Abstract
Temperature is one of the most important factors governing the activity of ectothermic species, and it plays an important but less studied role in the manifestation of invasive species impacts. In this study, we investigated temperature-specific feeding and metabolic rates of invasive and native crayfish, and evaluated how temperature regulates their ecological impacts at present and in future according to different climatic scenarios by bioenergetics modelling. We conducted a series of maximum food consumption experiments and measured the metabolic rates of cold-adapted native noble crayfish (Astacus astacus) and invasive signal crayfish (Pacifastacus leniusculus) originally from a warmer environment over a temperature gradient resembling natural temperatures in Finland. The maximum feeding rates and routine metabolic rates (RMR) of native noble crayfish were significantly higher at low temperatures (< 10 °C than the rates of invasive signal crayfish. The RMRs of the species crossed at 18 °C, and the RMRs of signal crayfish were higher at temperatures above 18 °C. These findings indicate that the invader’s thermal niche has remained stable, and the potential impacts per capita are lower at suboptimal cold temperatures than for the native species. Our bioenergetics modelling showed that the direct annual predation impact of noble and signal crayfish seem similar, although the seasonal dynamics of the predation differs considerably between species. Our results highlight that the temperature-specific metabolic and feeding rates of species need to be taken into account in the impact assessment instead of simple generalisations of the direction or magnitude of impacts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasion by non-native species is one of the main threats to global biodiversity and the function of ecosystems (Sala et al. 2000). Quantifying the impacts of invasive species and the recognition of the most harmful species are essential for effective invasive species management (e.g. Simberloff et al. 2013). However, species invasions are complex phenomena that depend on species-specific characteristics, the receiving community, and the introduction pathways (Richardson and Pysek 2012). A growing body of literature has shown that the recognition and assessment of ecological impacts can be a complex task (Simberloff et al. 2013). A more comprehensive understanding of invasive species’ impacts and the factors influencing them will improve management, because actions can then be targeted at the most harmful species and most vulnerable ecosystems (Strayer 2012; Lowry et al. 2013; Ricciardi et al. 2013).
Predicting invasions of non-native species in new areas and estimating their impacts are timely, because climate change is likely to increase species invasions (Strayer 2012; Lowry et al. 2013; Ricciardi et al. 2013). Models that assume niche conservatism—the tendency of species to maintain an ancestral ecological trait over time and space—have been widely used to predict the geographical distribution of invasive species. Niche conservatism is relevant to various traits from those that determine the species abiotic niche (e.g. thermal limits) and those that determine the species’ resource use (e.g. diet) (Wiens et al. 2010). However, the concept of niche conservatism is currently debated in invasion ecology (Wiens et al. 2010; Comte et al. 2017). This is because many successful invaders adapt to a wide range of habitats (Kolar and Lodge 2001), which may indicate weak niche conservatism in ecological traits. It may be that species are conservative regarding some ecological traits (e.g. thermal requirements) and more flexible for others (e.g. habitat or food source use). This would allow a species to occur in a certain region, but its impacts will be regulated by a suboptimal position at the environmental gradient (e.g. temperature range) (Ricciardi et al. 2013). This habitat filtering or environmental matching has been shown to limit the impacts of invasive plants by decreasing performance or population size, but wider evidence of the effects remains scarce (Ricciardi et al. 2013), which may increase uncertainty in impact predictions.
Invasive species are often shown to be more efficient in resource use than native counterparts (McCarthy et al. 2006; Twardochleb et al. 2013), which explains the severe declines or extinctions of native prey species directly by predation or indirectly via resource competition (Simberloff et al. 2013). However, these species-specific traits may vary in environmental conditions (Iacarella et al. 2015), potentially influencing the manifestation of impacts. Recent studies and meta-analyses have indicated that the ecological impacts of certain invasive species can vary across space, leading to the question of which factor or factors regulate the impacts (Ricciardi et al. 2013; Ercoli et al. 2015; Evangelista et al. 2019). For example, Ercoli et al. (2015) concluded that there is no clear evidence that the invasive signal crayfish is a more voracious predator than the native crayfish species in northern latitudes with a colder climate than in the native range of invasive signal crayfish.
Temperature is regarded as one of the most important abiotic factors that govern the impacts of ectothermic invasive species, because it regulates overall resource use (Velde et al. 2009; Kelley 2014), but species’ behavioural traits and resource use efficiency play important roles (Alexander et al. 2014; Iacarella et al. 2015; Veselý et al. 2019). Several recent studies have shown that certain invasive species are more efficient predators than natives (Dick et al. 2013; Rosewarne et al. 2016), and superiority may be explained by differences in metabolic rates (Taylor and Dunn 2018). However, laboratory studies are often conducted at species’ optimal temperature ranges (but see Iacarella et al. 2015), which can lead to false conclusions and overgeneralisation of results, because invasive species often populate semi-optimal environments with varying conditions (Ricciardi et al. 2013).
Bioenergetics models provide an opportunity to quantify the food consumption of an organism in variable environmental conditions and obtain data-driven estimates of individual- and population-level energy and prey requirements and interactions (Kitchell et al. 1977; Karjalainen et al. 2015; McHuron et al. 2020). A bioenergetics model applies an energy balance equation which balances total mass, waste loss, and growth by mass- and temperature-specific physiological functions or coefficients (Hanson et al. 1997). These models are especially powerful tools for conducting if-else modelling, and bioenergetics approaches have been applied in numerous ways to predict the impact of climate change on organisms, populations, and ecosystems (Humphries et al. 2004; Matzelle et al. 2015; Jørgensen et al. 2016).
