Abstract
We studied the historical prevalence of the invasive and pathogenic chytrid fungus Batrachochytrium dendrobatidis (Bd) among amphibians from the Bolivian Andes. Our aim was also to determine its geographic pattern of dispersion, and a potential host taxonomic signature. We collected frog tissue samples from nine museum collections covering a period from 1863 to 2005 and from the field during 2009–2016. Bd was diagnosed via quantitative PCR in 599 individuals of 17 genera and 54 species. We found an overall Bd prevalence of 41% among 44 species tested. The first incidence of Bd was from a Telmatobius culeus in 1863; this is the earliest report of detection for this pathogen in the world. Results reveal a non-random historical and geographical pattern of Bd occurrence and amphibian declines that suggests the presence of two different invasive strains, an ancient endemic and a more recent introduction. Prevalence of Bd increased significantly by the mid-1990s, particularly in the cloud-forests, and this is coincident with the timing of drastic amphibian declines. In contrast, amphibians occurring in drier altiplano habitats have persisted in spite of Bd presence. We hypothesize that the early 1990s, and the cloud-forests in central Bolivia were the center of an epidemic surge of Bd that took its toll on many species, especially in the genus Telmatobius. Further sampling of cloud-forest species, and ongoing genetic studies of Bd isolates from Bolivia should help resolve the history of this invasive pathogen and test hypotheses on the differential response of endangered hosts.
Similar content being viewed by others
Introduction
The declines and extinctions of amphibians from tropical montane communities known for their diversity and degree of endemism have concerned biologists since before the turn of the century (Weygoldt 1989; Stuart et al. 2004; Lips et al. 2005). In tropical America, amphibian losses have been documented from mountain systems in Mexico (Cheng et al. 2011; Frías-Álvarez et al. 2008; Lips et al. 2004), Guatemala (Mendelson III et al. 2004; Rovito et al. 2009), Honduras (Kolby et al. 2010; Puschendorf et al. 2006), Nicaragua (Sunyer et al. 2009), Costa Rica (Pounds and Crump 1994; Lips 1998; La Marca et al. 2005), Panama (Lips 1999; Lips et al. 2006), Puerto Rico (Burrowes et al. 2004), Colombia (Lynch and Grant 1988; Ruiz and Rueda-Almonacid 2008; Velásquez et al. 2008), Venezuela (Lampo et al. 2006, 2008), Ecuador (Ron and Merino 2000; Bustamante et al. 2005), Peru (Catenazzi et al. 2010), and Bolivia (De la Riva and Lavilla 2008; Cortez 2009; De la Riva and Burrowes 2011). In addition to direct anthropogenic causes, two factors have been commonly considered the main culprits of sudden losses of entire amphibian communities: climate change (Menéndez‐Guerrero and Graham 2013; Ron et al. 2003; Weygoldt 1989), and the invasive, pathogenic chytrid fungus Batrachochytrium dendrobatidis (Longcore et al. 1999; Skerratt et al. 2007). Whether these two factors act alone, or in synergy to exacerbate a host’s vulnerability to disease, has been a matter of discussion (Pounds et al. 2006; Lips et al. 2008; Pounds and Coloma 2008; Longo et al. 2010). Yet, regardless of the cause, we are concerned with the declines affecting areas where 20–30 years ago we were stunned by the numbers and the diversity of amphibians, many of which we described to science for the first time (Lynch and Burrowes 1990; De la Riva 2005, 2007; De la Riva et al. 2012).
When the culprit of declines and extinctions are emergent infectious diseases caused by an invasive pathogen, determining spatiotemporal patterns of its prevalence, and understanding the mechanisms that facilitate its dispersion can help identify geographical areas and host taxa at greater risk (Laurance et al. 1996). The amphibian pathogenic chytrid fungus, Bd, has shown evidence of directional movement across tropical montane landscapes of Central America (Lips et al. 2006; Cheng et al. 2011), and the Andes in South America (Lips et al. 2008). For the Andes, a pattern of multiple Bd introductions followed by four dispersion waves was hypothesized by Lips et al. (2008). If the rate of spread predicted by these authors for the southern Andes is correct, Bd should have entered Bolivia shortly after 1999. However, drastic amphibian declines and species extinctions in central Bolivia were apparent to one of the authors (I. De la Riva) in 1994, suggesting that if Bd was involved in these declines it should have been present in Bolivia earlier. Exhaustive herpetological work in Bolivian cloud-forests by De la Riva between 1987–1990 yielded a diverse and abundant amphibiofauna; but in 1994 cloud-forest habitats in central Bolivia were depauperate of certain amphibians; some species apparently vanished and have not been observed since (Aguayo 2000; De la Riva 2005; De la Riva and Lavilla 2008; De la Riva and Burrowes 2011; De la Riva and Reichle 2014).
Our knowledge of the evolution of Bd has improved considerably since Morehouse and collaborators first examined its genetic diversity (Morehouse et al. 2003), as was recently summarized by James et al. (2015). There are four distinct genotypes endemic to South Africa (Bd-Cape), Switzerland (Bd-Ch), Brazil (Bd-Brazil) and Korea (Bd-Korea), and a widespread hyper-virulent global pandemic lineage—Bd-GPL (Bataille et al. 2013; Farrer et al. 2011; Goka et al. 2009; James et al. 2015; Rodríguez et al. 2014; Rosenblum et al. 2013; Schloegel et al. 2012). While endemic lineages are presumably less virulent, Bd-GPL has been associated to epizootics responsible for amphibian declines in Europe, Australia, and North, Central and South America. Two genetically divergent clades have been identified within Bd-GPL (Schloegel et al. 2012), various haplotypes can be distinguished from variation in the ribosomal internal transcribed spacer (rRNA ITS1) (Schloegel et al. 2012; Rodríguez et al. 2014), and considerable multilocus genotypes can be recognized using genotyping by sequencing (Jenkinson et al. 2016). Nonetheless, these variants are generally referred to as lineages or strains, and Bd is still considered one species. Thus far two Bd lineages have been identified in South America, Bd-GPL2 and the endemic Bd-Brazil strain confined to the Brazilian Atlantic Forest (James et al. 2015; Jenkinson et al. 2016). Given the current status of amphibians in Bolivia, we expect that Bd-GPL2 is associated to the drastic declines observed in cloud-forest habitats, but the potential for one or more endemic strains in less affected habitats cannot be discarded.
