Abstract
The continuous growth and movement of the human population is increasing the frequency of translocating species from their native ranges to novel environments. However, biological invasions offer a rare opportunity to investigate how species can colonise and adapt to new conditions. In that sense, Ecological Niche Models (ENMs) can be a powerful tool to predict where invasive species will spread over the next decades, although they depend heavily on climatic niche conservatism between native and exotic ranges. To reduce these uncertainties, ENMs can be refined by accounting for dispersal constraints. The common wall gecko, Tarentola mauritanica is a native and widespread Mediterranean lizard that has been introduced across different tropical and sub-tropical regions. In this study, we aim to predict the potential and future distribution of T. mauritanica geckos using correlative models, its potential colonization regions under a dispersal model, and the niche overlap between native and introduced populations. The correlative models predict that the most suitable geographic areas for this gecko correspond to Mediterranean-type ecosystems, such as California, central Chile, the Cape Region of South Africa, around the Caspian Sea, south-eastern Asia, and south-western and southern Australia. The species distribution models projected to 2061–2080, forecast that the range of T. mauritanica is likely to shift towards northern latitudes but, surprisingly, not to expand. According to the dispersal models, T. mauritanica will be able to colonise a similar geographic range compared to the one obtained with the correlative models for the future. Finally, the niche overlap results demonstrate that T. mauritanica’s realised niche has not been conserved over space, as the naturalised climatic niche of the introduced populations differs significantly from its native one. The latter results suggest that there has been no climatic niche conservatism during the several introductions of T. mauritanica and that this species seems to be able to cope with novel and more humid environments, typical from the tropics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The movement of species beyond their native habitats, driven by human activities, is leading to new populations (Wilson et al. 2009). Some introduced species adapt and cause environmental problems, such as biodiversity loss and disrupted ecosystem services (Butchart et al. 2010; Gurevitch and Padilla 2004). Invasive reptiles significantly contribute to global biodiversity decline. A recent meta-analysis by Capinha et al. (2017) found nearly 200 reptile species established in various regions worldwide, with invasions increasing. This is especially concerning in biodiversity hotspots, where invasive species richness is high (Li et al. 2016).
Climate change compounds these issues by affecting the introduction of new invasive species and the distribution of existing ones (Hellmann et al. 2008). However, the effects vary among species, with some benefiting from climate change while others struggle. Species distribution models (SDMs), or ecological niche models (ENMs), are crucial in invasion biology (Srivastava et al. 2019). They predict invasion risk (e.g., Kramer et al. 2017; Tingley et al. 2018) and guide control strategies (e.g., Giljohann et al. 2011; Tulloch et al. 2014). ENMs also apply to other conservation areas, including assessing climate change impacts on genetic and functional diversity (e.g., Pauls et al. 2013; Weterings et al. 2019), and selecting protected areas (e.g., Guisan et al. 2013). ENM studies often reveal similarities in climatic niches between native and introduced species populations (e.g., Petitpierre et al. 2012; Zhu et al. 2020), and that models based on native distributions can predict spread in introduced areas (e.g., Thuiller et al. 2005). In general terms, the concordance between the realised climatic niches of a species’ native and introduced distributions may provide evidence of niche conservatism (e.g., Petitpierre et al. 2012; Wiens and Graham 2005). The assumption of niche conservatism entails that niche divergence will be negligible over the timeframe of prediction, and this assumption is used to predict the impacts of anthropogenic climate change on species distributions and persistence (e.g., Petitpierre et al. 2012). Yet, shifts between climatic niches of native and introduced populations are common (e.g., Atwater et al. 2018; Fernández and Hamilton 2015; Parravicini et al. 2015). Introduced populations’ shifts may provide insights into species’ responses to climate change (e.g., Fernández and Hamilton 2015; Guisan et al. 2014; Moran and Alexander 2014).
Gecko lizards excel in long-distance dispersals, natural or human-mediated (e.g., Agarwal et al. 2021; Perella and Behm 2020; Rocha et al. 2022). Their success is due to factors like their association with humans, small size, nocturnal habitats, calcareous eggs, and high population densities (Locey and Stone 2006; Rödder and Lötters 2009; Weterings and Vetter 2018). Some invasive geckos are nearly cosmopolitan (Baldo et al. 2008). The genus Hemidactylus, with over 165 species (Agarwal et al. 2021), includes about 10 with intercontinental distributions (Capinha et al. 2017; Weterings and Vetter 2018). The common wall gecko Tarentola mauritanica (Linnaeus, 1758), a species complex with six putative distinct taxa (Rato et al. 2016), is native to the west coastal Mediterranean Basin (Vogrin et al. 2017). However, due to its association with human settlements and long-distance dispersal (Carranza et al. 2000), it has been introduced to various regions in the eastern Mediterranean, Macaronesia, North and South America (e.g., Deso et al. 2020; Díaz-Fernández et al. 2019; Ortiz-Medina et al. 2019; Rato et al. 2015b). The environmental impacts of T. mauritanica in these new habitats are largely unknown (Baldo et al. 2008; Deso et al. 2020). In Croatia, they compete with Hemidactylus turcicus through spatial displacement (Lisičić et al. 2012). On Madeira Island, they may influence arthropod communities (Martins et al. 2022). Tarentola mauritanica is well adapted to living near humans, frequently feeding on insects attracted to artificial light (Valverde 1967). These behaviours contribute to their success, as they inadvertently disperse long distances via human transportation (e.g., Arredondo and Núñez 2014; Díaz-Fernández et al. 2019; Ortiz-Medina et al. 2019). While individuals reach various parts of the world, climatic conditions can limit their establishment. However, with climate change, some populations may thrive. Evidence shows a northward shift in the Iberian Peninsula and France (Geniez and Cheylan 2012; Moreno-Rueda et al. 2011), and expansion in Mediterranean-like regions of Madeira Island (Silva-Rocha et al. 2022). Climatic variables related to humidity and temperature seasonality drive niche shifts and genetic diversification in native T. mauritanica (Rato et al. 2015a). They also affect the successful establishment of the common wall gecko on Madeira Island (Silva-Rocha et al. 2022). Additionally, their plasticity in response to water loss is likely crucial for the success of introduced populations worldwide (Rato and Carretero 2015). Although the current distribution of both native and introduced T. mauritanica populations is well documented (Vogrin et al. 2017), attempts to infer their potential geographic distribution have been limited to a local scale (Geniez and Cheylan 2012; Moreno-Rueda et al. 2011; Silva-Rocha et al. 2022). Given their transcontinental dispersal, this knowledge gap on a broader scale is significant.