Our main question in the study concerns how temperature regulates the physiology and feeding of ectothermic invasive species, and whether the manifestation of the ecological impacts is linked to species-specific thermal and metabolic characteristics. To answer these questions, we conducted laboratory experiments and bioenergetics modelling with invasive signal crayfish (Pacifastacus leniusculus) and native noble crayfish (Astacus astacus) inhabiting the same geographical regions in Finland (Ruokonen et al. 2018). Our study species have been considered ecologically and functionally redundant (Westman et al. 2002), but they presumably differ in thermal requirements and tolerances, given that signal crayfish originates from the west coast of North America which has different annual temperatures than in Finland (Nyström 2002; Heinimaa and Pursiainen 2008). The optimal and lethal temperatures of signal crayfish have been shown to be higher than noble crayfish (Becker et al. 1975; Rutledge and Pritchard 1981; Rognerud et al. 1988; Firkins and Holdich 1993; Nakata et al. 2002; Nyström 2002), and empirical evidence indicates that low autumn and winter temperatures limit signal crayfish spread to northern latitudes in Finland (Heinimaa and Pursiainen 2008).
We studied maximum food consumption as a proxy for the direct impacts and metabolic rates of invasive and native crayfish species at a biologically relevant temperature gradient in Finland.
We also used bioenergetics modelling to evaluate how the direct impacts of study species varied between seasons, and how species-specific thermal characteristics influenced the impacts in a future climate scenario. We assumed that the thermal niche of invasive signal crayfish had remained stable and hypothesised that the thermal niche still reflected their original physiological characteristics with low metabolic and maximum consumption rates at low water temperatures. On the contrary, we expected that the noble crayfish was also adapted to forage actively at temperatures below 10 °C, because the water temperature in their habitats in Northern Europe can be less than 10 °C for seven or eight months (Korhonen 2002). We suggest that thermal niche conservatism is an important component that determines the impacts of ectothermic invasive species and can thus explain the variation in the impacts across a species’ distribution range (Ercoli et al. 2015).
Material and methods
The noble crayfish used in the laboratory experiments and respirometry were captured with baited traps (ca. 50–70 g of fresh fish flesh, Rutilus rutilus) from lakes Palvajärvi (62° 13′ 56; 26° 0′ 44) and Vesantojärvi (62° 53′ 44; 26° 28′ 48). Signal crayfish were collected from established populations in the crayfish ponds of the Laukaa aquaculture station of the Natural Resources Institute Finland (62° 28′ 10; 25° 52′ 42) and trapped from lakes Päijänne (62° 4′ 37; 25° 30′ 58) and Vesantojärvi (62° 53′ 44; 26° 28′ 48) and transported to the experimental unit of the University of Jyväskylä. All crayfish capture sites were located in the southern boreal region with similar general annual temperatures. Crayfish were acclimated to the laboratory conditions in flow-through, 20l plastic containers for 1–4 weeks before the experiments commenced. During the acclimation to experimental temperatures, crayfish were reared at 2–18 °C in similar light conditions (12:12 h light–dark cycle) and fed with raw sliced potatoes, fish, and commercial fish feed.
Maximum food consumption rate
The maximum food consumption rate (CMAX, g wet mass−1 24 h−1) of noble crayfish and signal crayfish was determined from 2 to 22 °C in 22 separate experiments in 2010–2016 (Table 1). Before the CMAX experiments, each crayfish individual was kept without food in a separate rearing pool for 4 days and acclimatised to the experimental temperature. After the acclimation period, a crayfish was placed in an experimental arena and offered fish eggs (offered total mass 1.5–3.3 g), chironomid larvae (offered total mass 2.5–12.0 g), and/or pea mussels (offered total mass 4.1 g) (Table 1) placed randomly on the bottom of the arena (plastic arena, height 15 cm x length 75 cm × width 55 cm, area = 0⋅4125 m2). The crayfish were allowed to feed ad libitum on the food sources in the dark, and the uneaten items were counted after the 12-h feeding period. The mass of consumed food was then calculated using the average wet mass (g) of pre-weighted food items (Chironomids: average mass 46.0 mg, SD ± 3.2; vendace eggs: average mass 2.2 mg, SD ± 0.09; whitefish eggs: average mass 12.1 mg SD ± 0.2; trout eggs: average mass 109.0 mg, SD ± 3.2; pea mussels: average mass 58.5 mg, SD ± 24.0). The extra moisture on the surface of food items was removed by turning the item onto moist towel paper before weighing. The exponential temperature functions CMAX = CA × e (CB × temperature) were used for the bioenergetics models of both crayfish. The mass parameter was excluded, because within the size range of crayfish (Table 1) which we used in the experiment, we did not observe any statistically significant effect.
Metabolic rates
Mass- and temperature-specific metabolic rate functions were estimated from the oxygen consumption of unfed noble (27.9–40.8 g wet mass) and signal crayfish (14.2–87.1 g wet mass) measured in an intermittent-flow respirometer connected to a fibre-optic oxygen sensor (Loligo Systems 4-channel OXY-4 sensors). Oxygen consumption was measured at temperature ranges from 4.3 to 29.4 °C and 3.6 to 29.1 °C for noble and signal crayfish respectively. When the crayfish were acclimatised to each experimental temperature, they were kept in the container, where the water temperature was changed at a maximum rate of 2 °C day−1 to achieve the experimental temperature in the respirometer. Before respirometry, the crayfish were kept without food for 48 h.