The aim of this work was to document the historical incidence of Bd in Bolivia, identify the taxa affected, and highlight geographic and ecological correlates that may have contributed to this pathogen’s spread through the complex Andean topography. We focused on amphibians from montane habitats, because in tropical regions Bd is more likely to grow in the cooler highlands where temperatures are within its optimal growth range of 17–25 °C (Piotrowski et al. 2004), and because the majority of the Bolivian amphibians considered threatened by the International Union for Conservation of Nature (IUCN) occur in this part of the country (De la Riva and Reichle 2014; http://www.iucnredlist.org/). Among our findings we report the earliest known record for Bd (1863), a non-random historic and geographic pattern of Bd occurrence that suggests the presence of more than one Bd strain, and a significant increase in Bd prevalence after the mid-1990s that is coincident with the timing of amphibian declines in Bolivia.
As with many other places affected by an epidemic outbreak of chytridiomycosis, there are no comprehensive data available on amphibian abundance for affected communities in Bolivia before and after the declines. This limits our potential to accurately quantify amphibian losses, and the ecological consequences that the loss of species may represent to affected areas. In addition, our data are limited for two other reasons: (1) herpetological work in Bolivia is incomplete and this is reflected in the kind and amount of material deposited in scientific collections, and (2) field work on Bolivian amphibians was scarce until 1987 when I. De la Riva started to work in the country, surveying intensively in the Amazonian and Andean regions until 1990. After this, his work became intermittent, and other herpetologists, from Bolivia and other foreign countries, also enriched the literature and scientific collections. In spite of the limitations mentioned above, this survey work is significant because it contributes to our knowledge of the historical invasion of a deadly pathogen in a biodiversity hotspot—the tropical Andes (Myers et al. 2000)—while underscoring the importance of environmental interactions in defining host response and its consequences to biodiversity loss. In addition, it provides the framework for testing hypotheses on potential genetic differences between pathogen strains that may explain the historical, geographic and taxonomic patterns of amphibian declines observed in a tropical region with the topographical complexity of Bolivia.
Methods
Sampling
Our sampling efforts were geographically focused on the Andean region of Bolivia, specifically the altiplano or highland plateau between the east and west cordilleras, the valleys, and the Amazonian slopes (Fig. 1). The diverse ecosystems of Bolivia have been described in detail by Ibisch (1996), and in the context of amphibian distribution by Köhler (2000) and De la Riva et al. (2000). We sampled three ecoregions in the Bolivian Andes: (1) High Andean Forests and treeless higlands known as Puna (HA)—located in the western altiplano region at elevations between 2500 and 4600 m a. s. l., with a mean annual temperature below 10 °C and a precipitation of 500–700 mm; it sustains 6–8 arid months/yr, and is characterized by puna grasslands and low evergreen mountain forests. (2) Inter-Andean Mesothermic Valleys (IAV)—located at elevations between 1300 and 3000 m a.s.l., with mean annual temperatures of 12–16 °C and precipitation of 500–700 mm; it sustains 6–8 arid months/yr and is characterized by deciduous and semidecidious forests of medium height. (3) Cloud-forests (CF)—comprising both the upper and lower humid montane forests locally known as “Yungas”, as well as the humid grasslands right above the elfin forests; this area is located at elevations between 500 and 3500 m a.s.l., with mean annual temperatures of 12–24 °C and precipitation of 2500–6000 mm; it sustains 0–2 arid months/yr and is characterized by evergreen mountain forests of medium height. In the remaining of this paper we will indicate in parentheses the ecoregion of each locality discussed.
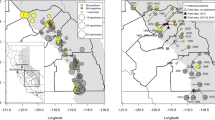
Map of central Bolivia showing ecoregions sampled in the Andes, color-coded as follows: High Andean Forests and Puna (HA) in white (yellow online), Inter-Andean Mesothermic Valleys (IAV) in grey (brown online), and Cloud-Forests (CF) in charcoal (green online). Dates indicate Bd first occurrence in a locality. Triangles denote localities where Bd was present before amphibian declines were observed (Pre-decline), and circles indicate localities where Bd was present after drastic amphibian declines (Post-decline). Specific locality information available in supplementary online resource—SM1
Museum collections consulted
We sampled Bd from live amphibians in the field in 2009, 2012, 2013 and 2016, and from museum specimens collected from 1863 to 2005. The decision as to which species and specimens to sample was based on one of the author's (De la Riva) observation of declines in the wild, listing in one of the IUCN Red list threat categories (Critically Endangered-CR, Endangered-EN, or Vulnerable-V), and/or availability in museum collections. The collections sampled were: Colección Boliviana de Fauna, Museo Nacional de Historia Natural, La Paz, Bolivia (CBF); Centro de Biodiversidad y Genética, Universidad de Cochabamba, Cochabamba, Bolivia (CBG); Museo de Historia Natural Alcide d’Orbigny, Cochabamba, Bolivia (MHNC); Museo de Historia Natural Noel Kempff Mercado, Santa Cruz, Bolivia (MNK); Museo Nacional de Ciencias Naturales-CSIC, Madrid, Spain (MNCN); Estación Biológica de Doñana-CSIC, Seville, Spain (EBD); American Museum of Natural History, New York, USA; Natural History Museum, The University of Kansas, Lawrence, Kansas, USA; and Carnegie Museum of Natural History, Pittsburgh, Pennsylvania, USA (for museum specimen numbers, species names, locality and Bd infection status, refer to supplementary online resource—SM1).