In this study, we aimed to (1) predict the potential and future distribution of T. mauritanica geckos using correlative models; (2) forecast their potential invasive range under future climates using dispersal models; and (3) evaluate niche overlap between native and introduced populations. Given the global geographic distribution of the model species, we expect a larger predicted range of habitat suitability than the one currently known. Considering its thermophilic nature, we anticipate that future climate change will expand suitable habitats. Finally, we also hypothesize divergent environmental niches between native and introduced populations due to the extensive geographic range of the latter and novel environmental conditions these populations are subject to.
Materials and methods
Species occurrence
Species occurrence records of Tarentola mauritanica from native and introduced ranges were compiled from the Global Biodiversity Information Facility (GBIF) database (November 2022, www.gbif.org), personal databases (Catarina Rato and Ricardo Rocha), and recently published records (Arredondo and Núñez 2014; Baldo et al. 2008; Díaz-Fernández et al. 2019; Huerta-Vera 2016; Mačát et al. 2014; Mizerakis and Strachinis 2017; Mizsei et al. 2017; Ortiz-Medina et al. 2019; Strachinis and Artavanis 2017; Strachinis and Pafilis 2018). From GBIF, we only included records containing animal pictures to be sure about the taxonomic assignment. The combination of all these different record sources allowed covering the entire known distribution (native and introduced) of T. mauritanica. Species occurrences were cleaned and filtered for duplicates (i.e., records from the same location) and erroneous geo-referenced records, and later removed from the final dataset. Additionally, to reduce the effects of sampling bias, we performed a spatial thinning on the native and introduced records which were less than 20- and 10 km apart respectively, with the spThin R package (Aiello-Lammens et al. 2015). In the end, a total of 723 records comprising the entire distribution (native and introduced), were used to perform all further analyses.
Environmental data
Using the function worldclim_global from the geodata R package (Hijmans et al. 2022), a set of 19 bioclimatic variables was downloaded from WorldClim v2.1 (http://www.worldclim.org) at a resolution of 2.5 arc minutes, used to estimate the realised ecological niche (sensu Sillero 2011) of the species across the globe. The bioclimatic variables were masked to a hypothesis of accessible area for T. mauritanica (Anderson and Raza 2010; Barve et al. 2011; Soberón and Peterson 2005). The mask was a 0.5-degree buffered spatial polygon using the occurrence points (Phillips et al. 2009). To test for multicollinearity among environmental variables, we used the function vif from the usdm R package (Naimi et al. 2014). To reduce the number of highly correlated variables, we applied a Pearson correlation with the function layerStats from the package raster (Hijmans 2022), and a hierarchical cluster analysis to select the most relevant variables among collinear variables with a correlation < |0.75|. In the end, the following variables were selected: Mean Diurnal Range (bio2), Temperature Seasonality (bio4), Max Temperature of Warmest Month (bio5), Min Temperature of Coldest Month (bio6), Mean Temperature of Wettest Quarter (bio8), Annual Precipitation (bio12), Precipitation Seasonality (bio15), Precipitation of Driest Quarter (bio17), Precipitation of Warmest Quarter (bio18), and Precipitation of Coldest Quarter (bio19).
Ecological niche models (ENMs)
Potential changes in the spatial distribution of the target species were implemented using six different algorithms/models in the biomod2 R package (Thuiller et al. 2009), a computer-based platform for ensemble forecasting of species distributions, enabling the treatment of a range of methodological uncertainties in models, and the examination of species–environment relationships (Araújo and New 2007; Thuiller et al. 2009). The algorithms we used were: GLM (generalised linear model), GBM (generalised boosted regression model), GAM (generalised additive model), CTA (classification tree analysis), RF (random forest), and the MAXENT.Phillips (maximum-entropy model).
Standard procedures were followed to compute the realised niche models (Sillero et al. 2021; Sillero and Barbosa 2021). Species occurrence data were coupled with 10,000 randomly generated pseudo-absences, following Barbet-Massin et al. (2012). Ten replicate models were built using 70% of the native and introduced occurrences and pseudo-absence data, and the remaining 30% were withheld for evaluating predictions. As not all algorithms have the same output range, we scaled all model outputs within a range between 0 and 1000 to calculate the ensemble modelling afterwards, using the parameter scale.models from the BIOMOD_Modeling() function. Hence, we evaluated a total of 60 models: 1 pseudo-absence set × 10 iterations × 6 models. Models’ performance was evaluated using two discrimination measures: the true skill statistic (TSS) (Allouche et al. 2006) and the area under the receiver operating characteristic (ROC) curve (AUC) (Fielding and Bell 1997). For the ensemble modelling, only those models with TSS and ROC scores above 0.8, and 0.9, respectively were considered (Allouche et al. 2006; Shabani et al. 2018). To choose the best models, a preliminary screening was carried out to investigate which variables and algorithms would be more suitable for predicting the target species’ ENMs, based on model evaluation (i.e., TSS, and ROC), and projections to the current conditions (See Supplementary Material). One of the main features of the biomod2 software is the ability to combine predictions made in single models in an ensemble.
For the best models, we projected the current potential distribution of T. mauritanica, using the BIOMOD_Projection function from biomod2, and generated an ensemble model as the mean of all models.
To project the potential species distribution under climate change (2061–2080), we used nine CMIP6 climate models (BCC-CSM2-MR, CanESM5, CNRM-CM6-1, CNRM-ESM2-1, CMCC-ESM2, IPSL-CM6A-LR, MIROC6, MIROC-ES2L, MRI-ESM2-0), each within the four shared socio-economic pathways (SSP126, SSP245, SSP370 and SSP585), downloaded from WorldClim v2.1, using the function cmip6_world from the geodata R package (Hijmans et al. 2022). The final future projections were performed using the BIOMOD_Projection function from biomod2, and an ensemble model was generated by averaging all climatic models for each SSP.