After acclimation, four crayfish were placed individually in random order in separate acrylic chambers (volume 451–2237 ml, depending on the size of the crayfish) in which oxygen consumption was measured for 15 min with a 9-min flush and waiting period over a 24-h period, resulting in a total of 60 observations per individual. The order of the different temperatures changed between different groups of crayfish. The routine rate of oxygen consumption (RMR) was estimated by the average of all observations, excluding the first 3-h measurements after placing the crayfish in the chamber to avoid the effect of handling the stress of crayfish in the RMR. The RMR is thus the average rate of metabolism during a 24-h period that includes the spontaneous activity of crayfish but does not include feeding-induced thermogenesis (Keskinen et al 2008). In the bioenergetics model, the feeding induced thermogenesis is modelled by the specific dynamic action (SDA) coefficient (Kitchell et al. 1977; Karjalainen et al. 2015).The standard rate (SMR) was based on the mean value of the three lowest oxygen consumption values during the 24-h measurement period. The SMR is an estimate of a minimum metabolic rate which excludes all activity- and feeding-induced thermogenesis. To exclude bacterial oxygen consumption, the oxygen consumption of empty chambers was measured before and after each crayfish measurement. The chambers were kept in the dark during the entire measurement period.
For the bioenergetics model, the parameters for the RMR functions (gO2 g−124 h−1) were applied from respiratory experiments. The equation (Kitchell et al. 1977) used was:
where RA is the intercept of the allometric mass function, W is crayfish wet mass (g), RB is the slope of the allometric mass function, and f(T) is the temperature dependence function. The temperature dependence function was
where
e = Euler’s number, constant,
where
RQ = Q10 rate.
RTO = optimum temperature of metabolia.
RTM = lethal temperature.
The RTM for signal crayfish was adopted from (Becker et al. 1975) and (Rutledge and Pritchard 1981). No lethal temperature estimate is available in the literature for noble crayfish, but (Rognerud et al. 1988) stated that “experiences of no extra mortality at 28 °C is known”. We tested different RTM combinations using R2 as selection criterion and found 31 °C to be the best RTM for noble crayfish. Non-linear regression fitting was performed by the IBM SPSS statistics v. 24 software.
Statistical analyses
Differences in the food consumption (CMAX) rate and the metabolic rate (routine rate RMR and standard rate SMR) between the species were analysed with the General Linear Model (GLM, IBM SPSS statistics version 24 software). Species was set as a fixed factor, and the experimental temperature was included as a covariate. The analysis was first performed with both temperature and mass of crayfish as covariates, but because the mass of crayfish was not significant (p > 0.25), the mass was excluded in the final analysis of CMAX, RMR, and SMR. CMAX, RMR, and SMR were LN-transformed before analyses.
Ecological impacts
The ecological impacts of both crayfish were evaluated by estimating their annual food consumption by the Wisconsin-type bioenergetics model (Kitchell et al. 1977). We applied the model construction of Karjalainen et al. (2015), but we used the CMAX and RMR functions fitted in this study (Fig. 1, Table 2). No separate activity coefficient was used in the model, because we used the RMR, which was assumed to correspond to the routine activity in the field (activity coefficient = 1, Karjalainen et al. 2015). The CMAX and RMR functions were species-specific, but all other model parameters were the same for both species. The excretion coefficient (0.18), SDA coefficient (0.01961), and egestion coefficients for each food category was according to Karjalainen et al. (2015). The egestion coefficients were 0.08 for fish eggs, fish carcass, and benthic invertebrates, 0.86 for terrestrial detritus, and 0.59 for aquatic plants. The energetic content of crayfish, fish eggs, fish carcass, benthic invertebrates, terrestrial detritus, and aquatic plants was 3684, 7262, 6380, 3349, 10,571 and 1407 J g−1 wet mass respectively. The composition of the food in the model was according to Karjalainen et al. (2015), except the fish eggs were assumed to be available only during the period from the middle of November to the end of April, when the proportions of food were 6.3, 5.4, 27.0, 43.2, and 18.1% for fish eggs, fish carcasses, benthic invertebrates, terrestrial detritus, and aquatic plants respectively. During the rest of the year, they were 8.4, 30.3, 43.2, and 18.1% for fish carcasses, benthic invertebrates, terrestrial detritus, and aquatic plants.
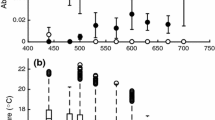
Maximum food consumption rate (CMAX, g wet mass g wet body mass −1 24 h−1) of noble (solid black line, open circle, exponential function CMAX = 0.029 ± 0.033 e0.076±0.008*temp, R2 = 0.433, n = 9, standard error of parameter given after ±), and signal (dashed black line, black circle, CMAX = 0.007 ± 0.002 e0.143±0.030*temp, R2 = 0.726, n = 12) crayfish in relation to the water temperature (temp) measured in the food consumption experiments. The grey curves are the same RMR curves as in Fig. 2
We estimated the food consumption of both species assuming that the wet body mass of individual crayfish was 37 and 55 g at the beginning and end of the simulation period, which lasted 365 days starting on 1 January and ending on 31 December. The change in mass was based on the information from Westman and Savolainen (2002). Three different annual daily water temperature curves (past, present, and future) from the littoral zone of two Finnish lakes were used: the mean daily temperature for 1971–2000 in Lake Päijänne (62° 3′ 49″ N, 25° 45′ 50″ E, Forsius et al. 2013), the mean daily temperature in 2019 in Lake Southern Konnevesi (5-day moving average of our own unpublished measurements with temperature loggers in the littoral of Kotilahti and Pitkälahti) and in the future scenario for 2070–2099 (emission scenario A2 according to IPCC (2007), Forsius et al. 2013) adjusted for Lake S Konnevesi (62° 35′ 08″ N, 26° 35′ 32″ E). A scenario adjustment for Lake S Konnevesi was made by adding the difference in daily temperatures between the Lake Päijänne past baseline (1971–2000) and A2 scenario temperatures from Forsius et al. (2013) to the daily temperature of Lake S Konnevesi in 2019. The used future scenario A2 is a high emission “alarm signal” scenario which predicts considerable changes in the climate, especially in the boreal region (IPCC 2007).