Laboratory methods and analysis
Museum specimens were rinsed with 70% ethanol before swabbing to decrease the probability of contamination with small skin pieces belonging to other frogs in the jar. Gloves were rinsed between individuals within the same jar, and changed when sampling frogs from a new jar. Tissue samples were taken by firmly running a fine-tip swab (Medical Wire, MW113) over the lower abdomen, pelvic patch, ventral sides of both thighs, and digits of all limbs of each specimen. Swabs were stored in vials with 70% ethanol at room temperature until processed, approximately one month later. DNA was extracted from swabs, using 50 µl of Prepman Ultra (Applied Biosystems 4318930) following Hyatt et al. (2007). We used quantitative Polymerase Chain Reaction (qPCR) to diagnose Bd following methods by Boyle et al. (2004) with Bd genomic equivalent (GE) standards of 1000, 100, 10, 1 and 0.1 GE, along with negative and positive controls. Using this method, Cheng et al. (2011) recovered Bd DNA from preserved amphibians in 83–90% of the samples that they had diagnosed Bd-positive via histological examination (Cheng et al. 2011), and other authors have been successful at detecting Bd from ancient specimens of amphibians (Bataille et al. 2013; Richards-Hrdlicka 2012; Rodríguez et al. 2014; Talley et al. 2015).
From all frogs sampled, we determined prevalence of infection through time, family, ecoregion, and reproductive mode (aquatic larvae or direct-developer) by counting the number of Bd positive individuals, divided by the total sampled in each category, and calculated respective binomial 95% Confidence Intervals (Rodríguez et al. 2014). Only samples with Bd GE of 0.1 or greater were considered positive for infection. We performed Exact Pearson Chi square tests in two-way contingency tables (Infected vs. not Infected) to determine if the probability of Bd infection was randomly distributed through time periods, phylogenetic history (families), ecoregions, and reproductive modes. Exact sample sizes per ecoregion are provided in supplementary online resource—SM2.
Results
Pattern of infection through time
We sampled a total of 599 specimens from eight amphibian families, 17 genera, and 54 species collected from 1863 to 2016. Prevalence of Bd infections ranged from 33% (95% CI 10–70) until the 1920s to 59% (95% CI 44–56) in the years 2000–2016 (Fig. 2). The earliest detection of infection was in a Telmatobius culeus from Lake Titicaca (HA) collected in 1863 (Fig. 1). Diagnostic qPCR from the original DNA extraction of this sample was repeated, again with positive results. To disregard the possibility of contamination, the individual (MNCN 4049) was swabbed again, and the second sample was also Bd-positive. Furthermore, in order to observe Bd structures, we performed histology of the skin with hematoxylin and eosin (H&E) and a more fungus specific stain—periodic acid-Schiff–PAS (Puschendorf and Bolaños 2006) and although structures resembling Bd sporangia with discharge tubes were observed (A. Pessier pers. comm.), results are inconclusive, potentially due to the bad quality of the skin in this old specimen. To our knowledge, this individual currently represents the oldest record of Bd presence. Unfortunately, this was the only Bolivian individual from the nineteenth century that we found in the museums consulted for this study, and the next year from which collections were available was 1917. From the six specimens sampled between 1917–1959, only two, a Rhinella veraguensis (1917) and a Hypsiboas riojanus (1921), tested positive for Bd. The localities where these two individuals were collected are approximately 447 and 225 linear km southwest of Lake Titicaca, respectively, in the Departments of Santa Cruz (IAV) and Cochabamba (HA) (Fig. 1). The number of specimens available in scientific collections increases after the 1970s, and so do our sample sizes (Fig. 2). All frogs that were Bd-positive in the 1970s occurred in dry habitats (HA and IAV) with specimens infected from the Departments of La Paz to Potosí (Fig. 1). The incidence of Bd appeared to remain stable throughout the 1980s, with a prevalence of 29% (Fig. 2), and until this decade most infected specimens were detected from the drier ecoregions of the Andes. Thus, by 1989, frogs infected with Bd were detected throughout the Bolivian altiplano and some lower inter-Andean mesothermic valleys (Fig. 1—triangles).
There is little change in the mean Bd prevalence in amphibians until the 1990s, during which time an increase in Bd was detected (Fig. 2). Prevalence of Bd among our samples is not randomly distributed across the periods before and after amphibian declines (X2 = 16.96, df = 1, p < 0.0001), and the probability of being infected after 1994 is 3.5 times greater than in the years before (OR 3.52, 95% CI 1.90–6.40; Fig. 2). Consequently, we divide the chronology of Bd prevalence in two time periods that bound a crucial turning point for the amphibians of Bolivia: before declines (1863–1990) and after drastic declines (1994 to present) symbolized with triangles versus circles in Fig. 1. Unfortunately, because documented fieldwork did not take place in the country from 1991 to 1993, there were no specimens collected during this critical period, which we hypothesize as the starting point and the peak of the Bd epidemic responsible for the substantial amphibian declines observed by I. De la Riva in central Bolivia in 1994.