Representation of the two types of overlap analyses used: full overlap (a) and background overlap (b). The background in b corresponds to the actual values of the climatic variables in the study region. Each ellipsoid represents the ecological niche of T. mauritanica species (the native niche represented in blue and the introduced one represented in red). Comparison of observed values of niche overlap against the null distribution of 1000 overlap values of random ellipsoids (c). Solid green line shows the observed values, and the dashed green line the 5 % confidence interval (CL)
Species dispersion model
A cellular automaton model was implemented using the MigClim R package (Engler and Guisan 2009; Engler et al. 2012) to incorporate dispersal constraints of T. mauritanica and predict the potential dispersal movements towards future climatic scenarios. Succinctly, a cellular automaton is a matrix of cells containing values that change over time based on regulations influenced by adjacent cells, as explained by Sarkar (2000). Datasets to simulate dispersal (i.e., the biomod2 models) had a resolution of 2.5 arcmin per pixel (~ 3.5 km). MigClim enables the implementation of species-specific dispersal constraints and demographic parameters into projections of species distribution models under environmental change and/or landscape fragmentation scenarios (Engler et al. 2012). It can equally well be used to simulate dispersal in stable environments and undisturbed landscapes (e.g., for modelling the potential spread of invasive species) (Engler et al. 2012). MigClim calculates the probability of each grid cell being colonised as a function of its distance to the source cells, its future habitat suitability, the dispersal ability of the species, and the propagule production potential of source cells over time.
We used MigClim to model the dispersal of T. mauritanica under a no-barrier dispersal scenario, that is, the species can disperse to any suitable cell (following Carranza et al. 2000). To do that, we provided MigClim with the following inputs: a map defining the species’ initial distribution, the species’ dispersal parameters, and maps of the current and future species’ habitat suitability obtained previously from biomod2. We chose a reclassification threshold of 0 (i.e., continuous mode), meaning that habitat suitability model output will not be converted into binary values (i.e. presence and absence). Instead, we used the raw values of the realised models, that is, the habitat suitability index values, as a conditional probability that a cell becomes colonized; thus, the habitat suitability index is interpreted as a value of “habitat invasibility”. We also defined a dispersal kernel of 0.1 (i.e., the probability of colonizing a directly adjacent cell). Long distance dispersal frequency was set to 0.1 and the min-max distance range as 25 cells and 2857 cells, respectively, since the minimum recorded dispersal event is 89 km from the Iberian Peninsula to Alborán islet (Rato et al. 2021a), and the maximum from France to Chile (10.000 km in Arredondo and Núñez 2014). Propagule production potential was set to 1. We had 2 environmental change steps (present, and future 2061–2080) with 30 dispersal steps in each environmental change step. Accordingly, the total number of dispersal steps simulated was equal to [envChgSteps] × [dispSteps], here 60, which corresponds to 60 years from 2023 to 2080. Simulations were repeated 100 times.
Ecological niche overlap
To characterise and compare the niches of both native and introduced populations of T. mauritanica, we created ellipsoidal envelope ecological niche models for each group of interest.
Ellipsoidal envelopes were built using the first three PCAs of the variables previously used in the species distribution modelling (see Environmental data). Then, we retrieved the environmental information of the occurrence points for each group and fit three-dimensional minimum volume ellipsoids (Van Aelst and Rousseeuw 2009). PCAs were obtained using the kuenm_rpca function from the kuenm R package (Cobos et al. 2019).
To measure overlap using these ellipsoids, we followed two different approaches. First, the full overlap, which considers the background data that overlap between the two ellipsoids. To this end, a cloud of points uniformly distributed in the multi-dimensional environmental space that covers the two ellipsoids is used to detect how those envelopes overlap (Qiao et al. 2016). Secondly, we measured the background overlap, which considers only the subset of the full overlap corresponding to environments that are accessible to the groups of interest (which may or may not fill all the volume of the two ellipsoids) (Nuñez-Penichet et al. 2021). The overlap is measured using the Jaccard Index (Mammola 2019), where the value of overlap is the ratio between the number of points within the intersection of two ellipsoids (E1 ∩ E2) and the total number of points contained by two ellipsoids (E1 ∪ E2): J = E1 ∩ E2/E1 ∪ E2. To perform the analyses of overlap using the environmental combinations accessible to each group, we used the values of the PCs for each group. To test the statistical significance of our observed values of overlap, we compared the observed results to a null distribution of 1,000 overlapped values derived from comparing ellipsoid envelopes created from points randomly sampled from each group’s accessible environment. The null hypothesis is that the two ellipsoids fitted to actual observations overlap at least as much as random-data ellipsoids. If the observed niche overlap value falls inside the upper 95% of the null distribution, the null hypothesis cannot be rejected. As the full overlap analysis uses points that are uniformly distributed, significance tests (which rely on available conditions) were performed only for analyses of background overlap. Analyses of niche overlap were carried out in R using the package ellipsenm (Cobos et al. 2020).
Results
All six algorithms (GLM, CTA, RF, GBM, GAM, and MAXENT.Phillips) produced species distribution models with TSS and ROC evaluation scores over 0.8 and 0.9, respectively (Fig. S1). However, both RF and CTA algorithms were removed from the modelling and projection analyses; the CTA exhibited a low mean ROC score (< 0.75; Fig. S2), and they both delivered unsuitable ENMs for T. mauritanica, such as Scandinavia (in CTA), Alaska, and Siberia (in RF) (Fig. S5). Moreover, most of the environmental variables contributed very little to the RF model (Fig. S3), which might explain its poor capacity to estimate accurately suitable habitats for T. mauritanica. As for the initially considered environmental variables, they were all kept for further analysis.
In general, the most suitable areas for T. mauritanica correspond to regions with low to moderate precipitation, moderate to high temperatures, low-temperature seasonality, and low to moderate mean diurnal range (Fig. S4).
