Abstract
Anthropogenic nutrient enrichment is one of the key global change pressures threatening the health of estuaries. This is evident at the persistently eutrophic Swartkops Estuary located along the warm temperate coast of South Africa. Augmented nutrient-rich baseflows (e.g., stormwater runoff, wastewater treatment work discharges) have resulted in the persistent growth of invasive alien aquatic plants (IAAPs), particularly water hyacinth (Pontederia crassipes), in the upper estuarine reaches. As such, the objective of this study was to investigate the temporal population dynamics of water hyacinth in the Swartkops Estuary to inform management options. Methods included five-weekly sampling campaigns in winter, spring, and summer, interspersed with monthly sampling over a year-long period. Physico-chemical variables and river inflow were measured in situ, while samples were collected for inorganic nutrients, phytoplankton biomass, and IAAP measurements (cover, biomass, and tissue nutrients). Model results indicated that increased water temperature and inorganic nitrogen (ammonium and NOx) levels promoted increased coverage of water hyacinth, while seasonal analysis highlighted reduced (p < 0.05) dissolved oxygen levels during the peak summer IAAP accumulation period. Notably, model results indicated declining IAAP tissue TN and TP concentrations with increasing water temperature, yet overall TN and TP storage was highest in summer due to the extensive water hyacinth cover observed during this period. Overall, the proportionally low and transient nature of nutrient storage by water hyacinth populations, together with the detrimental consequences they facilitate, highlights the need to integrate short-term control measures with catchment-scale management interventions geared towards mitigating the causative drivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal environments are vulnerable to anthropogenic activities that influence nutrient loading and alter hydrological conditions. These activities typically include inputs from agricultural practices, wastewater treatment facilities, and stormwater runoff. The ever-increasing growth of the human population, particularly in coastal watersheds, has necessitated increased development pressures that enhance anthropogenic nutrient loading and, in turn, threaten the functionality of estuarine ecosystems (Freeman et al. 2019). In such instances, estuaries become prone to eutrophication, a process whereby undesirable disturbances develop in response to a surplus of nutrients. When eutrophic conditions preside, the ecological and socio-economic value of estuaries are threatened by oxygen depletion, reduced biodiversity, habitat degradation, altered trophic pathways, and invasion by alien species (Bowen and Valiela 2001; Geletu 2023). Of these, the effects of invasive alien aquatic plant (IAAP) accumulations on estuaries are largely understudied in the literature, likely due to these communities typically favouring freshwater conditions. More specifically, water hyacinth (Pontederia crassipes Mart. formerly known as Eichhornia crassipes) is typically at the forefront of IAAP infestations in freshwater systems, globally (Lowe et al. 2000), yet the role that this species plays in shaping estuarine water quality is largely unknown. The growth and reproduction of water hyacinth is mainly determined by available nutrients and temperature, while establishment in coastal areas and estuaries is limited by salinity constraints, with upper salinity tolerance levels of approximately 5 (De Casabianca 1995; Mangas-Ramírez and Elías-Gutiérrez 2004). The widespread distribution of the plant is attributed to its high tolerance range to environmental conditions such as temperature, pH, and nutrient levels (Wilson et al. 2005; Coetzee and Hill 2012). Ideal environmental conditions for water hyacinth include water temperatures between 10 and 40 °C (Wilson et al. 2005), oligohaline conditions (salinity < 5; De Casabianca 1995), and pH between 6 and 8 (Gopal 1987).
In most instances, the rapid growth of IAAPs in their introduced range is attributed to a lack of natural competitors (Simberloff et al. 2013) and the possession of numerous highly competitive traits that allow them to proliferate and outcompete native plant species (Hill 2003; Hill et al. 2020). The ability of water hyacinth to dominate water columns stems from its buoyancy (i.e., maximise light attenuation) (Hussner et al. 2017) and ability to reproduce both vegetatively and sexually (Hill et al. 2020), thus enabling rapid growth (Hill et al. 2020; Djihouessi et al. 2023). Additionally, a single plant is capable of producing vast numbers of seeds that can remain viable for up to 20 years in the sediment (Djihouessi et al. 2023). When invasive species dominate their introduced habitat range, they can cause negative effects on the ecosystem by outcompeting the native plant species and changing the biotic and abiotic conditions in the affected ecosystem (Richardson et al. 2000), thus resulting in loss of biodiversity and ecosystem functionality (Tobias et al. 2019).
Microtidal estuaries, such as those in South Africa, are particularly susceptible to IAAP accumulations as they have longer retention times that can exacerbate the potential impacts of these communities. The rate of IAAP infestations is increasing in South African estuaries due to the cumulative effects of severe nutrient pollution, a lack of natural competitors, and altered freshwater inflow conditions (Adams et al. 2020; Nunes et al. 2020; Van Niekerk et al. 2022). The most damaging IAAP species occurring in South African estuaries include P. crassipes, Azolla filiculoides (water fern), Myriophyllum aquaticum (parrots feather), Pistia stratiotes (water cabbage), and Salvinia molesta (Kariba weed). A total of 36 estuaries (12%) have been reported to support IAAPs countrywide (Adams et al. 2020). Found in all estuary types, IAAPs are particularly common along the subtropical east coast of South Africa due to substantial wastewater inputs and the predominance of freshwater conditions (Van Niekerk et al. 2019, 2020), all of which favour IAAP growth. Despite possessing numerous ecophysiological advantages that facilitate rapid growth rates and high degrees of phenotypic plasticity, the distribution of IAAPs is limited in estuaries due to their relatively narrow salinity tolerance range, i.e., largely confined to oligohaline (0–5) conditions. As such, IAAPs typically colonise calm waters in the upper reaches of predominantly open estuaries (mouth inlet open to the sea more than 90% of the time), while potentially spreading throughout temporarily closed estuaries (also referred to as intermittently closed estuaries in other parts of the world) when conditions are fresh during closed mouth phases (Van Niekerk et al. 2020). The occurrence of IAAP species in estuaries is a concern given that dense accumulations of these communities have been shown to enhance evaporative losses, disrupt stream flow, reduce dissolved oxygen levels, and decrease light penetration throughout the water column. These conditions culminate in an environment that reduces pelagic productivity (i.e., phyto- and zooplankton) and submerged macrophyte cover, while simultaneously impacting fish movement patterns, recreational activities (e.g., boating), and property values. The socio-economic impact of these accumulations is highlighted by the US$ 3 million spent by the National Department of Forestry, Fisheries, and the Environment on herbicide control of P. crassipes (water hyacinth) in South Africa between 2010 and 2018 (Hill et al. 2020). According to Strange et al. (2019), the main objective of IAAP mitigation is not only to control the infestations but should instead address the anthropogenic pressures that drive their presence. This is because only removing floating macrophytes will result in an open niche that will likely be filled with a new set of invasive species resulting in a shift between steady states of invasions.