Pattern of infection through ecoregions
Overall Bd prevalence is significantly associated with ecoregions (X2 = 11.44, df = 2, p = 0.003), and the change in Bd occurrence per ecoregion is not independent of time (X2 = 50.72, df = 6, p < 0.0001). Approximately 30% of individuals sampled in the drier habitats (HA and IAV) were infected with Bd during the 1970s, and a marked increase in Bd prevalence is evident after the 1990s, both in the inter-Andean valleys (IAV) and in the cloud-forests (CF) (Fig. 3). In the cloud-forests, there is a significant linear increase in Bd prevalence of 2.69% per year since its first detection in 1982 until present (Y = 2.69X − 1.89, F(2,26) = 24.27, p < 0.001, R2 = 50.2%). In contrast to species from the HA and the IAV, many Bd-positive species in the CF have disappeared and declined drastically in the mid 1990s (De la Riva and Reichle 2014). For example, all nine cloud-forest species of Telmatobius became very rare since 1994, and are now absent from these humid montane habitats (Table 1). Interestingly, some populations of T. simonsi still occur in inter-Andean valleys (IAV), while those from cloud-forests habitats have disappeared (see Köhler 2000; R. Aguayo and A. Muñoz, pers. comm.).
Prevalence of Batrachochytrium dendrobatidis (Bd) infections through time in the different Bolivian ecoregions sampled: HA High Andes, IAV Inter-Andean Valleys, CF Cloud-forests and corresponding upper 95% confidence intervals. Sample sizes for each ecoregion per time vary—see supplementary online resource—SM2
Phylogenetic pattern of infection
All genera sampled except three—Cochranella, Hyalinobatrachium, and Noblella—had species infected with Bd (see SM1), and 81% (44/54) of the species were found infected with this pathogen at one time or another. Prevalence of Bd infection was not independent of families (X2 = 81.19, df = 7, p = 0.0001). Whereas the families Telmatobiidae, Leptodactylidae, and Hylidae had Bd prevalences above 48%, Hemiphractidae and Craugastoridae had lower prevalence with 32% of the individuals sampled being Bd-positive, and Bufonidae and Centrolenidae each had Bd-prevalences below 20% (Fig. 4). Overall, 84% of the species that were Bd-positive are currently placed in IUCN categories of risk. We are especially concerned with the Telmatobiidae (aquatic frogs with high endemism in Bolivia) because we sampled all the species in the country, all of them tested positive for Bd with an overall prevalence of 53%, and all are currently in IUCN categories of threat, either as Endangered or Critically Endangered (De la Riva and Reichle 2014; Fig. 4). The status of two other families—Craugastoridae and Bufonidae—is also of concern because over 50 and 30%—respectively—of the species within these families that occur in Bolivia are threatened, and we detected moderate Bd prevalence in member species (Fig. 4). Since one criterion for inclusion in IUCN threat categories is rarity of previously abundant species (De la Riva and Reichle 2014), these data suggest that Bd was involved in their declines.
Family level comparison between prevalence of Batrachochytrium dendrobatidis (Bd) with upper 95% confidence intervals and the percentages of Bolivian species that are in IUCN categories of threat (CR critical, EN endangered, VU vulnerable). For reference, we provide the percentage of species sampled for this study, and include the actual number on top of the corresponding bar. Sample size (n) for Bd prevalence is given beside each family name. We show only the families for which we sampled more than ten specimens
The reproductive mode of amphibians is generally constrained by evolutionary history (Duellman and Trueb 1994). Among the frogs sampled, those in the family Craugastoridae lay terrestrial eggs with direct development, while those in the other families mostly lay eggs that hatch into aquatic larvae. Although vulnerability to Bd has been more often associated with species that breed in water than with terrestrial direct-developers (Lips et al. 2003; Bielby et al. 2008), we did not find a significant difference in Bd prevalence among Bolivian species that reproduce either way.
Discussion
The tropical Andes are the world’s biodiversity hotspot with the highest number of endemic amphibians (Myers et al. 2000) and also the region with the greatest number (84.1% of the species) of so-called “enigmatic amphibian declines” (Stuart et al. 2004; Collins and Crump 2009). Thus, it is an important biogeographic region for the study of the effect of Bd, a pathogen responsible for amphibian extinctions and declines all over the world (Fisher et al. 2009; James et al. 2015). Here we show that the amphibians of the Bolivian Andes were exposed to Bd as early as 1863. However, the response of amphibians to Bd in Bolivia is complex, and our results reveal a historical, ecologic, and taxonomic signature. Historically, Bd is marked by a time period between 1990 and 1994 associated with a significant increase in prevalence (Fig. 2). Ecologically and in the same time frame, Bd occurrence increases significantly in the central cloud-forests where it is associated with drastic amphibian declines (Figs. 2, 3). Finally, the effect of this pathogen has not been independent of the phylogeny of its hosts, taking its toll especially among entirely aquatic frogs in the family Telmatobiidae from humid montane forests (De la Riva and Reichle 2014; Fig. 4). We did not find differences in Bd prevalence between amphibians that reproduce in water versus land, but this may be because we sampled mostly adults. Among tropical aquatic breeders, Bd prevalence is greater in larvae and metamorphosing young than in adults (Kolby et al. 2010), while in terrestrial-direct developing frogs, juveniles carry the highest Bd loads (Longo and Burrowes 2010). Considering that the infectious stage of Bd is an aquatic zoospore, we recommend broad sampling across ontogenetic stages to better discern potential vulnerability of amphibians to this pathogen (Scheele et al. 2015).