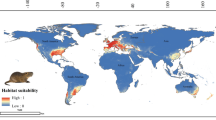
Tarentola mauritanica’s ensembled potential distribution is predicted to be wider than the range it is known today (Figs. 1 and 2). For instance, the regions around the Black and Caspian Seas present high habitat suitability, as well as south-eastern Asia, the south-western coast of South Africa and the southern coast of Australia. In Europe, suitable regions also include the northern latitudes of this continent.
As for the habitat suitability to 2061–2080 (Fig. 3), all SSP scenarios support very similar models for the future distribution of T. mauritanica. In Europe, a shift to northern latitudes is forecasted, reaching the southern UK and the southern Scandinavian Peninsula. In North America, the north-eastern region of the USA will become suitable in the future. Moreover, south-eastern Spain and North Africa are predicted to become less suitable for this gecko species. The same is observed for south-eastern Asia, Argentina, and Uruguay.
Again, the species dispersal models among the different future scenarios are very similar (Fig. 4), and they also predict that over the next 60 years, T. mauritanica will be able to colonise a similar geographic range compared to the one obtained with the correlative models for the future (Fig. 3).
The full niche overlap between native and introduced ellipsoids was 0.43, and the overlap using the available conditions was 0.58 (Fig. 5). The null hypothesis of niche overlap (using the background) was rejected (p-value = 0.00), with the observed values of niche overlap being smaller than expected, falling below the 5% confidence limit.
Discussion
Across its native Mediterranean geographic range, the common wall gecko, T. mauritanica is subject to mild wet winters, and warm and dry summers (Lionello et al. 2006). These specific climatic conditions coincide with the obtained response curves for the several temperature and precipitation variables used in this study. Not surprisingly, the correlative models predicted California, central Chile, the Cape Region of South Africa, around the Caspian Sea, south-eastern Asia, and south-western and southern Australia, as some of the most suitable geographic areas for this gecko to occur, which also correspond to the areas of the world with Mediterranean-type ecosystems (Esler et al. 2018; Sayre et al. 2020). Nevertheless, the cool temperate climate we find in Northern Europe is also predicted as moderately suitable for T. mauritanica.
Although this gecko species has been successfully introduced across several sub-tropical and tropical regions of the world (e.g., Madeira, Azores, Yucatán, Florida, Uruguay and Argentina in Arredondo and Núñez 2014; Báez and Biscoito 1993; Baldo et al. 2008; Barreiros et al. 2010; Díaz-Fernández et al. 2019; Huerta-Vera 2016; Jesus et al. 2008; Rato et al. 2015b), our ENMs have identified only one (not yet colonised) highly suitable tropical region located in south-western Iran, facing the United Arab Emirates Peninsula.
Despite some known limitations (Araújo and Peterson 2012), ENMs can be a powerful tool to predict where invasive species will spread next. However, until the last stage of invasion, an introduced species is not yet at equilibrium with its environment, as demonstrated in previous studies focusing on other invasive species (e.g., Barbet-Massin et al. 2018; Gallien et al. 2012; Václavík and Meentemeyer 2012). The equilibrium hypothesis is an important assumption, and its violation has to be acknowledged when interpreting ENM predictions (Garcia et al. 2012). Indeed, violating the equilibrium hypothesis has some consequences when modelling species distribution, such as the underestimation of the potential climatic niche of a species, which can in turn underestimate the geographical area the species can invade (Václavík and Meentemeyer 2012). Considering that the introduction of T. mauritanica into tropical ecosystems has taken place only in the last 20–30 years, it is reasonable to assume that these populations have not yet reached an equilibrium with the novel environmental conditions and that the habitat suitability models might be underestimating the probabilities of species occurrences. Actually, over the last three decades, the occurrence area of T. mauritanica on Madeira Island has increased by more than 20 km (Silva-Rocha et al. 2022). Moreover, the same study demonstrates that despite preferring Mediterranean-like climate areas, more humid regimes seem also suitable for the introduced populations of the common wall gecko in sub-tropical Madeira Island.
Furthermore, our niche overlap results demonstrate that T. mauritanica’s realised niche has not been conserved over space, as the naturalised climatic niche of the introduced populations differs significantly from its native one (e.g., Early and Sax 2014; Medley 2010; Parravicini et al. 2015). These results highlight two important aspects: first, there has been no climatic niche conservatism during the several introductions of T. mauritanica; and second, this species seems to be able to cope with novel and more humid environments.
Undoubtedly, biological invasions offer a rare opportunity to investigate how species colonise new environments (Kueffer et al. 2013; Richardson and Pyšek 2008; Sax et al. 2007), and whether they preserve their climatic niche in a new range (Pearman et al. 2008). Addressing this question has proven important in recent years as a test for ecological niche models, which depend heavily on climatic niche conservatism between native and exotic ranges (Colwell and Rangel 2009; Pearman et al. 2008; Peterson 2011). Evidence exists both for (e.g., Peterson 2011; Petitpierre et al. 2012; Strubbe et al. 2013), and against (e.g., Broennimann et al. 2007; Fitzpatrick et al. 2007; Lauzeral et al. 2011; Li et al. 2014; Medley 2010; Rödder and Lötters 2009) climatic niche conservatism during invasions, which is most likely related to the different types of niche change, biological and/methodological study contexts, data types, species characteristics, or methods being used (see references in Guisan et al. 2014).
The introduction and expansion of the common wall gecko populations across different tropical ranges (Arredondo and Núñez 2014; Baldo et al. 2008; Silva-Rocha et al. 2022), suggests that the species can cope with more humid environments. However, as the response curves demonstrate, when humidity is too high, the habitat becomes unsuitable for T. mauritanica. This is most likely due to the limited favourable conditions viable for gecko reproduction, particularly concerning humid environments. Like most gecko lizards, T. mauritanica produces rigid-shelled eggs (a pre-adaptation to arid environments) that in conditions of high humidity can limit embryo development (Pike et al. 2012). Moreover, evidence from the gecko Chondrodactylus turneri suggests that under high moisture conditions, fungal infections can decrease the viability of hard-shelled gekkotan eggs (Andrews 2015). While the excess of humidity/water vapour is a limitation to gecko groups with highly calcified rigid-shelled eggs (e.g., Sphaerodactylidae, Phyllodactylidae, and Gekkonidae), parchment-shelled eggs are highly permeable and benefit from water vapour (Andrews 2015). Hence, in a humid environment, depending on the gecko group, females will either choose drier microhabitats and/or reproduce during the drier seasons (if they have hard-shelled eggs) (e.g., Somaweera 2009), or deposit their eggs in humid places (if they lay parchment-shelled eggs). Unless the individuals inhabiting the tropics can select suitable micro-habitats for egg laying, the future of these populations might be compromised under current climatic conditions. Yet, the climate is changing at a global scale and at such a fast pace that species are more likely to change their distributions as a response than to adapt in situ (Bradshaw and Holzapfel 2006).