The Swartkops Estuary is a largely modified, predominantly open system (Van Niekerk et al. 2020) that flows through highly industrialised and urbanised areas in the Nelson Mandela Bay Metropole (33.8604° S, 25.6221° E) in South Africa. The water quality in the Swartkops Estuary has measurably changed due to land-use activities (Adams et al. 2019; Lemley et al. 2017, 2023). The natural hydrology of the Swartkops Estuary is modified by an upstream dam impoundment and continuous nutrient-rich discharges from three upstream wastewater treatment works (WWTWs) (Lemley et al. 2023), all of which have reduced the propensity for natural flushing events and facilitated stable hydrological conditions in the upper estuary. These conditions, together with the nutrient-rich nature of WWTW inflows, have led to the persistent growth of IAAPs, particularly water hyacinth (P. crassipes) (Van Niekerk et al. 2019; Adams et al. 2020). The plant is largely confined to the upper reaches of the estuary where salinity levels only occasionally become unfavourable for IAAP growth during spring high tides. In South Africa, water hyacinth is regarded as a Category 1 prohibited aquatic weed under the Conservation of Agricultural Resources Act 1983 (Van Niekerk et al. 2019; Hill et al. 2020). Control of IAAPs in South Africa became a focus of the Working for Water Programme in October 1995, which is geared towards water resource protection and job creation (Marais et al. 2004). In the Swartkops, the methods of control used were mainly chemical and biological, however, budget constraints have prevented the implementation of control measures since 2020 (A Heunis pers. Comm). As such, the aim of this study was to investigate the drivers of water hyacinth population dynamics in the upper Swartkops Estuary and propose solutions to improve the quality of the water entering the estuary. The main objective of this study was to identify temporal patterns of water hyacinth response variables, as well as to determine the effects of these populations on water quality. There is a need to find solutions to mitigate the effects of increased nutrient inputs to the Swartkops Estuary, as it is nationally one of the most important estuaries for maintaining biodiversity. Understanding the threats to the estuary will allow for proper monitoring and management to avoid further water quality deterioration. Such information is pertinent globally given the continued deterioration of estuarine health in the face of global change pressures (e.g., nutrient enrichment, warming) and will contribute to our understanding of how best to manage eutrophic estuaries prone to IAAP infestations.
Method and materials
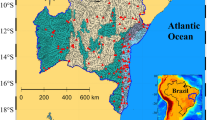
Field work for the study was conducted at the upper Swartkops Estuary with most of the measurements taking place at the upper tidal limit (Perseverance, i.e., 33° 48′ 39.2′′ S; 25° 31′ 48.5′′ E). The estuary is fed with freshwater inflow from the 155 km long Swartkops River. The river drains a 1354 km2 catchment area which includes agricultural lands, industries, and residential urban areas (formal and informal settlements). These activities have contributed to the deteriorating water quality in the Swartkops Estuary catchment due to the release of industrial waste, untreated wastewater, and domestic effluents (Adams et al. 2019; Lemley et al. 2023). The river itself is prone to invasion by floating IAAPs which reach the estuary as they are transported downstream during high flow. The study was conducted at the upper tidal limit (i.e., Fig. 1) on a weekly basis for five weeks at the beginning of each season (winter, spring, and summer). Thereafter, monthly sampling was conducted between each season and in autumn to determine temporal variation in IAAP cover and water column nutrient dynamics.

(Adapted from Taljaard et al. 2022)
Map of the Swartkops Estuary showing the location of the delineated reaches (A—lower, B—middle, and C—upper) as well as the location of the three WWTWs, sampling site at Perseverance and downstream point sources (i.e., MWC—Motherwell Canal; MMC—Markman Canal; CR—Chatty River)
Rainfall and flow
Rainfall data for the period of June 2021 to May 2022 for the Uitenhage catchment was provided by the South African Weather Service (https://www.weathersa.co.za/). This was averaged for monthly rainfall and used with flow data to investigate IAAP cover dynamics in the estuary. Stream depth measurements were taken on each sampling day by stretching a tape measure across the width of the river and using a metre staff to record the depth from the riverbed to the surface of the water at 5 m intervals. The float method, channel width, and water depth measurements were used to calculate the streamflow volumetric rate (m3 s−1). The float method used a neutrally buoyant item to calculate the time it took to travel over a given distance (m s−1). Flow velocity and volumetric flow rate were calculated using the following equation:
Physico-chemical variables
Physico-chemical variables were measured in situ using a YSI ProDSS multiparameter meter on each sampling occasion at the surface and bottom of the water column. Measured parameters included pH, dissolved oxygen (DO) (mg L−1), salinity, and temperature (°C). Additionally, 1 L water samples were also collected at the study site on each sampling occasion for further analysis of phytoplankton and inorganic nutrients in the laboratory.
Water nutrient analysis
The collected water was filtered in the laboratory using Ahlstrom Munksjӧ MGC glass-fibre filters (1.2 μm pore size, 47 mm diameter). The filters were frozen for analysis of phytoplankton biomass, while the filtrates were analysed using the SEAL AutoAnalyzer 3 HR (SEAL Analytical, Inc.) operated by the South African Environmental Observation Network (SAEON) Elwandle Node. Inorganic nutrient concentrations for orthophosphate (PO43−), ammonium (NH4+), total oxidised nitrogen (NOx, i.e., NO3− + NO2−), and silicate (SiO2) were analysed (Murphy and Riley 1962; Grasshoff et al. 1999).
Phytoplankton biomass
The frozen filter papers were thawed and used for chlorophyll-a (chl-a) analysis by placing the filters into glass vials. Thereafter, 10 ml of 95% ethanol was added to each vial for chl-a extraction (Merck 4114). The samples were then placed in a cold, dark room for 24 h. Thereafter, the extract was filtered, and spectrophotometric analyses were performed following the method described by Nusch (1980). Absorbance was read at a wavelength of 665 nm, before and after acidification with 1 N HCl.