Our deterministic bioenergetics model produced the annual food consumption estimates of both species without an uncertainty analysis of parameter variability. We carried out simple one parameter sensitivity analyses for RQ parameters in our noble and signal crayfish models using both low and high 95% confidence limits of the RQ estimated from our experimental data. The RQ parameter determines the temperature dependence of the RMR in our models (see Table 2).
Results
The CMAX of the noble and signal crayfish differed statistically significantly between species (GLM, F1, 21 = 8.756, p = 0.008), with the statistically significant temperature covariate (F = 10.480, p < 0.001). At low temperatures, the CMAX of noble crayfish was higher than the CMAX of signal crayfish. The fitted CMAX functions crossed at 18 °C and predicted a higher CMAX of signal crayfish at higher temperatures (Fig. 1).
The RMR of the noble and signal crayfish differed between species (GLM, F1, 113 = 19.385, p < 0.001) with the statistically significant temperature covariate (F = 242.52, p < 0.001). On the contrary, the SMR of these two species did not differ between species (GLM, F1, 113 = 1.138, p = 0.288), but the temperature covariate was statistically significant (F = 361.308, p < 0.001). The RMR of noble crayfish was higher at low temperatures. At temperatures above 20 °C, the RMR of signal crayfish was higher than the RMR of noble crayfish (Fig. 2A, Table 2). The RMR functions of medium-weight (35 g) crayfish crossed at 18 °C (Fig. 2A). The SMR diverged between species at high temperatures only, and the SMR functions crossed at 21 °C (Fig. 2B). The large variation in individual MRs in Fig. 2 (a circle represents the metabolic rate of an individual crayfish) is partly based on the variation in crayfish size.
(A) The routine (RMR gO2 g−1 24 h−1) and (B) standard metabolic rate (SMR gO2 g−1 24 h−1) of noble (solid line, open circle, n = 40) and signal (dashed line, black circle, n = 74) crayfish measured by the intermittent respirometer at different temperatures. The curves of the RMR are non-linear regression fittings, based on mass-specific RMRs of medium-weight (35 g) crayfish from our own measurements and temperature limits from the literature (see Table 2). In plot B, the grey curves are the same RMR curves as in plot A. Exponential functions are SMRnoble = 0.0003e0.049x (R2 = 0.435, n = 40) and SMRsignal = 0.0001e0.089x (R2 = 0.917, n = 74).
In the simulation, in which an individual crayfish of both species was set to grow from a size of 37 g to a size of 55 g, the total annual food consumption of noble crayfish was 17% higher than the food consumption of signal crayfish in the littoral temperature of Lake S Konnevesi in 2019 (Fig. 3A). During the cold periods from January to May and September to December, noble crayfish consumed considerably more food, but in July and August, the consumption of signal crayfish was higher (Fig. 3A and B). If the signal crayfish was allowed to consume the same amount of food (a simulation with a constant annual ration) as noble crayfish, the final wet body mass (73 g) was 33% higher than the final mass of noble crayfish (55 g).
(A) Total annual food consumption (J d−1) of an individual noble (solid line) and signal crayfish (dashed line) estimated by the bioenergetics model. The daily temperature in the littoral zone of Lake Southern Konnevesi in 2019 is represented by a dotted line. (B) The difference (% (Noble C-Signal C)/Noble C) in the monthly food consumption of noble and signal crayfish
Climate change scenarios from bioenergetics modelling showed that the total annual food consumption of both species increased from the past to the present and future scenarios (Fig. 4A, B). The total annual change in noble crayfish was 2% from past to present, and 8% from present to future, while the corresponding annual changes of signal crayfish were 4% from past to present, and 13% from present to future. Thus, according to our simulations based on the temperature-specific physiological differences between species, the predation impact of signal crayfish will approach the impact of noble crayfish in future scenarios, but the total annual food consumption of noble crayfish was still 12% higher than the total annual consumption of signal crayfish. However, our sensitivity analysis of RQ showed that a ± 18% change (lower and higher limits of 95% confidence limits) in the RQ parameter of the noble crayfish model caused a 12–19% change in the total annual food consumption of noble crayfish, and a ± 11% change in the RQ parameter of the signal crayfish model caused a 7–11% change in the total annual food consumption of noble crayfish (Table 3). A sensitivity analysis indicated that the differences in annual food consumption between species and scenarios were low compared to the variation caused by the parameter uncertainty in RQ alone (Table 3).