Indeed, the species distribution models projected to 2061–2080, forecast that the range of T. mauritanica is likely to shift towards northern latitudes. South-eastern Spain and parts of North Africa will become unsuitable, while the south of the UK and the Scandinavian Peninsula seem to offer more relevant environmental conditions for this gecko species. Furthermore, the climate in the tropics will become unsuitable, while parts of the north-eastern USA seem to favour the establishment of T. mauritanica. At least in the Iberian Peninsula and France, a northward shift in the range of the common wall gecko populations has already been documented as a response to global warming (Geniez and Cheylan 2012; Moreno-Rueda et al. 2011). According to Sumner et al. (2003), by the late twenty-first century, the far south of Spain will undergo a continued spread of aridity of the climate, extending westwards. This is already the hottest and one of the aridest parts of Spain, and the predicted environmental changes will be disastrous to several taxa of the region (e.g., Algyroides marchii in Rato et al. 2021b), including a thermophilic reptile such as T. mauritanica.
Contrary to what we obtained with the species distribution models, the dispersal models forecast that colonization by the common wall gecko will still take place across southern Spain and North Africa. Indeed, the largest colonisation is predicted to take place in the European continent, with T. mauritanica expanding its territory towards the northern and eastern regions. In South America, the dispersal models also predicted a potential expansion to the south, and towards the north following the Atlantic coast of Brazil. The population from California is also expected to disperse to more northern territories.
The common wall gecko occurs naturally in a typical Mediterranean climate and has mostly crepuscular activity. It thermoregulates during the first 2–3 h of the day near the refugium, to where it returns once its optimal temperature is achieved (Martínez-Rica 1974). In comparison to other sympatric similar-sized lacertid lizards, this gecko has a higher resistance to dehydration (García-Muñoz and Carretero 2013; Osojnik et al. 2013), with great plasticity among populations for this trait (Rato and Carretero 2015). Indeed, this feature conveys the common wall gecko with a great capacity to withstand long periods without water, and able to survive during transmarine trips. Moreover, future global warming would not be a limiting factor for this species, as already documented in previous studies (Geniez and Cheylan 2012; Moreno-Rueda et al. 2011). In fact, Moreno-Rueda et al. (2011) saw that the southern range of T. mauritanica has not been affected by global warming during the last 70 years. Therefore, both the predicted future range and colonization models are in line with the ecophysiological requirements and dispersal capacity of this species.
The way organisms respond to environmental change may be complex, since such responses are usually nonlinear, often have thresholds, and (as demonstrated in this study) could change with novel conditions (Beissinger and Riddell 2021; Huey et al. 2012). Therefore, there is a growing acknowledgment that we need to accurately characterize how organisms experience their environments and the biological mechanisms by which they respond to improve our predictions (Helmuth et al. 2005; Keith et al. 2008; Urban et al. 2016). In ectotherms, biophysical models can be used to estimate the body temperature of a single life stage in a particular microclimate (Levy et al. 2015). However, to identify T. mauritanica’s constraints and improve the results obtained here by the correlative models, body temperature predictions from biophysical models should ideally be combined with data on the temperature dependence of development, sex, activity, growth, survival, or reproduction.
Overall, globalisation is increasing the frequency of translocating species from their native ranges to novel transmarine environments. Over the last 30 years, the native Mediterranean common wall gecko, Tarentola mauritanica, has been introduced across several tropical and subtropical regions of the globe, where it has found novel environmental conditions. Our results confirm that the realised niche occupied by these introduced populations is significantly different from their native ones, highlighting the capacity of this species to cope with novel ecosystems, which is key to successful colonisation. Over the next 60 years, the geographic range of this gecko is likely to shift, benefiting from global climate change. The lack of knowledge on the effects it might have on local species and ecosystems reinforces the need for strong and serious monitoring actions.
Data availability
The datasets generated and/or analysed, and R scripts used during the current study are available in the Figshare repository [link will be made available upon acceptance of the manuscript].