IAAP cover and tissue nutrients
To estimate the IAAP cover at the study sites, aerial footage was used, taken on each sampling occasion using a drone (unmanned aerial vehicle (UAV)), approximately 500 m upstream from the sampling station and 500 m downstream. The footage was then used to map the water surface area (17.75 ha) and the extent of IAAP cover using ArcGIS Pro 2.9.1 software. At the sampling station, IAAP species composition was assessed by collecting IAAPs present using a 0.16 m2 quadrat. This was replicated five times on each sampling occasion. The IAAP species present at the upper Swartkops Estuary (Perseverance) was water hyacinth. The plants were then separated into leaves, stems/bulbs, and roots/rhizomes in the laboratory. The fresh weight of sorted plant parts from each replicate, were recorded. These were then oven-dried at 65 °C for 72 h, or until dry, after which the dry biomass was weighed and recorded (Xie and Yu 2003). The dry biomass (g/m2) was calculated for each plant part using the following formula:
where Area of quadrat = 0.16 m2
Subsequently, whole plant biomass was calculated by summing the biomass values of leaves, stems, and roots for each replicate and then calculating the mean from the five replicates. The sorted and dried plant material was then finely grounded into powder to analyse total nitrogen (TN) and total phosphorus (TP) using the simultaneous oxidation of nitrogen and phosphorus compounds with the persulphate method described by Koroleff (1999) consisting of sample digestion and spectrophotometer analysis. Using the dry biomass measurements and the nutrient concentration readings from the spectrophotometer, the amount of nutrients accumulated by the plants per hectare (kg/ha) was determined. Thereafter, total nutrient storage on each sampling occasion was quantified by scaling up the nutrient results using the measured IAAP cover data in relation to the surface water.
Data analysis
All plots and analyses were conducted in the R environment (RStudio version 4.3.2, R Core Team 2024). The Shapiro-Wilks test was used to test for normality of the study parameters. Seasonal differences in the mean concentrations of each variable were analysed using either the one-way ANOVA (parametric) or the Kruskal–Wallis (non-parametric) test. The post hoc Tukey (parametric) or Bonferroni Dunn (non-parametric) test was then used to compare data between specific seasons. The relationship between environmental parameters and selected water hyacinth response variables (i.e., cover, biomass, TN, and TP) were assessed using multivariate generalised linear modelling (GLM; ‘manyglm’, ‘negative binomial’ distribution: Wang et al. 2012). Temperature, salinity, dissolved oxygen, pH, SRP, NOx, ammonium, flow rate, phytoplankton biomass, and the DIN:DIP ratio were specified as possible predictors. Predictor importance was assessed using the ‘drop1’ function. Predictor variables were subsequentially excluded, and models compared in terms of Akaike Information Criterion (AIC) scores until the AIC was not improved (with ΔAIC ≤ 2: Zuur et al. 2009). The “anova” function (nBoot = 1000; p.uni = “adjusted”) was used to assess the explained deviance (D) of each predictor included in the parsimonious model (Wang et al. 2012). All of the analysed data were tested at an α priori significance level of α < 0.05 significance level.
Results
Rainfall and flow
The rainfall distribution pattern showed that most rainfall recorded during the study period occurred in austral spring and summer (Fig. 2). Winter months recorded the lowest rainfall measurements, with the minimum total rainfall recorded in June (2.6 mm) and maximum rainfall occurring in December (57.8 mm). Flow dynamics followed the same pattern as rainfall in that it increased from winter to spring/summer (Fig. 3). The lowest flow rate was measured in late June 2021 (0.31 m3 s−1), whereas the highest flow rate was recorded in mid-December (3.45 m3 s−1). A reduction in flow rate was observed during the January 2022 sampling period, followed by increased flow between February and March 2022.
Physico-chemical variables
Surface water temperature (Fig. 4A) displayed typical seasonal shifts relecting ambient climatic conditions. Thus, water temperature displayed significant seasonal changes (X2 = 17.42; df = 3), with summer conditions being markedly (p < 0.001) warmer than in winter. Winter surface temperatures ranged between 13.5 and 16.3 °C, while summer water temperatures ranged from 22.9 to 26.9 °C. Salinity levels ranged between 1 and 3 throughout the study period (Fig. 4B). However, reduced salinity was observed in summer compared to all other seasons (p < 0.01; X2 = 11.83; df = 3). A peak in salinity levels (4.8 ± 3.0) was recorded in early autumn (07/03/2022), which was above the salinity levels generally recorded at the site. The high salinity levels in the upper reaches of the estuary are attributed to tidal action downstream, usually a spring tide that pushes the relatively saline estuarine water from the middle reaches to the upper reaches. Dissolved oxygen (DO) concentrations (Fig. 4C) were highest between June and September 2021, with the peak concentration recorded during the last week of September (27/09/2021). Hypoxic conditions (DO < 2 mg/L) were primarily observed in late spring and early summer. DO concentrations displayed notable seasonal variability (X2 = 9.86; df = 3), with substantially higher concentrations recorded in spring and compared to summer (p < 0.05). The highest concentration of dissolved oxygen (DO) was 6.0 mg/L, while the lowest was 1.0 mg/L. The recorded mean pH levels (Fig. 4D) were notably higher in autumn and lower in summer (p < 0.01). Moreover, pH levels ranged from 7.0 to 8.1 throughout the study period and displayed notable seasonal shifts (F = 5.99; df = 3), whereby summer values were lower than those recorded in autumn (p < 0.01) and winter (p < 0.05).
Water column nutrients
Soluble reactive phosphorus (SRP) concentrations (Fig. 5A) ranged from 1.6 ± 0.09 mg/L (winter) to 5.4 mg/L ± 0.02 mg/L (autumn), with significantly higher levels recorded in autumn compared to the other seasons (p < 0.05; X2 = 7.63; df = 3). Similarly, ammonium (NH4+) concentrations (Fig. 5B) were higher (X2 = 9.75; df = 3) during autumn compared to spring and winter (both p < 0.05). During winter, NH4+ concentrations decreased weekly from the first to the fifth week. Conversely, spring and summer weekly sampling showed a weekly increasing trend in NH4+ concentrations. The highest concentration of NH4+ was measured in mid-autumn (6.42 ± 0.05 mg/L) and the lowest concentration was measured in early spring (0.06 ± 0.004 mg/L). Total oxidised nitrogen (NOx) (Fig. 5C) ranged between 0.08 and 2.12 mg/L, yet seasonal differences were absent (p > 0.05). Similarly, DIN:DIP ratios (Fig. 5D) remained low (< 1:1) throughout the study period and were comparable between seasons (p > 0.05).
IAAP cover
Water hyacinth cover (Figs. 6 and 7) was concentrated above the sampling station on the first day of sampling in winter (02/06/2021), covering 1.31 ha (7.5%) of the waterbody. Similarly, plant cover was also concentrated in the upper part of the sampling station at the beginning of the second seasonal sampling occasion in spring (06/09/2021), covering 1.54 ha (8.8%) of the waterbody. During the third seasonal weekly sampling programme in summer, water hyacinth cover increased notably both above and below the sampling station. The upper part of the waterbody was completely covered in water hyacinth with the plant covering up to 6.21 ha (35%) of the waterbody. The last day of the sampling period in autumn (10/05/2022) indicated a decrease in IAAP cover at the upper and lower reaches of the Perseverance waterbody (4.44 ha; 25%). Overall, the lowest IAAP cover (1.18 ha; 6.7%) was measured in winter (16/06/2021), while the highest coverage (6.84 ha; 39%) was recorded in summer (25/01/2022).