(A) Total annual cumulative food consumption (kJ d-1) of an individual noble (solid line) and signal crayfish (dashed line) estimated by the bioenergetics model for three different annual temperature developments. (B) Water temperatures in the littoral zone of two Finnish lakes: mean daily temperature for 1971–2000 in Lake Päijänne (past, blue dotted line), mean daily temperature in 2019 in Lake S Konnevesi (present, black dashed line), and in the future scenario for 2070–2099 (emission scenario A2, IPCC 2007) in Lake S Konnevesi (red solid line)
Discussion
Native noble crayfish and invasive signal crayfish had divergent temperature-specific metabolic and food consumption rates in our experiments. Noble crayfish, which are adapted to colder conditions, had higher routine metabolic rates (RMR) and consumed more food items at temperatures below 10 °C. Our results suggest that the RMR and maximum food consumption of invasive signal crayfish were higher at temperatures above 18 °C. However, food consumption and the RMR were quite similar between species in the middle of the experimental temperature gradient, indicating similar activity and per capita impacts on that temperature area. These results support our hypothesis that invaders’ performance and direct impacts at suboptimal temperatures are lower than those of native crayfish. The recent divergent empirical findings concerning the impacts of invasive species (e.g. Lagrue et al. 2014; Ercoli et al. 2015) could thus be at least partly explained by differences in their activity and metabolic rates, which leads to lower predation efficiency and net impacts in sub-optimal conditions. Temperature is among the most important determinants of the activity and function of ectothermic animals (Velde et al. 2009; Kelley 2014), but its role in the impact assessment of aquatic invasive species is not highlighted accordingly.
Aquatic ectotherms are often active during cold seasons in cold waters, but adaptation to cold and activity usually has costs due to physiological or behavioural adaptations (Pörtner 2002). Lozán (2000) studied the general activity of several crayfish species over a wide temperature range (4–20 °C). Signal crayfish were less active at low temperatures and more active at high temperatures than noble crayfish, which was in line with our results. In some earlier laboratory and field studies, the activity and feeding of signal crayfish in relation to temperature (e.g. Guan and Wiles 1998; Bubb et al. 2002). Bubb et al. (2002) suggested that a limited metabolic capacity could restrict the ability of invasive signal crayfish to outcompete native crayfish species. Standard metabolic rates (SMR) were similar between species through the studied temperature gradient. Taylor and Dunn (2018) reported a similar finding between two crayfish species (A. pallipes, P. leniusculus) and Chinese mitten crab (E. sinesis). With support from the literature, they suggested that the energy needed for vital functions and tissue maintenance ran at a similar rate in the studied species. The temperature-specific differences in the RMR between noble and signal crayfish are therefore mainly due to the differences in their activity. At low temperatures, noble crayfish have the potential to move, search for food, and feed more efficiently than signal crayfish.
Activity and energy production ability in the cold may be linked to elevated enzyme capacity and/or to increased mitochondrial densities or functions, which are reflected as higher metabolic rates (Pörtner 2002). It is possible to generate more energy in the muscle mitochondria, which enables higher locomotory activity and feeding rates, and leads to higher oxidative capacity and oxygen consumption in the cold. This widely observed metabolic cold adaptation (Sommer and Pörtner 2004; White et al. 2012) may provide a physiological explanation for the differences between noble and signal crayfish, but there are no crayfish-specific studies on mitochondrial densities or efficiency. The lower optimal and maximum temperature of noble crayfish compared with signal crayfish (Nyström 2002) indicates that the metabolic scope of noble crayfish is reduced at high temperatures due to increased oxygen demand and the overloading of the oxygen transport system (Pörtner 2002). However, according to our future simulations, severe detrimental temperature-induced impacts on noble crayfish are not anticipated in the near future.
Our results indicate that the thermal niche of signal crayfish has remained somewhat stable, indicating only a slow—if any—adaptation to a colder thermal environment over about 50 years (12–15 generations) in their new range (Ruokonen et al. 2018). Recent studies and meta-analyses have shown that the evolution of thermal performance and adaptation in ectotherms appears slow (Kristensen et al. 2020; Logan et al. 2020), and most invasive species conserve their climatic niche (Liu et al. 2020). In our case, the adaptation process may be slowed down because of low genetic diversity, as small numbers of initial propagules were introduced from single origin sources (Froufe et al. 2015; Ruokonen et al. 2018). The current distribution area of signal crayfish is the southern and central part of Finland (Ruokonen et al. 2018). Further spread is thought to be restricted by the unsuitable climate conditions in the northern part of the country (Heinimaa and Pursiainen 2008), which supports low adaptability and high conservatism in relation to the thermal niche.
The general view has been that invasive species, including crayfish, usually have stronger impacts on other biota than native conspecifics (e.g. Nyström and Strand 1996; Nyström et al. 1999; McCarthy et al. 2006; Twardochleb et al. 2013). Recently, some studies have reported a functional redundancy between invasive and native crayfish. Lagrue et al. (2014) and Ercoli et al. (2015) found that invasive signal crayfish and native noble crayfish had similar impacts on macroinvertebrate communities. The impacts’ magnitude may also depend on population density (Hansen et al. 2013; Ruokonen et al. 2016), vary between habitats (e.g. Ruokonen et al. 2014), and intraspecific variation in traits between invasive populations may also play an important role (Evangelista et al. 2019). Doherty-Bone et al. (2019) also showed that redundancy may differ between targeted organisms or ecosystem functions. These findings, combined with our results, show that the impact assessment of invasive crayfish is a multifaceted complex that is affected by the physiological differences between species, their habitats, and the diverse environmental factors in each ecosystem.
