ (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Darbah JNT, Kubiske ME, Nelson N, Kets K, Riikonen J, Sober A, Rouse L, Karnosky DF (2010) Will photosynthetic capacity of aspen trees acclimate after long-term exposure to elevated CO2 and O3? Environ Pollut 158:983–991
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Change Biol 4:217–227
Dawes MA, Hagedorn F, Handa IT, Streit K, Ekblad A, Rixen C, Körner C, Hättenschwiler S (2013) An alpine treeline in a carbon dioxide-rich world: synthesis of a nine-year free-air carbon dioxide enrichment study. Oecologia 171:623–637
Day FP, Schroeder RE, Stover DB, Brown ALP, Butnor JR, Dilustro J, Hungate BA, Dijkstra P, Duval BD, Seiler TJ, Drake BG, Hinkle CR (2013) The effects of 11 yr of CO2 enrichment on roots in a Florida scrub-oak ecosystem. New Phytol 200:778–787
De Graaff MA, Van Groenigen KJ, Six J, Hungate B, Van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Change Biol 12:2077–2091
Diaz S, Grime JP, Harris J, McPherson E (1993) Evidence of a feedback mechanism limiting plant response to elevated carbon dioxide. Nature 364:616–617
Dieleman WI, Luyssaert S, Rey A, de Angelis P, Barton CV, Broadmeadow MS, Broadmeadow SB, Chigwerewe KS, Crookshanks M, Dufrêne E, Jarvis PG, Kasurinen A, Kellomäki S, Le Dantec V, Liberloo M, Marek M, Medlyn B, Pokorný R, Scarascia-Mugnozza G, Temperton VM, Tingey D, Urban O, Ceulemans R, Janssens IA (2010) Soil [N] modulates soil C cycling in CO2-fumigated tree stands: a meta-analysis. Plant Cell Environ 33:2001–2011
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Treseder KK, Schlesinger WH, DeLucia EH, Finzi AC (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357
Drigo B, Kowalchuk GA, van Veen JA (2008) Climate change goes underground: effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biol Fert Soils 44:667–679
Edwards NT, Tschaplinski TJ, Norby RJ (2002) Stem respiration increases in CO2-enriched sweetgum trees. New Phytol 155:239–248
Ellsworth DS, Thomas R, Crous KY, Palmroth S, Ward E, Maier C, DeLucia EH, Oren R (2012) Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: a synthesis from Duke FACE. Glob Change Biol 18:223–242
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009) Global patterns in belowground communities. Ecol Lett 12:1238–1249
Franklin O, McMurtrie RE, Iversen CM, Crous KY, Finzi AC, Tissue DT, Ellsworth DS, Oren R, Norby RJ (2009) Forest fine-root production and nitrogen use under elevated CO2: contrasting responses in evergreen and deciduous trees explained by a common principle. Glob Change Biol 15:132–144
Fransson P (2012) Elevated CO2 impacts ectomycorrhiza-mediated forest soil carbon flow: fungal biomass production, respiration and exudation. Fungal Ecol 5:85–98
Garten CT, Iversen CM, Norby RJ (2011) Litterfall N15 abundance indicates declining soil Nitrogen availability in a free-air CO2 enrichment experiment. Ecology 92:133–139
Hagedorn F, Hiltbrunner D, Streit K, Ekblad A, Lindahl BD, Miltner A, Frey B, Handa IT, Hättenschwiler S (2013) Nine years of CO2 enrichment at the alpine treeline stimulates soil respiration but does not alter soil microbial communities. Biogeochemistry 57:390–400
Hamilton JG, DeLucia EH, George K, Naidu SL, Finzi AC, Schlesinger WH (2002) Forest carbon balance under elevated CO2. Oecologia 131:250–260
Handa IT, Hagedorn F, Hättenschwiler S (2008) No stimulation in root production in response to 4 years of in situ CO2 enrichment at the Swiss treeline. Funct Ecol 22:348–358
Hättenschwiler S, Körner C (1998) Biomass allocation and canopy development in spruce model ecosystems under elevated CO2 and increased N deposition. Oecologia 113:104–114
Hättenschwiler S, Miglietta F, Raschi A, Körner C (1997) Thirty years of in situ tree growth under elevated CO2: a model for future forest responses? Glob Change Biol 3:463–471
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Högberg P, Nordgren A, Agren GI (2002) Carbon allocation between tree root growth and root respiration in boreal pine forest. Oecologia 132:579–581