Effect of environmental interactions
The patterns of Bd prevalence and amphibian declines observed in Bolivia appear to involve interactions between pathogen, host’s evolutionary history, and environmental factors, as has been documented for other amphibian communities threatened by chytridiomycosis (Retallick et al. 2004; Murray et al. 2009; Longo et al. 2010; Becker et al. 2010; Becker and Zamudio 2011). The fact that species in drier habitats (HA and IAV) have persisted with Bd for so long, while frogs from the more humid, lower-elevation cloud-forests declined drastically, suggests that environmental factors typical of those ecoregions may have had a role in modulating the effect of chytridiomycosis. Temperature and moisture, for example, can influence growth, survival, and transmission of Bd (Johnson and Speare 2003; Piotrowski et al. 2004). The dry climate of altiplano and high-elevation valley habitats (HA and IAV) can hinder Bd spread because its infectious state is an aquatic zoospore (Longcore et al. 1999). Environmental transmission of zoospores shed by other infected hosts (Kolby et al. 2015b) seems highly unlikely here because the spores would desiccate rapidly from terrestrial substrates. In addition, the elevated UV radiation in the Bolivian high Andes (Piazena 1996), which is known to affect freshwater zooplankton (Cabrera et al. 1997), may also be detrimental to Bd zoospores. In this way, the high Andean puna and mesothermic valleys (HA and IAV) could serve as refugia from chytridiomycosis, as has been suggested of drier habitats in Costa Rica for Craugastor ranoides, or in Australia for Litoria lorica (Puschendorf et al. 2009, 2011). These sort of environmental safe havens from Bd, could explain the persistence of populations of Rhinella veraguensis and Telmatobius simonsi at IAV sites, after disappearing from cloud-forest habitats. Similarly in Peru, a new species of Telmatobius was recently described from dry Andean forests, while conspecific populations from humid forests (known only from museum specimens) seem to be extinct (Ttito et al. 2016).
Cloud-forests are characterized by a milder temperature regime (12–24 °C) and much more precipitation than the Andean dry puna and altiplano habitats (De la Riva et al. 2000; Köhler 2000). While favoring amphibians, together these two climatological conditions also render optimal requirements for Bd growth (Piotrowski et al. 2004), and thus turn the mid-elevation cloud-forests into an area of high risk for chytridiomycosis. The role of climate warming at making the mid-elevation belt in the Andes especially apt for Bd growth, and thus promoting amphibian declines, was discussed by Pounds et al. (2006) with dissenting opinions offered by other scientists (Lips et al. 2008; Rohr et al. 2008). Although we cannot definitely link amphibian declines and extinctions in the Bolivian cloud-forests directly to Bd because we were not there during the early 1990s to witness die offs, and although we cannot address whether the effect of chytridiomycosis has been exacerbated due to climate warming in the Andes, both threats co-occur, and may have had a synergistic detrimental effect on Bolivian amphibians, as has been suggested for other Neotropical mountain regions (Burrowes et al. 2004; Catenazzi 2011; Catenazzi et al. 2014; De la Riva and Burrowes 2011; Seimon et al. 2007).
Thus far we have discussed the potential for different environmental factors in ecoregions to modulate the effect of chytridiomycosis, presumably caused by a single Bd strain. However, if environmental conditions in the different ecoregions of Bolivia have acted as drivers of Bd evolutionary change, particularly in virulence traits, it is also possible the we are dealing with local divergent strains that can potentially illicit different responses on amphibian hosts.
Historical scenarios
Despite sampling limitations, the apparent absence of Bd from cloud-forests until 1982, the concurrent increase in Bd prevalence (Fig. 3), and the drastic decline of most forest Telmatobius between 1990 and 1994 in central Bolivia, resemble the reaction of naïve hosts to a novel pathogen and signal an epidemic event of chytridiomycosis that is not evident in the Bolivian altiplano and other dry habitats. Accordingly, our data suggest two possible scenarios to explain the history of Bd in Bolivia (Fig. 5), that are incompatible with the timing of a directional southern Andean wave entering from Peru at the turn of the century (see Lips et al. 2008).
Flow chart representing two potential scenarios to explain the history of Bd in the Bolivian Andes, and the potential implications to amphibian declines. In Scenario 1 we consider one endemic Bd strain that may have been pathogenic (or not), and in Scenario 2, we consider two Bd strains: an endemic (that behaves like in Scenario 1), and a virulent, recently-introduced strain in the central cloud-forests. HA High Andes, IAV Inter-Andean Valleys, CF Cloud-forests
In one scenario, an ancient strain of Bd is present in the northern part of the Bolivian altiplano well before the 1900s (Fig. 5, Scenario 1). The impact of this Bd strain on the amphibians then, is impossible to appraise due to the lack of scientific surveys from that time frame. What we do know is that all the amphibian species that occur in the altiplano at present survived this chytrid and were apparently healthy and abundant throughout the 1980s and 1990s, with declines observed only recently in some species of Rhinella and Telmatobius (De la Riva and Reichle 2014). The fact that museum specimens from cloud-forests in Bolivia did not test positive for Bd until 1982 (Fig. 2), when species were still abundant in this ecoregion, suggests that this ancient Bd would have moved slowly down the mountains, and this supports the hypothesis of a less virulent, endemic strain. Under this scenario, another stressor (possibly environmental), would have affected the amphibians in the cloud-forests during the mid-1990s, making them more susceptible and resulting in the high Bd prevalence (Figs. 2, 3), and drastic declines observed thereafter (De la Riva and Reichle 2014).
The second scenario (Fig. 5, Scenario 2) involves the presence in Bolivia of two different Bd strains: an ancient endemic (and perhaps less virulent) strain all over the country, and a very virulent strain of Bd (likely the global pandemic lineage-GPL2) introduced more recently in the central cloud-forests of Cochabamba (Fig. 1, blue circles). The latter would be responsible for an epidemic wave that expanded to the east and west over the humid montane forests of the Amazonian slopes of the Bolivian Cordillera Oriental. This outbreak eventually caused the disappearance of all species of forest Telmatobius (Table 1) and the decline of many other species, as for example, Atelopus tricolor, Rhinella quechua, or Rhinella veraguensis (De la Riva and Reichle 2014). The non-directional spread of Bd throughout the Bolivian landscapes before and after amphibian declines (Fig. 1) could have been facilitated by wind and rain patterns (Kolby et al. 2015a) or by aquatic Andean birds (Garmyn et al. 2012; Burrowes and De la Riva 2017). We detected Bd in the feet of several species of aquatic birds in the Bolivian altiplano from museum specimens collected from 1982 to 1997 (Burrowes and De la Riva 2017). These birds use water sources that drain into the cloud-forests, and potentially, could spread the pathogen there and across the complex topography of the Andes (Burrowes and De la Riva 2017).