Seasonal analysis of IAAP cover (Fig. 8) indicated substantially higher (X2 = 17.21; df = 3) water hyacinth coverage in summer (p < 0.001) and autumn (p < 0.05) compared to winter. Additionally, the summer period was characterised by elevated IAAP cover compared to that observed during spring (p < 0.05). Thus, cover increased with season from winter to summer and then subsequently decreased during the autumn months toward winter (Fig. 8).
Phytoplankton biomass
Overall, phytoplankton biomass (Fig. 9) displayed marginally significant seasonal variability (p = 0.05; X2 = 7.89; df = 3), with the spring season displaying notably higher concentrations than those recorded in autumn and winter. Phytoplankton biomass ranged from 6.7 ± 1.8 µg/L (late autumn) to 410.1 ± 20.2 µg/L (mid-spring) throughout the study period. There was a general pattern of an increase in biomass between winter and spring followed by a decrease recorded between summer and autumn.
Plant tissue nutrients
The difference in total phosphorus (TP) and total nitrogen (TN) plant nutrient concentration, per hectare, was observable (Fig. 10), with TN concentrations typically being higher than TP throughout the study period. TN concentrations ranged between 4 and 35 kg/ha, whereas TP concentrations ranged between 4 and 15 kg/ha. A marked seasonal trend in plant TN concentrations was observed (F = 7.18; df = 3), with higher concentrations observed in winter compared to summer (p < 0.01) and autumn (p < 0.05). Plant TP concentrations remained relatively low and did not show considerable changes with season (p > 0.05). The dry biomass of water hyacinth ranged between 136.9 and 711.4 g m−2 (Fig. 10) and varied with season (F = 4.49; df; 3), peaking in autumn and being lowest in spring and summer (both p < 0.05).
For the different plant parts, increased storage was observed within the stems of the water hyacinth. While that was the case, storage within the leaves and roots seemed to interchange between winter and summer seasons. For TP (Fig. 11A), the roots stored more of this nutrient in winter than in the leaves, whereas in summer, the leaves stored relatively more TP than the roots. Overall nutrient accumulation at the site indicated low TP storage during the winter sampling occasions, while storage in summer was relatively higher. TP storage was as low as 10.2 kg in winter and as high as 65.4 kg in summer. Conversely, TN storage was also predominantly in the stems (Fig. 11B). However, unlike TP, the leaves stored more TN than the roots in summer and in winter, both the roots and leaves stored relatively similar concentrations of TN. TN storage was also greater in summer than in winter and spring months. The highest TN storage was 70.8 kg and the lowest was 15.5 kg.
Of the environmental predictors considered for the most parsimonious model for the drivers of IAAP response variables, salinity, pH, dissolved oxygen, flow, phytoplankton biomass, SRP, and DIN:DIP were omitted through stepwise AIC comparison. In the most parsimonious model, water temperature (53.2% D; p < 0.001), ammonium (20.5% D; p < 0.01), and NOx (9.7% D; p < 0.05) all demonstrated significant overall interactions with the IAAP response variables (Table 1). Results for individual response variables, nested within the overall multivariate model, demonstrated that increased water temperature drives increased IAAP cover (33.9% D, p < 0.001) and lower tissue TN concentrations (18.1% D, p < 0.001). Similar, albeit less pronounced, inverse relationships (p ≤ 0.1) with temperature were observed for IAAP biomass and TP concentrations. Increased water column concentrations of ammonium (17.6% D, p < 0.001) and NOx (8.9% D, p < 0.05) were also shown to facilitate the increased coverage of water hyacinth populations. Similar to its interaction with temperature, tissue TN concentrations displayed a marginally inverse relationship (2.9% D, p ≤ 0.1) with ammonium levels. Notably, IAAP biomass and TP concentrations were not influenced by the availability of dissolved inorganic nitrogen (all p > 0.6).
Discussion
Abiotic conditions that characterised the upper reaches of the Swartkops Estuary were suitable for the growth and invasion of Pontederia crassipes (water hyacinth). Temporal variability of pH (7–8) and temperature (13–26 °C) were within the optimal condition ranges for water hyacinth growth (Gopal 1987; Wilson et al. 2000; Mironga et al. 2011). Salinity levels at the upper tidal limit reflected persistent oligohaline conditions (0.5–5) and, thus, were typically below the maximum salinity tolerance level of 5. Salinity is the major limiting variable for the growth of aquatic macrophytes, and IAAPs are unable to survive in high salinity environments (De Casabianca 1995; Henderson 2020). Temperature was the main variable influencing plant growth and coverage, while dissolved inorganic nitrogen (ammonium and NOx) and dissolved oxygen were the only abiotic variables influenced by water hyacinth dynamics.
Temperature is an important factor influencing plant growth as it influences the extensibility of the cell wall, osmotic potential, conductivity, and turgor (Shu et al. 2014). Low temperatures reduce plant growth, whereas higher temperatures accelerate plant growth (Hatfield and Prueger 2015). Wilson et al. (2000) assumed a logistic growth model for water hyacinth population dynamics in temperate regions. Macrophytes in temperate rivers follow a seasonal growth pattern, with high growth rates in spring and early summer, resulting in high biomass accumulation during mid and late summer (Cavalli et al. 2016), followed by a biomass die-back in autumn and winter (Riis et al. 2020). Water hyacinth population dynamics are temperature dependent, and their growth is determined by warm temperatures and lack of frost in the winter months (Nikkel et al. 2023). Although temperature was determined to be the most important abiotic factor influencing water hyacinth response variables, there are other variables that were not measured in this study which may have played a role. For instance, light intensity is an important environmental factor affecting the growth of water hyacinth (Shu et al. 2014; Driesen et al. 2020). Both light and temperature influence essential metabolic activities (e.g. photosynthesis, transpiration, and carbohydrate metabolism) (Monneveux et al. 2003; López-Pozo et al. 2023). That said, the influence of water temperature was observable in this study when water hyacinth increased in spring and had maximum coverage during mid to late summer, followed by a decrease in cover from autumn as temperatures started to decrease. However, plant biomass was higher in winter than in summer and displayed an inverse relationship with water temperature (Table 1). This can be explained by the differences in plant morphology in relation to season. The leaves, stems, and roots of the water hyacinth were smaller in comparison to the larger leaves and elongated stems and roots in summer months. This resulted in generally stunted plants allowing for more dense accumulations in winter, whereas more elongated and larger plants resulted in less dense, but more spatially extensive accumulations in summer.