Our model simulations based on temperature-specific differences in the physiology of the species (Fig. 3) illustrate that the impacts of native and invasive crayfish can vary significantly among seasons, and small changes in latitude or the habitat microclimate can be important. Although the instantaneous season- and system-specific impacts may differ, the long-term ecological impacts seem to approach each other. In considering the probable level of uncertainty based on our bioenergetics model simulations, the annual food consumption of noble and signal crayfish was similar, and significant differences cannot be seen in future climate scenarios either. Keskinen et al. (2008) conducted a comprehensive uncertainty analysis of multiple input parameters for a similar Wisconsin-type bioenergetics model for pikeperch (Sander lucioperca) to the one we used in this study for crayfish. They showed that the width of the 80% probability range for food consumption estimates from an evaluation of uncertainty was typically 20% of the median value. Our one-parameter sensitivity analysis of the RQ resulted in a 7–19% variation in annual food consumption.
In our model scenarios, we assumed that the food composition of both species was similar. Seasonal differences in activity and feeding may cause changes in annual diet composition or the predation impact on specific prey. For example, signal crayfish are a potential predator of the eggs of autumn-spawning fish during the autumn and winter (Karjalainen et al. 2015), but according to our new results, noble crayfish may be even more active and therefore potentially more efficient at preying on fish eggs at low temperatures. However, the littoral and sublittoral zones of large oligotrophic lakes are not the typical habitat of noble crayfish, whereas signal crayfish have spread widely in the large lakes of southern and central Finland (Ruokonen et al. 2014, 2018). Nyström and Strand (1996) reported that signal crayfish had a stronger grazing impact on aquatic macrophytes than noble crayfish. The difference was most evident at higher temperatures (15 and 22 °C). Nyström et al. (1999) also found that signal crayfish had stronger impacts on macrophytes and benthic macroinvertebrates in an experiment conducted at summer temperatures. An analysis of the seasonal dietary changes of noble and signal crayfish and the availability of their prey was beyond the scope of our study, but would be an important additional perspective in achieving a more comprehensive conception of their ecological impacts.
A rising ambient temperature, which is one of the most significant elements of climate change, poses a particular challenge to temperature sensitive organisms such as crustacea. Small changes in the ambient temperature may strongly influence the physiology and behaviour of crustaceans (Foucreau et al. 2013), which will undoubtedly be reflected in their impacts on the ecosystem and potential to invade the north. The energetic costs resulting from the rising temperature are usually compensated for by increasing food intake or changing the diet to energy sources with a higher energy content (Woodward et al. 2010). The rising temperature may also enhance predatory activity, thereby increasing impacts on other biota. Recently, Pellan et al. (2016) have suggested that the impact of invasive crustaceans is likely to increase with warming, but the generality of results remains scarce, and a mechanistic understanding of climate change impacts requires more research. Our simulations showed that the direct annual predation impact of noble and signal crayfish seems to be similar at present and in future, although the seasonal dynamics of the predation differs considerably. In all, the divergent temperature-specific metabolic and feeding rate of crayfish species needs to be taken into account in the invasion control and impact assessment of non-native crayfish instead of simple generalisations of the direction or magnitude of impacts.
Data availability
Data available from the authors on request.
References
Alexander ME, Dick JTA, Weyl OLF, Robinson TB, Richardson DM (2014) Existing and emerging high impact invasive species are characterized by higher functional responses than natives. Biol Lett 10:20130946
Becker CD, Genoway RG, Merril JA (1975) Resistance of northwestern crayfish, Pacifastacus leniusculus, to elevated temperatures. Trans Am Fish Soc 104:374–387
Bubb D, Lucas M, Thom T (2002) Winter movements and activity of signal crayfish Pacifastacus leniusculus in an upland river, determined by radio telemetry. Hydrobiologia 483:111–119
Comte L, Cucherousset J, Olden JD (2017) Global test of Eltonian niche conservatism of nonnative freshwater fish species between their native and introduced ranges. Ecography 40:384–392
Dick J, Gallagher K, Avlijas S, Clarke H, Lewis S, Leung S, Minchin D, Caffrey J, Alexander M, Maguire C, Harrod C, Reid N, Haddaway N, Farnsworth K, Penk M, Ricciardi A (2013) Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol Invas 15:837–846
Doherty-Bone T, Dunn AM, Jackson FL, Brown LE (2019) Multi-faceted impacts of native and invasive alien decapod species on freshwater biodiversity and ecosystem functioning. Freshw Biol 64:461–473
Ercoli F, Ruokonen T, Erkamo E, Jones R, Hämäläinen H (2015) Comparing the effects of introduced signal crayfish and native noble crayfish on the littoral invertebrate assemblages of boreal lakes. Freshw Sci 34:555–563