Given the observed pattern of amphibian declines in Bolivia, our expectations were that we would not find Bd prior to the 1990s. However, our results show that Bd was present at least a century before (and perhaps, it was always there). Had specimens been more densely sampled through time, it is highly plausible that we would have detected more Bd-positive individuals in cloud-forest habitats where the environment favors its growth. Nevertheless, it is not until the early 1990s that severe amphibian declines started. Of course, we cannot discard the hypothesis that other decline episodes took place in the past and went unnoticed. But what is certain is that in the late 1980s, a diverse and abundant anuran fauna was present in Bolivian cloud-forests, and a catastrophic event changed that state of things a few years later. Thus, the second two-strain scenario (Fig. 5, Scenario 2) is our favored hypothesis to explain the historical impact of Bd in Bolivia. Further sampling of cloud-forest amphibians, together with our ongoing efforts to obtain Bd isolates from different ecoregions in Bolivia and study their potential genetic divergence, should provide data to test the hypothesized scenarios (Fig. 5).
Current situation
Our sampling of live specimens since 2009 revealed that Bd is now widespread in the Bolivian Andes (Fig. 1) and even in the lower Amazonian slopes. This suggests that the epidemic has passed and that Bd is currently enzootic in Bolivia. However this does not mean that amphibians are now safe from the effects of this pathogen. Empirical work has demonstrated that species persisting with Bd may become more susceptible to chytridiomycosis under unfavorable environmental conditions like severe droughts (Longo et al. 2010), and that amphibians living with this disease have reduced population recruitment over time, resulting in further declines (Murray et al. 2009; Longo and Burrowes 2010; Scheele et al. 2014a, 2015). Thus, it is crucial that we support conservation efforts like “Bolivian Amphibian Initiative” (http://bolivianamphibianinitiative.org/) which, in line with mitigation strategies suggested for amphibians at risk of chytridiomycosis (Scheele et al. 2014b), perform periodical population and disease monitoring and maintain captive breeding colonies of endangered amphibians.
References
Aguayo R (2000) Ecología de la comunidad de anuros en dos pisos bioclimáticos del Parque Nacional Carrasco (Cochabamba-Bolivia). MS Thesis, Universidad Mayor de San Simón, Cochabamba
Bataille A, Fong JJ, Cha M, Wogan GOU, Baek HJ, Lee H, Min M-S, Waldman B (2013) Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol Ecol 22:4196–4209
Becker CG, Zamudio KR (2011) Tropical amphibian populations experience higher disease risk in natural habitats. Proc Natl Acad Sci (USA) 108(24):9893–9898
Becker CG, Loyola RD, Haddad CFB, Zamudio KR (2010) Integrating species life-history traits and patterns of deforestation in amphibian conservation planning. Divers Distrib 16(1):10–19
Bielby J, Cooper N, Cunningham AA, Garner TWJ, Purvis A (2008) Predicting susceptibility to future declines in the world’s frogs. Conserv Lett 1(2):82–90
Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60:141–148
Burrowes PA, Joglar RL, Green DE (2004) Potential causes of amphibian declines in Puerto Rico. Herpetologica 60(2):141–154
Burrowes PA, De la Riva I (2017) Detection of the amphibian chytrid fungus batrachochytrium dendrobatidis in museum specimens of andean aquatic birds: implications for pathogen dispersal. J Wildl Dis. doi:10.7589/2016-04-074
Bustamante MR, Ron SR, Coloma LA (2005) Cambios en la diversidad en siete comunidades de anuros en los Andes de Ecuador. Biotropica 37:180–189
Cabrera S, López M, Tartarotti B (1997) Phytoplankton and zooplankton response to ultraviolet radiation in a high-altitude Andean lake: short-versus long-term effects. J Plankton Res 19(11):1565–1582
Catenazzi A (2011) Temperature constraint of elevational range of tropical amphibians: response to Forero-Medina. Conserv Biol 25:425–426 (author reply 426-7)
Catenazzi A, Lehr E, Rodríguez LO, Vredenburg VT (2010) Batrachochytrium dendrobatidis and the collapse of anuran species richness and abundance in the upper Manu National Park, southeastern Peru. Conserv Biol 25(2):382–391
Catenazzi A, Lehr E, Vredenburg VT (2014) Thermal physiology, disease, and amphibian declines on the eastern slopes of the Andes. Conserv Biol 28:509–517
Cheng TL, Rovito SM, Wake DB, Vredenburg VT (2011) Coincident mass extirpation of Neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci (USA) 108:9502–9507
Collins JP, Crump ML (2009) Extinction in our times: global amphibian decline. Oxford University Press, New York
Cortez C (2009) Anfibios del Valle de Zongo (La Paz, Bolivia): I. Evaluación del estado de conservación. Ecol Bol 44(2):109–120
De la Riva I (2005) Bolivian frogs of the genus Telmatobius (Anura: Leptodactylidae): synopsis, taxonomic comments, and description of a new species. In: Lavilla EO, De la Riva I (eds) Studies on the Andean Frogs of the Genera Telmatobius and Batrachophrynus, Monografías de Herpetología, vol 7. Asociación Herpetológica Española, Valencia, Spain, pp 65–101
De la Riva I (2007) Bolivian frogs of the genus Phrynopus, with the description of twelve new species (Anura: Brachycephalidae). Herpetol Monogr 21(1):241–277
De la Riva I, Burrowes PA (2011) Rapid assessment of the presence of Batrachochytrium dendrobatidis in Bolivian Andean frogs. Herpetol Rev 42:372–375