Decreased dissolved oxygen levels in the water column with increased water hyacinth cover was also evident. This is aligned with other studies (i.e., Rommens et al. 2003; Mangas-Ramirez and Elias-Gutierrez 2004; Wang et al. 2013) that have shown reduced oxygen levels with increased water hyacinth cover. Hypoxic conditions occurred when water hyacinth covered over 20% of the surface water area. Such reductions in dissolved oxygen affect biodiversity in the water by reducing phytoplankton production, which, in turn, decreases the abundance and diversity of zooplankton, invertebrates, and fish populations (Wang et al. 2013). This pattern was observed in this study by spring phytoplankton bloom concentrations (> 100 µg Chl-a L−1) being displaced during periods of extensive IAAP coverage and low oxygen levels in summer and autumn, despite otherwise suitable conditions (e.g., nutrient-rich, warm). The dense mats created by water hyacinth block sunlight from penetrating the water column, reducing oxygen release by other primary producers (Wang et al. 2013). Furthermore, the mats can prevent oxygen diffusion between the air and water (Villamagna and Murphy 2010). The recovery rate for dissolved oxygen is dependent on the flow velocity in the channel, and dissolved oxygen levels can return to normal within two weeks in flowing water systems (Shu et al. 2014). The accumulation of water hyacinth at the upper tidal limit of the Swartkops Estuary during late spring could also be attributed to increased rainfall during this period that transported water hyacinth from the river to the downstream estuary. This is because flow dynamics influence the distribution and abundance of macrophytes (Slinger et al. 2017). However, large dense mats of floating IAAPs, particularly water hyacinth, have been shown to decrease flow dynamics (Chamier et al. 2012). As such, this could explain the decrease in flow rates measured during the summer sampling dates when IAAP cover was at its peak.
Nutrient supply, particularly nitrogen (Hill et al. 2020), is also a key factor that influences the growth and abundance of IAAPs (Reddy and Tucker 1983; Auchterlonie et al. 2021). Thus, as evidenced by model results (Table 1), the increased coverage of P. crassipes observed during the summer growth period was facilitated by excessive dissolved inorganic nitrogen (ammonium and NOx) availability. The high in situ concentrations of dissolved inorganic nutrients observed in this study (i.e., DIN and DIP both > 2 mg/L) primarily stem from anthropogenic inputs, particularly the three upstream WWTWs. These WWTWs are estimated to contribute significant daily DIN (209 kg) and DIP (127 kg) loads to the estuary (Lemley et al. 2023). This favours the extensive growth of water hyacinth in the upstream river and the upper reaches of the estuary, where they act as temporary nitrogen and phosphorus buffers for the downstream estuary. This was evidenced in this study by the observed increase in plant nutrient storage (both TN and TP; Fig. 11) as IAAP cover extent expanded. Moreover, the particularly strong interaction of ammonium with IAAP cover (Table 1) highlights the causative role of ammonium-rich WWTW effluents in this process. As such, aquatic macrophytes play an important role in nutrient transformation and reduction for downstream coastal areas (Pastor et al. 2023).
Correlations between plant tissue nutrients and dry biomass have been documented widely in water hyacinth plants (Reddy et al. 1989; Ho and Wong 1994; Xie and Yu 2003; Fox et al. 2008; Zainuddina et al. 2022). This trend was observed in this study, whereby TN and TP concentrations increased as plant biomass increased (Fig. 10). This is consistent with other studies that have investigated the relationship between nutrient uptake and total dry biomass and have found a strong correlation between the two variables in laboratory and pond experiments (Reddy et al. 1989; Fox et al. 2008). When investigating the nutrient storage in relation to cover dynamics, there was a clear increase in storage with increasing coverage. This suggests that plant cover plays a vital role in nutrient storage because although whole plant nutrient concentrations were lower, nutrient storage increased when considering the extent of plant cover in the water column. The potential for water hyacinth to reduce nutrient concentrations in the water column is reported to be dependent on the extent of populations (Pinto-Coelho and Greco 1999). Therefore, a possible explanation for the observed decrease in plant tissue nutrient concentration with increasing temperature may be attributed to the individuals within the population not having reached their nutrient uptake capacity during the exponential summer growth period (Xie and Yu 2003), i.e., nutrients less concentrated due to the increasing number of plants in the water column.
Management of invasive plants in aquatic systems is often through biological, chemical, and mechanical control measures. However, in most instances, the use of herbicides is often the preferred measure to control infestation by invasive plants, but this can be challenging when it is the only mode of management control employed (Sohrabi et al. 2023). As such, the combined use of chemical and biological methods can be advantageous in terms of managing IAAPs, however, these methods can lead to reduced water quality. Although extensive water hyacinth cover often reduces dissolved oxygen levels, spraying the hyacinth mats with herbicides as a means of control can result can also facilitate hypoxic conditions (Waltham and Fixler 2017). Potentially persistent hypoxic conditions induced by chemical control methods occur when the rate of oxygen consumption required for decomposition (i.e., decaying plants post-intervention) exceeds that of oxygen diffusion into the water column (O’Connell et al. 2000). In contrast, biological control methods, such as the use of weevils, requires long-term monitoring as it can take up to a decade before its impacts can be observed (Paterson et al. 2023). However, in some instances, host-specific biological control agents can significantly reduce infestations in enclosed impoundments. For example, a recent study by Coetzee et al. (2022) showed successful control of water hyacinth using the biocontrol agent Megamelus scutellaris at the Hartbeespoort Dam, in South Africa between 2015 and 2021. Additionally, the study showed a reduction in water hyacinth cover from 37% in December 2020 to less than 6% in April 2021. Biocontrol agents cause feeding scars on the leaves that affect the physiology of the plants by making them waterlogged and photosynthetically inefficient (Otieno et al. 2022). Notably, however, a study by Otieno et al. (2022) reported an increase in ammonium in an experimental pond after weevils were introduced in the experimental pond. This method of control results in the decomposition of plant tissues which, in turn, enhances in situ remineralisation processes by contributing to autochthonous nutrient stocks when previously absorbed nutrients are leached back into the system (Ouma et al. 2005; Dersseh et al. 2019). This creates a negative feedback loop, whereby in situ nutrient release has the potential to facilitate microalgal blooms (Otieno et al. 2022). As a result, the mechanical removal of water hyacinth after the implementation of chemical or biological control measures is considered the best approach to prevent the reintroduction of the accumulated nutrients.