Hungate BA, Dijkstra P, Wu Z, Duval BD, Day FP, Johnson DW, Megonigal JP, Brown AL, Garland JL (2013) Cumulative response of ecosystem carbon and nitrogen stocks to chronic CO2 exposure in a subtropical oak woodland. New Phytol 200:753–766
Inauen N, Körner C, Hiltbrunner E (2012) No growth stimulation by CO2 enrichment in alpine glacier forefield plants. Glob Change Biol 18:985–999
Iversen CM (2010) Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–357
Iversen CM, Hooker TD, Classen AT, Norby RJ (2011) Net mineralization of N at deeper soil depths as a potential mechanism for sustained forest production under elevated CO2. Glob Change Biol 17:1130–1139
Iversen CM, Keller JK, Garten CT, Norby RJ (2012) Soil carbon and nitrogen cycling and storage throughout the soil profile in a sweetgum plantation after 11 years of CO2-enrichment. Glob Change Biol 18:1684–1697
Jackson RB, Cook CW, Pippen JS, Palmer SM (2009) Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 90:3352–3366
Janouš D, Pokorný R, Brossaud J, Marek MV (2000) Long-term effects of elevated CO2 on woody tissues respiration of Norway spruce studied in open-top chambers. Biol Plant 43:41–46
Jastrow JD, Miller RM, Matamala R, Norby RJ, Boutton TW, Rice CW, Owensby CE (2005) Elevated atmospheric carbon dioxide increases soil carbon. Glob Change Biol 11:2057–2064
Jenkinson DS, Fox RH, Rayner JH (1985) Interactions between fertilizer nitrogen and soil nitrogen–the so-called ‘priming’ effect. J Soil Sci 36:425–444
King JS, Hanson PJ, Bernhardt E, De Angelis P, Norby RJ, Pregitzer KS (2004) A multiyear synthesis of soil respiration responses to elevated atmospheric CO2 from four forest FACE experiments. Glob Change Biol 10:1027–1042
Körner C (1994) Biomass fractionation in plants: a reconsideration of definitions based on plant functions. In: Roy J, Garnier E (eds) A whole plant perspective on carbon-nitrogen interactions. SPB Acad Publishing, The Hague, pp 173–185
Körner C (2000) Biosphere responses to CO2 enrichment. Ecol Appl 10:1590–1619
Körner C (2006) Plant CO2 responses: an issue of definition, time and resource supply. New Phytol 172:393–411
Körner C, Asshoff R, Bignucolo O, Hättenschwiler S, Keel SG, Pelaez-Riedl S, Pepin S, Siegwolf RTW, Zotz G (2005) Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science 309:1360–1362
Lebègue C, Laitat È, Perrin D, Pissard G (2004) Mise en œuvre de carottages de sol et de minirhizotrons pour l’étude à long terme de la réponse des fines racines d’épicéa (Picea abies (l.) Karst.) à l’augmentation de la concentration en CO2 dans l’atmosphère et la nutrition minérale. Biotechnol Agron Soc 8:41–53
Leuzinger S, Körner C (2007) Water savings in mature deciduous forest trees under elevated CO2. Glob Change Biol 13:2498–2508
Leuzinger S, Bader MKF (2012) Experimental vs. modeled water use in mature norway spruce (Picea abies) exposed to elevated CO2. Front Plant Sci 3:229
Leuzinger S, Hättenschwiler S (2013) Beyond global change: lessons from 25 years of CO2 research. Oecologia 171:639–651
Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C (2011) Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol Evol 26:236–241
Lloyd J, Taylor JA (1994) On the temperature-dependence of soil respiration. Funct Ecol 8:315–323
Lukac M, Calfapietra C, Godbold DL (2003) Production, turnover and mycorrhizal colonization of root systems of three Populus species grown under elevated CO2 (popFACE). Glob Change Biol 9:838–848
Luo Y, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739
Matamala R, Gonzàlez-Meler MA, Jastrow JD, Norby RJ, Schlesinger WH (2003) Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science 32:1385–1387
Mildner M, Bader MKF, Leuzinger S, Siegwolf RTW, Körner C (2014) Long-term 13C labeling provides evidence for temporal and spatial carbon allocation patterns in mature Picea abies. Oecologia 175:747–762. doi:10.1007/s00442-014-2935-5