De la Riva I, Lavilla EO (2008) Essay 9.2: Conservation status of the Andean frogs of the genera Telmatobius and Batrachophrynus. In: Stuart SN, Hoffmann M, Chanson JS, Cox NA, Berridge R, Ramani P, Young BE (eds) Threatened Amphibians of the World, Lynx Ediciones, with IUCN—The World Conservation Union, Conservation International, and NatureServe, Barcelona, Spain, p 101
De la Riva I, Reichle S (2014) Diversity and conservation of the amphibians of Bolivia. In: Heatwole H, Barrio-Amorós C, Wilkinson JW (eds) Status of decline of amphibians: Western Hemisphere. Amphibian biology. Chapter 13, Part 4 of Volume 9. Herpetology Monographs, vol 28, pp 46–65
De la Riva I, Köhler J, Lötters S, Reichle S (2000) Ten years of research on Bolivian amphibians: updated checklist, distribution, taxonomic problems, literature and iconography. Rev Esp Herp 14:19–164
De la Riva I, Trueb L, Duellman WE (2012) A new species of Telmatobius (Anura: Telmatobiidae) from montane forests of southern Peru, with a review of osteological features of the genus. S Am J Herpetol 7(2):91–109
Duellman WE, Trueb L (1994) Biology of amphibians. Johns Hopkins University Press, Baltimore
Farrer RA, Weinert LA, Bielby J et al (2011) Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci USA 108:18732–18736
Fisher MC, Garner TW, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63:291–310
Frías-Álvarez P, Vredenburg VT, Familiar-López M, Longcore JE, González-Bernal E, Santos-Barrera G, Zambrano L, Parra-Olea G (2008) Chytridiomycosis survey in wild and captive mexican amphibians. EcoHealth 5:18–26
Garmyn A, Van Rooij P, Pasmans F, Hellebuyck T, Van Den Broeck W, Haesebrouck F, Martel A (2012) Waterfowl: potential environmental reservoirs of the chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 7:e35038
Goka K, Yokoyama JUN, Une Y et al (2009) Amphibian chytridiomycosis in Japan: distribution, haplotypes and possible route of entry into Japan. Mol Ecol 18:4757–4774
Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Hero JM, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Coiling A (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Organ 73:175–192
Ibisch PL (1996) Neotropische Epiphytendiversitat—das Beispiel Bolivien. Arch Naturw Diss (M. Galuder Verlag) 1:1–356
James TY, Toledo LF, Rödder D, da Silva LD, Belasen AM, Betancourt-Román CM, Jenkinson TM, Lambertini C, Longo AV, Ruggeri J, Collins JP, Burrowes PA, Lips KR, Zamudio KR, Longcore JE (2015) Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecol Evol 5(18):4079–4097
Jenkinson T, Román B, Lambertini C, et al. (2016) Amphibian-killing chytrid in Brazil comprises both locally endemic and globally expanding populations. Mol Ecol 25:2978–2996
Johnson ML, Speare R (2003) Survival of Batrachochytrium dendrobatidis in water: quarantine and disease control implications. Emerg Infect Dis 9:922–925
Köhler J (2000) Amphibian diversity in Bolivia: a study with special reference to montane forest regions. Bonn Zool Monogr 48:1–243
Kolby JE, Padgett-Flohr GE, Field R (2010) Amphibian chytrid fungus Batrachochytrium dendrobatidis in Cusuco National Park, Honduras. Dis Aquat Organ 92:245–251
Kolby JE, Ramirez SD, Berger L et al (2015a) Presence of amphibian chytrid fungus (Batrachochytrium dendrobatidis) in rainwater suggests aerial dispersal is possible. Aerobiologia 31:411–419
Kolby JE, Ramirez SD, Berger L et al (2015b) Terrestrial dispersal and potential environmental transmission of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). PLoS ONE 10:e0125386
La Marca E, Lips KR, Lötters S, Puschendorf R, Ibáñez R, Rueda-Almonacid JV, Schulte R, Marty C, Castro F, Manzanilla-Puppo J, García-Pérez JE, Bolaños F, Chaves G, Pounds JA, Toral E, Young BE (2005) Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 37:190–201
Lampo M, Rodríguez-Contreras A, La Marca E, Daszak P (2006) A chytridiomycosis epidemic and a severe dry season precede the disappearance of Atelopus species from the Venezuelan Andes. Herpetol J 16(4):395–402
Lampo M, Sánchez D, Nicolás A, Márquez M, Nava-González F, García CZ, Rinaldi M, Rodríguez-Contreras A, León F, Han BA, Chacón-Ortiz A (2008) Batrachochytrium dendrobatidis in Venezuela. Herpetol Rev 39(4):449
Laurance WF, McDonald KR, Speare R (1996) Epidemic disease and the catastrophic decline of Australian rainforest frogs. Conserv Biol 10:406–413
Lips KR (1998) Decline of a tropical montane amphibian fauna. Conserv Biol 12:106–117
Lips KR (1999) Mass mortality and population declines of Anurans at an upland site in Western Panama. Conserv Biol 13:117–125
Lips KR, Reeve JD, Witters LR (2003) Ecological traits predicting amphibian population declines in Central America. Conserv Biol 17(4):1078–1088
Lips KR, Mendelson JR III, Muñoz-Alonso A, Canseco-Márquez L, Mulcahy DG (2004) Amphibian population declines in montane southern Mexico: resurveys of historical localities. Biol Conserv 119(4):555–564
Lips KR, Burrowes PA, Mendelson JR III, Parra-Olea G (2005) Amphibian declines in Latin America: widespread population declines, extinctions, and impacts. Biotropica 37(2):163–165
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP (2006) Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proc Natl Acad Sci USA 103:3165–3170
Lips KR, Diffendorfer J, Mendelson JR III, Sears MW (2008) Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol 6(3):e72
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227
Longo AV, Burrowes PA (2010) Persistence with chytridiomycosis does not assure survival of direct-developing frogs. EcoHealth 7(2):185–195