Overall, study results identified the seasonal dynamics and drivers of water hyacinth accumulations in the upper reaches of a persistently eutrophic estuary. Despite playing a transient role as a nutrient buffer to downstream coastal waters, the presence and excessive growth of P. crassipes highlighted in this study stems from its affinity for nutrient-enriched conditions. Notably, some studies argue that the nutrient uptake potential of water hyacinth may be negligible when contextualised with the extent of anthropogenic nutrient loading to the system. For example, Carrol and Curtis (2021) investigated the uptake capacity of water hyacinth at the Hartbeespoort Dam in South Africa and concluded that although the plant can accumulate pollutants, only a small proportion is removed from the system. Similarly, a study by Tshuthukhe et al. (2021) reported that non-native floating plants in the Swartkops Estuary could assimilate heavy metals, however, due to the constant input of pollutants in the river system, water quality improvement downriver was insignificant. Therefore, the constant influx of nutrient-rich discharges from WWTWs and urban stormwater canals to the Swartkops River (Lemley et al. 2023) likely renders the potential nutrient buffering capacity of IAAPs as largely inconsequential. Ultimately, the purpose of restoration is to improve the health and natural ecosystem functionality of the focus system. With this in mind, the reduction of anthropogenic nutrient loading should be the most important step for restoration and water quality management in eutrophic systems, after which other strategies for ecosystem restoration can be considered (Rezania et al. 2015). In the case of the Swartkops Estuary, catchment-scale nutrient reduction measures, particularly the removal of discharges from the upstream WWTWs, have been identified as critical to improving ecosystem health and preventing the continued loss of important ecosystem services (Adams et al. 2021). In the absence of catchment-based nutrient reduction strategies, the continued implementation of short-term ad hoc measures (e.g., in situ IAAP control) is likely to increase the pressure on downstream estuaries and coastal waters. As such, management authorities need to adopt a catchment-to coast approach to effectively improve water quality and prevent the detrimental consequences (e.g., oxygen depletion, biodiversity loss, reconfigured food webs, flow obstruction, faunal mortalities) associated with eutrophication. However, for such initiatives to be effective, the dynamics of anthropogenic nutrient loading (e.g., quantity, timing) and global change indicators (e.g., IAAPs) must be assessed on a case-by-case basis. This will enable the formulation of tailored management approaches that will maximise the efficacy thereof.
Data availability
Data will be made available upon reasonable request.
References
Adams JB, Pretorius L, Snow GC (2019) Deterioration in the water quality of an urbanised estuary with recommendations for improvement. Water SA 45:86–96. https://doi.org/10.4314/wsa.v45i1.10
Adams JB, Taljaard S, Van Niekerk L, Lemley DA (2020) Nutrient enrichment as a threat to the ecological resilience and health of South African microtidal estuaries. Afr J Aquat Sci 45:23–40. https://doi.org/10.2989/16085914.2019.1677212
Adams JB, Hughes D, James N, Kibble R, Lemley D, Rishworth G, Riddin T, Strydom N, Taljaard S, Tsipa V, Van Niekerk L (2021) Swartkops estuary: present ecological status and future restoration scenarios. Re-evaluating the present ecological status and recommended ecological category, as well as consequences of future climate change, urbanisation, and restoration scenarios. Institute for Coastal and Marine Research Report No. 48. Nelson Mandel University, South Africa
Auchterlonie J, Eden CL, Sheridan C (2021) The phytoremediation potential of water hyacinth: a case study from Hartbeespoort Dam, South Africa. S Afr J Chem Eng 37:31–36. https://doi.org/10.1016/j.sajce.2021.03.002
Bowen JL, Valiela I (2001) The ecological effects of urbanization of coastal watersheds: historical increases in nitrogen loads and eutrophication of Waquoit Bay estuaries. Can J Fish Aquat Sci 58:1489–1500. https://doi.org/10.1139/f01-094
Carroll ASD, Curtis CJ (2021) Increasing nutrient influx trends and remediation options at Hartbeespoort Dam, South Africa: a mass-balance approach. Water SA 47:210–220
Cavalli G, Baattrup-Pedersen A, Riis T (2016) Nutrient availability and nutrient use efficiency in plants growing in the transition zone between land and water. Plant Biol 18:301–306. https://doi.org/10.1111/plb.12397
Chamier J, Schachtschneider K, le Maitre DC, Ashton PJ, van Wilgen BW (2012) Impacts of invasive alien plants on water quality, with a particular emphasis on South Africa. Water SA 38:345–356. https://doi.org/10.4314/wsa.v38i2.19
Coetzee JA, Hill MP (2012) The role of eutrophication in the biological control of water hyacinth, Eichhornia crassipes, in South Africa. Biocontrol 57:247–261. https://doi.org/10.1007/s10526-011-9426-y
Coetzee JA, Miller BE, Kinsler D, Sebola K, Hill MP (2022) It’s a numbers game: inundative biological control of water hyacinth (Pontederia crassipes), using Megamelus scutellaris (Hemiptera: Delphacidae) yields success at a high elevation, hypertrophic reservoir in South Africa. Biocontrol Sci Techn 32:1302–1311. https://doi.org/10.1080/09583157.2022.2109594
De Casabianca M (1995) Large-scale production of Eichhornia crassipes on paper industry effluent. Bioresour Technol 54:35–38. https://doi.org/10.1016/0960-8524(95)00111-5
Dersseh MG, Melesse AM, Tilahun SA, Abate M, Dagnew DC (2019) Water hyacinth: review of its impacts on hydrology and ecosystem services—Lessons for management of Lake Tana. In: Melesse AF, Abtew W, Senay G (eds) Extreme hydrology and climate variability. Elsevier Inc, Amsterdam. https://doi.org/10.1016/B978-0-12-815998-9.00019-1
Djihouessi MB, Olokotum M, Chabi LC, Mouftaou F, Aina MP (2023) Paradigm shifts for sustainable management of water hyacinth in tropical ecosystems: a review and overview of current challenges. Environ Chall 11:100705. https://doi.org/10.1016/j.envc.2023.100705
Driesen E, Van den Ende W, De Proft M, Saeys W (2020) Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: a review. Agronomy 10:1975. https://doi.org/10.3390/agronomy10121975