References
Agarwal I, Ceríaco LMP, Metallinou M et al (2021) How the African house gecko (Hemidactylus mabouia) conquered the world. R Soc Open Sci 8:210749
Aiello-Lammens ME, Boria RA, Radosavljevic A et al (2015) spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38:541–545
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232
Anderson RP, Raza A (2010) The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J Biogeogr 37:1378–1393
Andrews RM (2015) Rigid shells enhance survival of gekkotan eggs. J Exp Zool Part A Ecol Genet Physiol 323:607–615
Araújo MB, New M (2007) Ensemble forecasting of species distributions. Trends Ecol Evol 22:42–47
Araújo MB, Peterson AT (2012) Uses and misuses of bioclimatic envelope modeling. Ecology 93:1527–1539
Arredondo C, Núñez H (2014) Tarentola mauritanica (Linnaeus, 1758), a new species of lizard for Chile (Reptilia, Phyllodactylidae). Bol Museo Nacl Hist Nat Chile 63:73–76
Atwater DZ, Ervine C, Barney JN (2018) Climatic niche shifts are common in introduced plants. Nat Ecol Evol 2:34–43
Báez M, Biscoito M (1993) First record of Tarentola mauritanica (Linneus, 1758) from the island of Madeira (NE Atlantic). In: First symposium of fauna and flora of the Atlantic islands. Funchal, Madeira
Baldo D, Borteiro C, Brusquetti F et al (2008) Reptilia, Gekkonidae, Hemidactylus mabouia, Tarentola mauritanica: distribution extension and anthropogenic dispersal. Check List 4:434–438
Barbet-Massin M, Jiguet F, Albert CH et al (2012) Selecting pseudo‐absences for species distribution models: how, where and how many? Methods Ecol Evol 3:327–338
Barbet-Massin M, Rome Q, Villemant C et al (2018) Can species distribution models really predict the expansion of invasive species? PLoS ONE 13:e0193085
Barreiros JP, Elias RB, Lourenço J et al (2010) First record of Tarentola mauritanica (Linnaeus, 1758) (Reptilia; Gekkonidae) in the Azores. Arquipélago Life Mar Sci 27:73–75
Barve N, Barve V, Jiménez-Valverde A et al (2011) The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Model 222:1810–1819
Beissinger SR, Riddell EA (2021) Why are species’ traits weak predictors of range shifts? Annu Rev Ecol Evol Syst 52:47–66
Bradshaw WE, Holzapfel CM (2006) Evolutionary response to rapid climate change. Science 312:1477–1478
Broennimann O, Treier UA, Müller-Schärer H et al (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709
Butchart SH, Walpole M, Collen B et al (2010) Global biodiversity: indicators of recent declines. Science 328:1164–1168
Capinha C, Seebens H, Cassey P et al (2017) Diversity, biogeography and the global flows of alien amphibians and reptiles. Divers Distrib 23:1313–1322
Carranza S, Arnold EN, Mateo JA et al (2000) Long-distance colonization and radiation in gekkonid lizards, Tarentola (Reptilia: Gekkonidae), revealed by mitochondrial DNA sequences. Proc R Soc Lond B 267:637–649
Cobos ME, Peterson AT, Barve N et al (2019) Kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ 7:e6281
Cobos M, Osorio-Olvera L, Soberón J et al (2020) Ellipsenm: ecological niche’s characterizations using ellipsoids. R package
Colwell RK, Rangel TF (2009) Hutchinson’s duality: the once and future niche. Proc Nat Acad Sci 106:19651–19658
Deso G, Renet J, Gomez MC et al (2020) Documenting the introduction of the moorish gecko Tarentola Mauritanica (Linnaeus, 1758)(Squamata: Phyllodactylidae) on the Levant and Port-Cros Islands (Hyères Archipelago, Var department, France). Herpetol Notes 13:809–812
Díaz-Fernández L, Paz A, Valdecantos S (2019) First checked arrival of Tarentola mauritanica (Linnaeus, 1758) in Salta, Argentina (Squamata; Phyllodactylidae). Herpetol Notes 12:853–854
Early R, Sax DF (2014) Climatic niche shifts between species’ native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Glob Ecol Biogeogr 23:1356–1365
Engler R, Guisan A (2009) MigClim: predicting plant distribution and dispersal in a changing climate. Divers Distrib 15:590–601
Engler R, Hordijk W, Guisan A (2012) The MIGCLIM R package–seamless integration of dispersal constraints into projections of species distribution models. Ecography 35:872–878
Esler KJ, Jacobsen AL, Pratt RB (2018) The biology of Mediterranean-type ecosystems. Oxford University Press, Oxford
Fernández M, Hamilton H (2015) Ecological niche transferability using invasive species as a case study. PLoS ONE 10:e0119891
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49
Fitzpatrick MC, Weltzin JF, Sanders NJ et al (2007) The biogeography of prediction error: why does the introduced range of the fire ant over-predict its native range? Glob Ecol Biogeogr 16:24–33
Gallien L, Douzet R, Pratte S et al (2012) Invasive species distribution models–how violating the equilibrium assumption can create new insights. Glob Ecol Biogeogr 21:1126–1136
Garcia RA, Burgess ND, Cabeza M et al (2012) Exploring consensus in 21st century projections of climatically suitable areas for African vertebrates. Glob Change Biol 18:1253–1269
García-Muñoz E, Carretero MA (2013) Comparative ecophysiology of two sympatric lizards. Laying the groundwork for mechanistic distribution models. Acta Herpetol 8:123–128
Geniez P, Cheylan M (2012) Les Amphibiens et les reptiles du Languedoc-Roussillon et régions limitrophes-Atlas biogéographique. Biotope, Mèze
Giljohann KM, Hauser CE, Williams NS et al (2011) Optimizing invasive species control across space: willow invasion management in the Australian Alps. J Appl Ecol 48:1286–1294
Guisan A, Tingley R, Baumgartner JB et al (2013) Predicting species distributions for conservation decisions. Ecol Lett 16:1424–1435
Guisan A, Petitpierre B, Broennimann O et al (2014) Unifying niche shift studies: insights from biological invasions. Trends Ecol Evol 29:260–269
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474
Hellmann JJ, Byers JE, Bierwagen BG et al (2008) Five potential consequences of climate change for invasive species. Conserv Biol 22:534–543
Helmuth B, Kingsolver JG, Carrington E (2005) Biophysics, physiological ecology, and climate change: Does mechanism matter? Annu Rev Physiol 67:177–201
Hijmans R (2022) raster: geographic data analysis and modeling. R package version 3.6–11. https://CRAN.R-project.org/package=raster