Evangelista C, Cucherousset J, Lecerf A (2019) Contrasting ecological impacts of geographically close invasive populations. Oecologia 189:529–536
Firkins I, Holdich DM (1993) Thermal studies with three species of freshwater crayfish. Freshw Crayfish 9:241–248
Forsius M, Anttila S, Arvola L, Bergström I, Hakola H, Heikkinen HI, Helenius J, Hyvärinen M, Jylhä K, Karjalainen J, Keskinen T, Laine K, Nikinmaa E, Peltonen-Sainio P, Rankinen K, Reinikainen M, Setälä H, Vuorenmaa J (2013) Impacts and adaptation options of climate change on ecosystem services in Finland: a model based study. Curr Opin Env Sust 5:26–40
Foucreau N, Piscart C, Puijalon S, Hervant F (2013) Effect of Climate-related change in vegetation on leaf litter consumption and energy storage by Gammarus pulex from continental or Mediterranean populations. PLoS ONE 8:e77242
Froufe E, Varandas S, Teixeira A, Sousa R, Filipová L, Petrusek A, Edsman L, Lopes-Lima M (2015) First results on the genetic diversity of the invasive signal crayfish Pacifastacus leniusculus (Dana, 1852) in Europe using novel microsatellite loci. J Appl Genet 56:375–380
Guan RZ, Wiles PR (1998) Feeding ecology of the signal crayfish Pacifastacus leniusculus in a British lowland river. Aquaculture 169:177–193
Hansen GJA, Hein CL, Roth BM, Vander Zanden MJ, Gaeta JW, Latzka AW, Carpenter SR (2013) Food web consequences of long-term invasive crayfish control. Can J Fish Aquat Sci 70:1109–1122
Hanson PC, Johnson TB, Schindler DE, Kitchell JF (1997) Fish Bioenergetics 3.0 for Windows. University of Wisconsin-Madison center for limnology and University of Wisconsin Sea Grant Inst., Madison, WI, USA
Heinimaa S, Pursiainen M (2008) Signal crayfish Pacifastacus leniusculus at northerly latitudes: a search for the distribution limits. Freshw Crayfish 16:37–41
Humphries MM, Umbanhowar J, McCann KS (2004) Bioenergetic prediction of climate change impacts on northern mammals. Integr Comp Biol 44:152–162
Iacarella JC, Dick JTA, Alexander ME, Ricciardi A (2015) Ecological impacts of invasive alien species along temperature gradients: testing the role of environmental matching. Ecol Appl 25:706–716
IPCC (2007) Climate change 2007: The scientific basis. In Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (Eds.) Contributions of working group I to the fourth assessment report of the intergovernmental panel on climate change: “The Physical Science Basis”. Cambridge University Press, Cambridge, pp 996
Jørgensen C, Enberg K, Mangel M (2016) Modelling and interpreting fish bioenergetics: a role for behaviour, life-history traits and survival trade-offs. J Fish Biol 88:389–402
Karjalainen J, Ruokonen TJ, Marjomäki TJ, Martikainen A, Pursiainen M, Sarvala J, Tarvainen M, Ventelä, (2015) Predation by signal crayfish Pacifastacus leniusculus on fish eggs and its consequences for coregonid recruitment. J Fish Biol 86:651–667
Kelley AL (2014) The role thermal physiology plays in species invasion. Conserv Physiol 2:cou45
Keskinen T, Jääskeläinen J, Marjomäki TJ, Karjalainen MT, J, (2008) Bioenergetics model for pikeperch Sander lucioperca – construction, validation and evaluation of uncertainty of multiple input parameters. Trans Am Fish Soc 137:1741–1755
Kitchell JF, Stewart DJ, Weininger D (1977) Applications of a bioenergetics model to yellow perch (Perca flavescens) and walleye (Stizostedion vitreum vitreum). J Fish Res Bd Can 34:1922–1935
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Korhonen J (2002) Suomen vesistöjen lämpötilaolot 1900-luvulla. Luonto Ja Luonnonvarat 566:1–116
Kristensen TN, Ketola T, Kronholm I (2020) Adaptation to environmental stress at different timescales. Ann N Y Acad Sci 476:5–22
Lagrue C, Podgorniak T, Lecerf A, Bollache L (2014) An invasive species may be better than none: invasive signal and native noble crayfish have similar community effects. Freshw Biol 59:1982–1995
Liu C, Wolter C, Xian W, Jeschke JM (2020) Most invasive species largely conserve their climatic niche. Proc Natl Acad Sci USA 117:23643
Logan ML, Minnaar IA, Keegan KM, Clusella-Trullas S (2020) The evolutionary potential of an insect invader under climate change. Evolution 74:132–144
Lowry E, Rollinson EJ, Laybourn AJ, Scott TE, Aiello-Lammens ME, Gray SM, Mickley J, Gurevitch J (2013) Biological invasions: a field synopsis, systematic review, and database of the literature. Ecol Evol 3:182
Lozán JL (2000) On the threat to the European crayfish: a contribution with the study of the activity behaviour of four crayfish species (Decapoda: Astacidae). Limnologica 30:156–161
Matzelle AJ, Sarà G, Montalto V, Zippay M, Trussell GC, Helmuth B (2015) A bioenergetics framework for integrating the effects of multiple stressors: opening a ‘black box’ in climate change research. Am Malac Bull 33:1–11
McCarthy JM, Hein CL, Olden JD, Vander Zanden MJ (2006) Coupling long-term studies with meta-analysis to investigate impacts of non-native crayfish on zoobenthic communities. Freshw Biol 51:224–235
McHuron EA, Luxa K, Pelland NA, Holsman K, Ream R, Zeppelin T, Sterling JT (2020) Practical application of a bioenergetic model to inform management of a declining fur seal population and their commercially important prey. Front Mar Sci 7:597973
Nakata K, Hamano T, Hayashi K, Kawai T (2002) Lethal limits of high temperature for two crayfishes, the native species Cambaroides japonicus and the alien species Pacifastacus leniusculus in Japan. Fish Sci 68:763–767