Freeman LA, Corbett DR, Fitzgerald AM, Lemley DA, Quigg A, Steppe CN (2019) Impacts of urbanization and development on estuarine ecosystems and water quality. Estuar Coasts 42:1821–1838. https://doi.org/10.1007/s12237-019-00597-z
Fox LJ, Struik PC, Appleton BL, Rule JH (2008) Nitrogen phytoremediation by water hyacinth (Eichhornia crassipes (Mart.) Solms). Water Air Soil Pollut 194:199–207. https://doi.org/10.1007/s11270-008-9708-x
Geletu TT (2023) Lake eutrophication: control of phytoplankton overgrowth and invasive aquatic weeds. Lakes Reserv Res Manag 28:e12425. https://doi.org/10.1111/lre.12425
Gopal B (1987) Water hyacinth. J Trop Ecol 4:92–93
Grasshoff K, Ehrhardt M, Kremling K (1999) Methods of seawater analysis, 3rd edn. Verlag Chemie, John Wiley & Sons, Weinheim
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extrem 10:4–10. https://doi.org/10.1016/j.wace.2015.08.001
Henderson L (2020) Invasive alien plants in South Africa. Plant Protection Research Institute Handbook No 21. Agricultural Research Council
Hill MP (2003) The impact and control of alien aquatic vegetation in South African aquatic ecosystems. Afr J Aquat Sci 28:19–24. https://doi.org/10.2989/16085914.2003.9626595
Hill MP, Coetzee JA, Martin GD, Smith R, Strange EF (2020) Invasive alien aquatic plants in South African freshwater ecosystems. In: van Wilgen B, Measey J, Richardson D, Wilson J, Zengeya T (eds) Biological invasions in South Africa. Springer, Switzerland. https://doi.org/10.1007/978-3-030-32394-3_4
Ho YB, Wong WK (1994) Growth and macronutrient removal of water hyacinth in a small secondary sewage treatment plant. Resour Conserv Recycl 11:161–178. https://doi.org/10.1016/0921-3449(94)90087-6
Hussner A, Stiers I, Verhofstad MJJM et al (2017) Management and control methods of invasive alien freshwater aquatic plants: a review. Aquat Bot 136:112–137. https://doi.org/10.1016/j.aquabot.2016.08.002
Koroleff F (1999) Simultaneous determination of total nitrogen and total phosphorus. In: Grasshoff K, Ehrhardt M, Kremling K (eds) Methods of seawater analysis, 3rd edn. Verlag Chemie, Weinheim
Lemley DA, Adams JB, Strydom NA (2017) Testing the efficacy of an estuarine eutrophic condition index: Does it account for shifts in flow conditions? Ecol Indic 74:357–370. https://doi.org/10.1016/j.ecolind.2016.11.034
Lemley DA, Human LRD, Rishworth GM, Whitfield E, Adams JB (2023) Managing the seemingly unmanageable: water quality and phytoplankton dynamics in a heavily urbanised low-inflow estuary. Estuar Coast 46:2007–2022. https://doi.org/10.1007/s12237-022-01128-z
López-Pozo M, Adams WW III, Polutchko SK, Demmig-Adams B (2023) Terrestrial and floating aquatic plants differ in acclimation to light environment. Plants 12(10):1928. https://doi.org/10.3390/plants12101928
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species. A selection from the global invasive species database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), p 12
Mangas-Ramírez E, Elías-Gutiérrez M (2004) Effect of mechanical removal of water hyacinth (Eichhornia crassipes) on the water quality and biological communities in a Mexican reservoir. Aquat Ecosyst Health 7:161–168. https://doi.org/10.1080/14634980490281597
Marais C, Van Wilgen BW, Stevens D (2004) The clearing of invasive alien plants in South Africa: a preliminary assessment of costs and progress: working for water. S Afr J Sci 100:97–103
Mironga JM, Mathooko JM, Onywere SM (2011) The effect of water hyacinth (Eichhornia crassipes) infestation on phytoplankton productivity in Lake Naivasha and the status of control. J Environ Eng Sci 5:1252–1260
Monneveux P, Pastenes C, Reynolds MP (2003) Limitations to photosynthesis under light and heat stress in three high-yielding wheat genotypes. J Plant Physiol 160:657–666
Murphy J, Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nikkel E, Clements DR, Anderson D, Williams JL (2023) Regional habitat suitability for aquatic and terrestrial invasive plant species may expand or contract with climate change. Biol Invasions 25:1–18. https://doi.org/10.1007/s10530-023-03139-8
Nunes M, Adams JB, Van Niekerk L (2020) Changes in invasive alien aquatic plants in a small closed estuary. S Afr J Bot 135:317–329. https://doi.org/10.1016/j.sajb.2020.09.016
Nusch EA (1980) Comparison of different methods for chlorophyll and phaeopigment determination. Arch Für Hydrobiol 14:14–36
O’Connell T, Jackson L, Brooks R (2000) Bird guilds as indicators of ecological condition in the Central Appalachians. Ecol Appl 10:1706–1721. https://doi.org/10.1890/1051-0761(2000)010[1706:BGAIOE]2.0.CO;2
Otieno D, Nyaboke H, Nyamweya C, Odoli C, Aura C, Outa N (2022) Water hyacinth (Eichhornia crassipes) infestation cycle and interactions with nutrients and aquatic biota in Winam Gulf (Kenya), Lake Victoria. Lakes Reserv Res Manag 27:12391. https://doi.org/10.1111/lre.12391
Ouma YO, Shilaby A, Tateishi R (2005) Dynamism and abundance of water hyacinth in the Winam Gulf of Lake Victoria: evidence from remote sensing and seasonal-climate data. Int J Environ Stud 62:449–465. https://doi.org/10.1080/00207230500197011
Pastor A, Holmboe CM, Pereda O, Giménez-Grau P, Baattrup-Pedersen A, Riis T (2023) Macrophyte removal affects nutrient uptake and metabolism in lowland streams. Aquat Bot 189:103694
Paterson ID, Motitsoe SN, Coetzee JA, Hill MP (2023) Recent post-release evaluations of weed biocontrol programmes in South Africa: a summary of what has been achieved and what can be improved. Biocontrol 68:1–3. https://doi.org/10.1007/s10526-023-10215-4
Pinto-Coelho RM, Barcelos Greco MK (1999) The contribution of water hyacinth (Eichhornia crassipes) and zooplankton to the internal cycling of phosphorus in the eutrophic Pampulha Reservoir, Brazil. Hydrobiologia 411:115–127. https://doi.org/10.1023/A:1003845516746
Reddy KR, Tucker JC (1983) Productivity and nutrient uptake of water hyacinth, Eichhornia crassipes: effect of nitrogen source. Econ Bot 37:237–247. https://doi.org/10.1007/BF02858790
Reddy KR, Agami M, Tucker JC (1989) Influence of nitrogen supply rates on growth and nutrient storage by water hyacinth (Eichhornia crassipes) plants. Aquat Bot 36:33–43
Rezania S, Ponraj M, Talaiekhozani A, Mohamad SE, Din MF, Taib SM, Sabbagh F, Sairan FM (2015) Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J Environ Manag 163:125–138. https://doi.org/10.1016/j.jenvman.2015.08.018
Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Dane PF, West CJ (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107. https://doi.org/10.1046/j.1472-4642.2000.00083.x