Hijmans R, Ghosh A, Mandel A (2022) _geodata: Download Geographic Data_. R package version 0.4–13, https://CRAN.R-project.org/package=geodata
Huerta-Vera S (2016) Registros de Gecko Mediterráneo, Tarentola mauritanica (Linnaeus 1758) (Squamata, Phyllodactylidae), en zona semi-urbana de Peñalolén, Región Metropolitana. Bol Chileno Herpetol 3:24–25
Huey RB, Kearney MR, Krockenberger A et al (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos Trans R Soc B Biol Sci 367:1665–1679
Jesus J, Lemos A, Gonçalves R et al (2008) First record of Tarentola mauritanica (Linnaeus, 1758) on Porto Santo Island. Herpetozoa 20:175–177
Keith DA, Akçakaya HR, Thuiller W et al (2008) Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biol Lett 4:560–563
Kramer AM, Annis G, Wittmann ME et al (2017) Suitability of Laurentian Great Lakes for invasive species based on global species distribution models and local habitat. Ecosphere 8:e01883
Kueffer C, Pyšek P, Richardson DM (2013) Integrative invasion science: model systems, multi-site studies, focused meta‐analysis and invasion syndromes. New Phytol 200:615–633
Lauzeral C, Leprieur F, Beauchard O et al (2011) Identifying climatic niche shifts using coarse-grained occurrence data: a test with non‐native freshwater fish. Glob Ecol Biogeogr 20:407–414
Levy O, Buckley LB, Keitt TH et al (2015) Resolving the life cycle alters expected impacts of climate change. Proc R Soc B Biol Sci 282:20150837
Li Y, Liu X, Li X et al (2014) Residence time, expansion toward the equator in the invaded range and native range size matter to climatic niche shifts in non-native species. Glob Ecol Biogeogr 23:1094–1104
Li X, Liu X, Kraus F et al (2016) Risk of biological invasions is concentrated in biodiversity hotspots. Front Ecol Environ 14:411–417
Lionello P, Malanotte-Rizzoli P, Boscolo R et al (2006) The Mediterranean climate: an overview of the main characteristics and issues. Elsevier, Amsterdam, pp 1–26
Lisičić D, Drakulić S, Herrel A et al (2012) Effect of competition on habitat utilization in two temperate climate gecko species. Ecol Res 27:551–560
Locey KJ, Stone PA (2006) Factors affecting range expansion in the introduced Mediterranean Gecko, Hemidactylus turcicus. J Herpetol 40:526–530
Mačát Z, Starcová M, Červenka J et al (2014) A molecular assessment and first record of Tarentola mauritanica (Squamata: Phyllodactylidae) on Corfu, Greece. Salamandra 50:172–176
Mammola S (2019) Assessing similarity of n-dimensional hypervolumes: Which metric to use? J Biogeogr 46:2012–2023
Martínez-Rica JP (1974) Contribución al estudio de la biología de los gecónidos ibéricos (Rept. Sauria). Publicaciones del Centro Pirenaico de Biología Experimental, CSIC 5:7–293.
Martins B, Silva-Rocha I, Rocha R et al (2022) Trophic interactions of an invasive gecko in an endemic-rich oceanic island: insights using DNA metabarcoding. Front Ecol Evol 10:1044230
Medley KA (2010) Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob Ecol Biogeogr 19:122–133
Mizerakis V, Strachinis I (2017) New record of Tarentola mauritanica (Squamata: Phyllodactylidae) from Lesvos island, Greece. Herpetol Notes 10:157–159
Mizsei E, Jablonski D, Végvári Z et al (2017) Distribution and diversity of reptiles in Albania: a novel database from a Mediterranean hotspot. Amphibia-Reptilia. https://doi.org/10.1163/15685381-00003097
Moran EV, Alexander JM (2014) Evolutionary responses to global change: lessons from invasive species. Ecol Lett 17:637–649
Moreno-Rueda G, Pleguezuelos JM, Pizarro M et al (2011) Northward shifts of the distributions of Spanish reptiles in association with climate change. Conserv Biol 26:278–286
Naimi B, Hamm NAS, Groen TA et al (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37:191–203
Nuñez-Penichet C, Cobos ME, Soberon J (2021) Non-overlapping climatic niches and biogeographic barriers explain disjunct distributions of continental Urania moths. Front Biogeogr 13:e52142
Ortiz-Medina JA, Cabrera-Cen DI, Chan-Noh MM et al (2019) First record of the Moorish Gecko, Tarentola mauritanica (Linnaeus, 1758) (Squamata: Phyllodactylidae), in Mexico. Herpetol Notes 12:971–974
Osojnik N, Žagar A, Carretero MA et al (2013) Ecophysiological dissimilarities of two sympatric lizards. Herpetologica 69:445–454
Parravicini V, Azzurro E, Kulbicki M et al (2015) Niche shift can impair the ability to predict invasion risk in the marine realm: an illustration using Mediterranean fish invaders. Ecol Lett 18:246–253
Pauls SU, Nowak C, Bálint M et al (2013) The impact of global climate change on genetic diversity within populations and species. Mol Ecol 22:925–946
Pearman PB, Guisan A, Broennimann O et al (2008) Niche dynamics in space and time. Trends Ecol Evol 23:149–158
Perella CD, Behm JE (2020) Understanding the spread and impact of exotic geckos in the greater Caribbean region. Biodivers Conserv 29:1109–1134
Peterson AT (2011) Ecological niche Conservatism: a time-structured review of evidence. J Biogeogr 38:817–827
Petitpierre B, Kueffer C, Broennimann O et al (2012) Climatic niche shifts are rare among terrestrial plant invaders. Science 335:1344–1348
Phillips SJ, Dudík M, Elith J et al (2009) Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl 19:181–197
Pike DA, Andrews RM, Du W-G (2012) Eggshell morphology and gekkotan life-history evolution. Evol Ecol 26:847–861
Qiao H, Peterson AT, Campbell LP et al (2016) NicheA: creating virtual species and ecological niches in multivariate environmental scenarios. Ecography 39:805–813
Rato C, Carretero MA (2015) Ecophysiology tracks phylogeny and meets ecological models in an Iberian gecko. Physiol Biochem Zool 88:564–575
Rato C, Harris DJ, Perera A et al (2015) A combination of divergence and conservatism in the niche evolution of the moorish gecko, Tarentola mauritanica (Gekkota: Phyllodactylidae). PLoS ONE 10:e0127980–e0127980
Rato C, Resendes R, Tristão da Cunha R et al (2015) First record of Tarentola substituta Joger, 1984, and genetic identification of Tarentola mauritanica (Linnaeus, 1758) in the Azores. Herpetozoa 27:182–187