Nyström P (2002) Ecology. In: Holdich DM (ed) Biology of freshwater crayfish. Blackwell Publishing Ltd., Oxford, pp 193–235
Nyström P, Bronmak C, Graneli W (1999) Influence of an exotic and a native crayfish species on a littoral benthic community. Oikos 85:545–553
Nyström P, Strand JA (1996) Grazing by a native and an exotic crayfish on aquatic macrophytes. Freshw Biol 36:673–682
Pellan L, Médoc V, Renault D, Spataro T, Piscart C (2016) Feeding choice and predation pressure of two invasive gammarids, Gammarus tigrinus and Dikerogammarus villosus, under increasing temperature. Hydrobiologia 781:43–54
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol 132:739–761
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of nonnative species. Ecol Monog 83:263–282
Richardson DM, Pysek P (2012) Naturalization of introduced plants: ecological drivers of biogeographical patterns. New Phytol 196:383–396
Rognerud S, Apperberg M, Eggereide A, Pursiainen M (1988) Water quality and effluents. In: Skurdal J, Westman K, Bergan PI (eds) Crayfish Culture in Europe, Reports from the Workshop on Crayfish Culture, Trondheim, pp 18–28
Rosewarne PJ, Mortimer RJG, Newton RJ, Grocock C, Wing CD, Dunn AM (2016) Feeding behaviour, predatory functional responses and trophic interactions of the invasive Chinese mitten crab (Eriocheir sinensis) and signal crayfish (Pacifastacus leniusculus). Freshw Biol 61:426–443
Ruokonen TJ, Ercoli F, Hämäläinen H (2016) Are the effects of an invasive crayfish on lake littoral macroinvertebrate communities consistent over time? Knowl Manag Aquat Ecosyst 417:31
Ruokonen TJ, Sjövik R, Erkamo E, Tulonen J, Ercoli F, Kokko H, Jussila J (2018) Introduced alien signal crayfish (Pacifastacus leniusculus) in Finland - uncontrollable expansion despite numerous crayfisheries strategies. Knowl Manag Aquat Ecosyst 419:27
Ruokonen TJ, Karjalainen J, Hämäläinen H (2014) Effects of an invasive crayfish on the littoral macroinvertebrates of large boreal lakes are habitat specific. Freshw Biol 59:12–25
Rutledge PS, Pritchard AW (1981) Scope for activity in the crayfish Pacifastacus leniusculus. Am J Phys 240:87–92
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Biodiversity - global biodiversity scenarios for the year 2100. Science 287:1770–1774
Simberloff D, Martin J, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, Garcia-Berthou E, Pascal M, Pysek P, Sousa R, Tabacchi E, Vila M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Sjövik R (2016) Impacts of temperature on growth of signal crayfish (Pacifastacus leniusculus Dana) and food density on food acquisition of signal and noble crayfish (Astacus astacus L.). MS thesis, University of Jyväskylä
Sommer AM, Pörtner HO (2004) Mitochondrial function in seasonal acclimatization versus latitudinal adaptation to cold in the Lugworm Arenicola marina (L.). Physiol Biochem Zool 77:174–186
Strayer DL (2012) Eight questions about invasions and ecosystem functioning. Ecol Lett 15:1199–1210
Taylor N, Dunn A (2018) Predatory impacts of alien decapod Crustacea are predicted by functional responses and explained by differences in metabolic rate. Biol Invas 20:2821–2837
Twardochleb LA, Olden JD, Larson ER (2013) A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw Sci 32:1367–1382
Velde G, Leuven RSEW, Platvoet D, Bacela K, Huijbregts MAJ, Heniks HWM, Kruijt D (2009) Environmental and morphological factors influencing predatory behaviour by invasive non-indigenous gammaridean species. Biol Invas 11:2043–2054
Veselý L, Boukal DS, Buřič M, Kuklina I, Fořt M, Yazicioglu B, Prchal M, Kozák P, Kouba A, Sentis A (2019) Temperature and prey density jointly influence trophic and non-trophic interactions in multiple predator communities. Freshw Biol 64:1984–1993
Westman K, Savolainen R (2002) Growth of the signal crayfish, Pacifastacus leniusculus, in a small forest lake in Finland. Boreal Environ Res 7:53–61
Westman K, Savolainen R, Julkunen M (2002) Replacement of the native Crayfish Astacus astacus by the Introduced Species Pacifastacus leniusculus in a small, enclosed Finnish Lake: a 30-year study. Ecography 25:53–73
White CR, Alton LA, Frappell PB (2012) Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc Royal Soc B 279:1740–1747
Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Jonathan Davies T, Grytnes JA, Harrison SP, Hawkins BA, Holt RD, McCain CM, Stephens PR (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13:1310–1324
Woodward G, Perkins DM, Brown LE (2010) Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos Trans Royal Soc B Biol Sci 365:2093–2106
Acknowledgements
We thank Heini Hyvärinen, Antti Martikainen, Jani Pulkkinen, Rosanna Sjövik, and Saana Tepsa for their diligent work during the feeding experiments and respirometry.
Funding
Open access funding provided by Natural Resources Institute Finland (LUKE). Work was partly funded from the Finnish Fisheries Environmental Programme by the European Maritime and Fisheries Fund.
Author information
Authors and Affiliations
Contributions
TR: Planning, experimental work, supervision, writing, reviewing. JK: Planning, experimental work, data analysis and statistics, bioenergetic modelling, writing, reviewing, editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruokonen, T.J., Karjalainen, J. Divergent temperature-specific metabolic and feeding rates of native and invasive crayfish. Biol Invasions 24, 787–799 (2022). https://doi.org/10.1007/s10530-021-02687-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02687-1