Riis T, Tank JL, Reisinger AJ, Aubenau A, Roche KR, Levi PS, Baattrup-Pedersen A, Alnoee AB, Bolster D (2020) Riverine macrophytes control seasonal nutrient uptake via both physical and biological pathways. Freshw Biol 65:178–192. https://doi.org/10.1111/fwb.13412
Rommens W, Maes J, Dekeza N, Inghelbrecht P, Nhiwatiwa T, Holsters E, Ollevier F, Marshall B, Brendonck L (2003) The impact of water hyacinth (Eichhornia crassipes) in a eutrophic subtropical impoundment (Lake Chivero, Zimbabwe). I. Water quality. Arch Hydrobiol 158:373–388. https://doi.org/10.1127/0003-9136/2003/0158-0373
Shu X, Zhang Q, Wang W (2014) Effects of temperature and light intensity on growth and physiology in purple root water hyacinth and common water hyacinth (Eichhornia crassipes). Environ Sci Pollut Res 21:12979–12988. https://doi.org/10.1007/s11356-014-3246-4
Simberloff J-L, Martin D, Genovesi P et al (2013) Impacts of biological invasions—what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013
Slinger JH, Taljaard S, Largier JL (2017) Modes of water renewal and flushing in a small intermittently closed estuary. Estuar Coast Shelf S 193:346–359. https://doi.org/10.1016/j.ecss.2017.07.002
Sohrabi S, Vilà M, Zand E, Gherekhloo J, Hassanpour-bourkheili S (2023) Alien plants of Iran: impacts, distribution and managements. Biol Invasions 25:97–114. https://doi.org/10.1007/s10530-022-02884-6
Strange EF, Landi P, Hill JM, Coetzee JA (2019) Modeling top-down and bottom-up drivers of a regime shift in invasive aquatic plant stable states. Front Plant Sci 10:1–9. https://doi.org/10.3389/fpls.2019.00889
Taljaard S, Lemley DA, Van Niekerk L (2022) A method to quantify water quality change in data-limited estuaries. Estuar Coast Shelf Sci 272:107888. https://doi.org/10.1016/j.ecss.2022.107888
Tobias VD, Conrad JL, Mahardja B, Khanna S (2019) Impacts of water hyacinth treatment on water quality in a tidal estuarine environment. Biol Invasions 21:3479–3490. https://doi.org/10.1007/s10530-019-02061-2
Tshithukhe G, Motitsoe SN, Hill MP (2021) Heavy metals assimilation by native and non-native aquatic macrophyte species: a case study of a river in the Eastern Cape Province of South Africa. Plants 10:2676. https://doi.org/10.3390/plants10122676
Van Niekerk L, Adams JB, Lamberth SJ, MacKay F, Taljaard S, Turpie JK Weerts, S (2019) South African National Biodiversity Assessment 2019a: Technical report. Volume 3: Estuarine environment. Report number: SANBI/NAT/NBA2018/2019/ Vol3/A. Pretoria, South Africa: South African National Biodiversity Institute
Van Niekerk L, Adams JB, James NC, Lamberth SJ, MacKay CF, Turpie JK, Rajkaran A, Weerts SP, Whitfield AK (2020) An estuary ecosystem classification encompassing biogeography, size and types of diversity that supports estuarine protection, conservation and management. Afr J Aquat Sci 45:199–216. https://doi.org/10.2989/16085914.2019.1685934
Van Niekerk L, Taljaard S, Lamberth SJ, Adams JB, Weerts SP, MacKay CF (2022) Disaggregation and assessment of estuarine pressures at the country-level to better inform management and resource protection–the South African experience. Afr J Aquat Sci 47:127–148. https://doi.org/10.2989/16085914.2022.2041388
Villamagna AM, Murphy BR (2010) Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biol 55:282–298. https://doi.org/10.1111/j.1365-2427.2009.02294.x
Waltham NJ, Fixler S (2017) Aerial herbicide spray to control invasive water hyacinth (Eichhornia crassipes): water quality concerns fronting fish occupying a tropical floodplain wetland. Trop Conserv Sci 10:1–10. https://doi.org/10.1177/1940082917741592
Wang Y, Naumann U, Wright ST, Warton DI (2012) Mvabund-an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474
Wang Z, Zhang Z, Zhang Y, Zhang J, Yan S, Guo J (2013) Nitrogen removal from Lake Caohai, a typical ultra-eutrophic lake in China with large scale confined growth of Eichhornia crassipes. Chemosphere 92(2):177–183
Wilson JR, Rees M, Holst N, Thomas M, Hill G (2000) Water hyacinth population dynamics. In: Australian centre for international agricultural research proceedings. ACIAR; 1998, pp 96–104
Wilson JR, Holst N, Rees M (2005) Determinants and patterns of population growth in water hyacinth. Aquat Bot 81:51–67. https://doi.org/10.1016/j.aquabot.2004.11.002
Xie Y, Yu D (2003) The significance of lateral roots in phosphorus (P) acquisition of water hyacinth (Eichhornia crassipes). Aquat Bot 75:311–321. https://doi.org/10.1016/S0304-3770(03)00003-2
Zainuddina NA, Dinb MFM, Nuida M, Halima KA, Salima NAA, Eliasb SH, Lazima ZM (2022) The phytoremediation using water hyacinth and water lettuce: correlation between sugar content, biomass growth rate, and nutrients. J. Kejuruter. 34:915–924. https://doi.org/10.17576/jkukm-2022-34(5)-19
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
Opinions expressed and conclusions arrived at are those of the authors and do not necessarily represent the official views of the funding agencies. Mr Riaan Weitz and Ms Shulamy Ntsoeu are thanked for assisting with field and laboratory work. Dr Monique Nunes and Daniel Buttner are also thanked for assistance with statistical analyses. We are also grateful to the South African Environmental Observation Network (SAEON) Elwandle node for logistic assistance in the field and laboratory through the Shallow Marine and Coastal Research Infrastructure (SMCRI) programme. Finally, we thank three anonymous reviewers for their constructive comments which helped improve this manuscript.
Funding
Open access funding provided by Nelson Mandela University. This work is based on research financially supported by the Water Research Commission (Project: C2020/2021–00076) and the DSI/NRF Research Chair in Shallow Water Ecosystems (UID: 84375). The Water Research Commission (Project No. C2020/2021–00076) and the Nelson Mandela University are thanked for providing funding to CPL and DAL, respectively.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Sample collection, preparation, and analysis were performed by CPL and DAL. JBA secured project funding. The first draft of the manuscript was written by CPL and DAL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lakane, C.P., Adams, J.B. & Lemley, D.A. Drivers of seasonal water hyacinth dynamics in permanently eutrophic estuarine waters. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03347-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03347-w