Moore DJP, Gonzalez-Meler MA, Taneva L, Pippen JS, Kim HS, DeLucia EH (2008) The effect of carbon dioxide enrichment on apparent stem respiration from Pinus taeda L. is confounded by high levels of soil carbon dioxide. Oecologia 158:1–10
Negisi K (1979) Bark respiration rate in stem segments detached from young Pinus densiflora trees in relation to velocity of artificial sap flow. J Jpn For Sci 61:88–93
Norby RJ, Zak DR (2011) Ecological lessons from free-air CO2 enrichment (FACE) experiments. Annu Rev Ecol Evol Syst 42:181–203
Norby RJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulemans R (1999) Tree responses to rising CO2 in field experiments: implications for the future forest. Plant Cell Environ 22:683–714
Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG (2004) Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. P Natl Acad Sci USA 101:9689–9693
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. P Natl Acad Sci USA 107:19368–19373
Palmroth S, Oren R, McCarthy HR, Johnsen KH, Finzi AC, Butnor JR, Ryan MG, Schlesinger WH (2006) Aboveground sink strength in forests controls the allocation of carbon below ground and its CO2-induced enhancement. Proc Natl Acad Sci USA 103:19362–19367
Pepin S, Körner C (2002) Web-FACE: a new canopy free-air CO2 enrichment system for tall trees in mature forests. Oecologia 133:1–9
Phillips RP, Meier IC, Bernhardt ES, Grandy AS, Wickings K, Finzi AC (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett 15:1042–1049
Pokorný R, Tomášková I, Marek MV (2013) Response of Norway spruce root system to elevated atmospheric CO2 concentration. Acta Physiol Plant 35:1807–1816
Pregitzer KS, Zak DR, Curtis PS, Kubiske ME, Teeri JA, Vogel CS (1995) Atmospheric CO2, soil-nitrogen and turnover of fine roots. New Phytol 129:579–585
Pregitzer KS, Burton AJ, King JS, Zak DR (2008) Soil respiration, root biomass, and root turnover following long-term exposure of northern forests to elevated atmospheric CO2 and tropospheric O3. New Phytol 180:153–161
Pritchard SG, Rogers HH, Davis MA, Van Santen E, Prior SA, Schlesinger WH (2008) Fine root dynamics in a loblolly pine forest are influenced by free-air- CO2-enrichment: a six-year-minirhizotron study. Glob Change Biol 14:588–602
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raich JW, Schlesinger WH (1992) The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44:81–99
Ryan MG, Waring RH (1992) Maintenance respiration and stand development in a subalpine lodgepole pine forest. Ecology 73:2100–2108
Schleppi P, Bucher-Wallin I, Hagedorn F, Körner C (2012) Increased nitrate availability in the soil of a mixed mature temperate forest subjected to elevated CO2 concentration (canopy FACE). Glob Change Biol 18:757–768
Sigurdsson BD, Medhurst JL, Wallin G, Eggertsson O, Linder S (2013) Growth of mature boreal Norway spruce was not affected by elevated CO2 and/or air temperature unless nutrient availability was improved. Tree Physiol 33:1192–1205
Smith AR, Lukac M, Bambrick M, Miglietta F, Godbold DL (2013) Tree species diversity interacts with elevated CO2 to induce a greater root system response. Glob Change Biol 19:217–228
Spinnler D, Egh P, Körner C (2002) Four-year growth dynamics of beech-spruce model ecosystems under CO2 enrichment on two different forest soils. Trees-Struct Funct 16:423–436
Teskey RO, McGuire MA (2002) Carbon dioxide transport in xylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant Cell Environ 25:1571–1577
Tingey DT, Lee EH, Waschmann R, Johnson MG, Rygiewicz PT (2006) Does soil CO2 efflux acclimatize to elevated temperature and CO2 during long-term treatment of douglas-fir seedlings? New Phytol 170:107–118
Waring RH, Schlesinger WH (1985) Forest ecosystems: concepts and management. Academic Press, London
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147:201–222