Rato C, Harris DJ, Carranza S et al (2016) The taxonomy of the Tarentola mauritanica species complex (Gekkota: Phyllodactylidae): bayesian species delimitation supports six candidate species. Mol Phylogenet Evol 94:271–278
Rato C, Marques V, Paracuellos M et al (2021) Alborán Island, a small meeting point for three invasive lizards, whose geographic origin is uncovered by molecular analysis. BioInvasions Rec 10:977–990
Rato C, Sillero N, Ceacero F et al (2021) A survival story: evolutionary history of the Iberian algyroides (Squamata: Lacertidae), an endemic lizard relict. Biodivers Conserv. https://doi.org/10.1007/s10531-021-02217-4
Richardson DM, Pyšek P (2008) Fifty years of invasion ecology–the legacy of Charles Elton. Wiley Online Library, Hoboken, pp 161–168
Rocha S, Trinks A, Harris DJ et al (2022) The global and western Indian Ocean dispersal of house geckos from Asia using historical and mitochondrial DNA perspectives. Front Ecol Evol 10:791762
Rödder D, Lötters S (2009) Niche shift versus niche Conservatism? Climatic characteristics of the native and invasive ranges of the Mediterranean house gecko (Hemidactylus turcicus). Glob Ecol Biogeogr 18:674–687
Sarkar P (2000) A brief history of cellular automata. ACM Comput Surv (CSUR) 32:80–107
Sax DF, Stachowicz JJ, Brown JH et al (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471
Sayre R, Karagulle D, Frye C et al (2020) An assessment of the representation of ecosystems in global protected areas using new maps of World Climate regions and World ecosystems. Global Ecol Conserv 21:e00860
Shabani F, Kumar L, Ahmadi M (2018) Assessing accuracy methods of species distribution models: AUC, specificity, sensitivity and the true skill statistic. Glob J Hum Soc Sci 18:6–18
Sillero N (2011) What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol Model 222:1343–1346
Sillero N, Barbosa AM (2021) Common mistakes in ecological niche models. Int J Geograph Inform Sci. https://doi.org/10.1080/13658816.2020.1798968
Sillero N, Arenas-Castro S, Enriquez-Urzelai U et al (2021) Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol Model 456:109671
Silva-Rocha I, Santos JM, Rocha R et al (2022) Bioclimatic and local drivers modulating the expansion of an introduced temperate reptile in a subtropical island. Global Ecol Conserv 37:e02164
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers Inf 2:1–10
Somaweera R (2009) Reproductive ecology of the Kandyan Day Gecko, Cnemaspis kandiana, in Gannoruwa Forest Reserve. J Natl Sci Found Sri Lanka 37:13–22
Srivastava V, Lafond V, Griess VC (2019) Species distribution models (SDM): applications, benefits and challenges in invasive species management. CABI Rev 14:1–13
Strachinis I, Artavanis D (2017) Additions to the known herpetofauna of the island of Ithaki, Ionian Sea, Greece. Herpetozoa 30(1/2):64–66
Strachinis I, Pafilis P (2018) First record of Tarentola mauritanica (Linnaeus, 1758), from Athens, Greece. Herpetozoa 31(1/2):98–99
Strubbe D, Broennimann O, Chiron F et al (2013) Niche conservatism in non-native birds in Europe: niche unfilling rather than niche expansion. Glob Ecol Biogeogr 22:962–970
Sumner GN, Romero R, Homar V et al (2003) An estimate of the effects of climate change on the rainfall of Mediterranean Spain by the late twenty first century. Clim Dyn 20:789–805
Thuiller W, Lavorel S, Araújo MB (2005) Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob Ecol Biogeogr 14:347–357
Thuiller W, Lafourcade B, Engler R et al (2009) BIOMOD–a platform for ensemble forecasting of species distributions. Ecography 32:369–373
Tingley R, García-Díaz P, Arantes CRR et al (2018) Integrating transport pressure data and species distribution models to estimate invasion risk for alien stowaways. Ecography 41:635–646
Tulloch AI, Tulloch VJ, Evans MC et al (2014) The value of using feasibility models in systematic conservation planning to predict landholder management uptake. Conserv Biol 28:1462–1473
Urban MC, Bocedi G, Hendry AP et al (2016) Improving the forecast for biodiversity under climate change. Science 353:aad8466
Václavík T, Meentemeyer RK (2012) Equilibrium or not? Modelling potential distribution of invasive species in different stages of invasion. Divers Distrib 18:73–83
Valverde JA (1967) Estructura de una comunidad mediterránea de vertebrados terrestres. Monografías de Ciencias Moderna, 76. CSIC, Madrid
Van Aelst S, Rousseeuw P (2009) Minimum volume ellipsoid. WIRE Comput Stat 1:71–82
Vogrin M, Corti C, Mellado VP et al (2017) Tarentola mauritanica. The IUCN red list of threatened species 2017: e.T61578A63716927. https://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T61578A63716927.en. Accessed 23 Feb 2021
Weterings R, Vetter KC (2018) Invasive house geckos (Hemidactylus spp.): their current, potential and future distribution. Curr Zool 64:559–573
Weterings R, Barbetti M, Buckley HL (2019) Hypothesis: Do invasive house geckos exacerbate dengue Fever epidemics? Biol Invasions 21:3533–3543
Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Ann Rev Ecol Evol Syst 36:519–539
Wilson JR, Dormontt EE, Prentis PJ et al (2009) Something in the way you move: dispersal pathways affect invasion success. Trends Ecol Evol 24:136–144
Zhu G, Gutierrez Illan J, Looney C et al (2020) Assessing the ecological niche and invasion potential of the Asian giant hornet. Proc Natl Acad Sci 117:24646–24648
Funding
Open access funding provided by FCT|FCCN (b-on). CR is supported by a postdoctoral contract from Fundação para a Ciência e Tecnologia (FCT), Portugal (DL57/2016/CP1440/CT0005). ISR was supported by FCT through a post-doc grant within project PTDC/BIA-EVL/27958/2017.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Catarina Rato and Iolanda Silva-Rocha. The first draft of the manuscript was written by Catarina Rato and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rato, C., Silva-Rocha, I. & Sillero, N. What does the future hold for a thermophilic and widely introduced gecko, Tarentola mauritanica (Squamata: Phyllodactylidae)?. Biol Invasions 26, 1061–1074 (2024). https://doi.org/10.1007/s10530-023-03229-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03229-7