Zha TS, Kellomäki S, Wang KY, Ryyppö A (2005) Respiratory responses of scots pine stems to 5 years of exposure to elevated CO2 concentration and temperature. Tree Physiol 25:49–56
Acknowledgments
We are particularly indebted to E. Amstutz and G. Grun who kept the free air CO2 enrichment system running. We are also notably obliged to S. Jakob, and several student helpers for their support in data collection and sample processing. We thank T. Klein for providing additional fine root biomass and preliminary stem basal increment data that supported our argumentation. Thanks to T. Baisden and the invaluable comments from three anonymous reviewers that greatly helped in improving earlier drafts. Funding came from the Swiss National Science Foundation (Grant Nos 31003AB-126028 and 31003A_140753, 31-67775.02, 3100-059769.99, 3100-067775.02, and 3100AO-111914/1). The crane was sponsored by the Swiss Federal Office of the Environment (FOEN).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: W. Troy Baisden.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10533_2015_84_MOESM1_ESM.pdf
Fig. S1 Raw data of soil respiration (R soil) and soil temperature at 10 cm depth of mature Picea abies exposed to ambient, or elevated atmospheric CO2 concentrations in 2008, 2009, 2010, and 2011 (ambient CO2: n = 5 trees; eCO2: n = 5 trees; mean ± SE). Soil temperature under elevated and ambient CO2 did not differ (n.s.). Therefore, the mean of all trees is plotted (n = 10). The grey-shaded areas on top of the panels denote the FACE periods
10533_2015_84_MOESM2_ESM.pdf
Fig. S2 Raw data of stem respiration (R stem) and bark surface temperature of mature Picea abies exposed to ambient, or elevated atmospheric CO2 concentrations in 2009, 2010, and 2011 (ambient CO2: n = 5 trees; eCO2: n = 5 trees; mean ± SE). Bark surface temperature under elevated and ambient CO2 did not differ (n.s.). Therefore, the mean of all trees is plotted (n = 10). The grey-shaded areas on top of the panels denote the FACE periods
10533_2015_84_MOESM3_ESM.pdf
Fig. S3 Picea abies stem respiration (R stem) response to bark surface temperature (upper panels), and soil respiration (R soil) response to soil temperature 10 cm below ground (lower panels) during the FACE periods of the years 2009, 2010, and 2011. Here, we show raw data of R stem and R soil (i.e. uncorrected for the pre-treatment difference observed between control and treated trees). The inset diagrams in 2009 depict the pre-treatment uncorrected R stem (upper inset) and R soil (lower inset) response in the period before the initiation of FACE in 2009. All respiration measurements were fitted with Lloyd and Taylor (1994) functions. Trees were exposed to ambient (open symbols, dashed line), or elevated atmospheric CO2 concentrations (filled symbols, solid line). Each symbol represents the mean R stem or R soil rates measured per tree (n = 2–4) and measurement campaign. The Q 10 values indicate the mean increase in the R stem or R soil rate per 10 °C temperature increase (from 5 °C to 15 °C)
10533_2015_84_MOESM4_ESM.pdf
Fig. S4 Tree distribution map at the SCC site near Basel, Switzerland. Outside the perimeter of the crane’s jib, only the Picea abies control trees are depicted, not the surrounding trees
10533_2015_84_MOESM5_ESM.pdf
Fig. S5 Photosynthetic enhancement ratio of one-year old (2008) and current-year (2009) needles prior to the start of CO2 enrichment (pre-treatment, left panel) and 4 weeks after FACE initiation (FACE, right panel). White bars indicate control trees, grey bars indicate trees selected for CO2 enrichment and black bars denote trees receiving CO2 enrichment. Mean ± SE (n = 5 per group/treatment)
Rights and permissions
About this article
Cite this article
Mildner, M., Bader, M.KF., Baumann, C. et al. Respiratory fluxes and fine root responses in mature Picea abies trees exposed to elevated atmospheric CO2 concentrations. Biogeochemistry 124, 95–111 (2015). https://doi.org/10.1007/s10533-015-0084-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0084-5