Longo AV, Burrowes PA, Joglar RL (2010) Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Dis Aquat Organ 92:253–260
Lynch JD, Burrowes PA (1990) The frogs of the genus Eleutherodactylus (Family Leptodactylidae) at the La Planada Reserve in southwestern Colombia with descriptions of eight new species. Occup Pap Mus Nat Hist Univ 136:1–31
Lynch JD, Grant T (1988) Dying frogs in western Colombia: catastrophe or trivial observation? Rev Acad Colomb Cienc 22:149–152
Mendelson JR III, Brodie JRED, Malone JH, Acevedo ME, Baker MA, Smatresk NJ, Campbell JA (2004) Factors associated with the catastrophic decline of a cloudforest frog fauna in Guatemala. Rev Biol Trop 52(4):991–1000
Menéndez-Guerrero PA, Graham CH (2013) Evaluating multiple causes of amphibian declines of Ecuador using geographical quantitative analyses. Ecography 36:756–769
Morehouse EA, James TY, Ganley AR et al (2003) Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol 12:395–403
Murray KA, Skerratt L, Speare R, McCallum H (2009) Impact and dynamics of disease in species threatened by the amphibian chytrid fungus, Batrachochytrium dendrobatidis. Conserv Biol 23(5):1242–1252
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772):853–858
Piazena H (1996) The effect of altitude upon the solar UV-B and UV-A irradiance in the tropical Chilean Andes. Sol Energy 57(2):133–140
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15
Pounds JA, Coloma LA (2008) Beware the lone killer. Nat Rep Clim Chang 2:57–59
Pounds JA, Crump ML (1994) Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv Biol 8:72–85
Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MP, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439(7073):161–167
Puschendorf R, Bolaños F (2006) Detection of Batrachochytrium dendrobatidis in Eleutherodactylus fitzingeri: effects of skin sample location and histologic stain. J Wildl Dis 42:301–306
Puschendorf R, Castaneda F, McCranie JR (2006) Chytridiomycosis in wild frogs from Pico Bonito National Park, Honduras. EcoHealth 3(3):178–181
Puschendorf R, Carnaval AC, VanDerWal J, Zumbado-Ulate H, Chaves G, Bolaños F, Alford RA (2009) Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: proposing climatic refuges as a conservation tool. Divers Distrib 15(3):401–408
Puschendorf R, Hoskin CJ, Cashins SD, McDonald KE, Skerratt LF, Vanderwal J, Alford RA (2011) Environmental refuge from disease-driven amphibian extinction. Conserv Biol 25(5):956–964
Retallick RW, McCallum H, Speare R (2004) Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol 2(11):e351
Richards-Hrdlicka KL (2012) Extracting the amphibian chytrid fungus from formalin-fixed specimens. Methods Ecol Evol 3:842–849
Rodríguez D, Becker CG, Pupin NC, Haddad CFB, Zamudio KR (2014) Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol Ecol 23:774–787
Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ (2008) Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA 105(45):17436–17441
Ron SR, Merino A (2000) Amphibian declines in Ecuador: overview and first report of chytridiomycosis from South America. Froglog 42:2–3
Ron SR, Duellman WE, Coloma LA et al (2003) Population decline of the Jambato toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. J Herpetol 37:116–126
Rosenblum EB, James TY, Zamudio KR et al (2013) Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc Natl Acad Sci USA 110:9385–9390
Rovito SM, Parra-Olea G, Vásquez-Almazán CR, Papenfuss TJ, Wake DB (2009) Dramatic declines in neotropical salamander populations are an important part of the global amphibian crisis. Proc Natl Acad Sci USA 106:3231–3236
Ruiz A, Rueda-Almonacid JV (2008) Batrachochytrium dendrobatidis and chytridiomycosis in anuran amphibians of Colombia. EcoHealth 5(1):27–33
Scheele BC, Guarino F, Osborne W, Hunter DA, Skerratt LF, Driscoll DA (2014a) Decline and re-expansion of an amphibian with high prevalence of chytrid fungus. Biol Conserv 170:86–91
Scheele BC, Hunter DA, Grogan LF, Berger LE, Kolby JE, McFadden MS, Marantelli G, Skerratt LF, Driscoll DA (2014b) Interventions for reducing extinction risk in chytridiomycosis-threatened amphibians. Biol Conserv 28(5):1195–1205
Scheele BC, Hunter DA, Skerratt LF, Brannelly LA, Driscoll DA (2015) Low impact of chytridiomycosis on frog recruitment enables persistence in refuges despite high adult mortality. Biol Conserv 182:36–43
Schloegel LM, Toledo LF, Longcore JE et al (2012) Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol Ecol 21:5162–5177
Seimon TA, Seimon A, Daszak P, Halloy SR, Schloegel LM, Aguilar CA, Sowell P, Hyatt AD, Konecky B, Simmons JE (2007) Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Glob Chang Biol 13(1):288–299
Skerratt L, Berger L, Speare R et al (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306(5702):1783–1786
Sunyer J, Páiz G, Dehling DM, Köhler G (2009) A collection of amphibians from Río San Juan, southeastern Nicaragua. Herpetol Notes 2:189–202
Talley B, Muletz C, Vredenburg V et al (2015) A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biol Conserv 182:254–261
Ttito A, Landauro CZ, Venegas PJ et al (2016) A new species of Telmatobius Wiegmann, 1834, from the Eastern Cordillera Central of the Andes, Peru (Anura: Telmatobiidae), with description of its tadpole, and range extension of T. mendelsoni De La Riva et al., 2012. Ann Carnegie Mus 83:255–268
Velásquez BE, Castro F, Bolívar W, Herrera MI (2008) Infección por el hongo quítrido Batrachochytrium dendrobatidis en anuros de la Cordillera Occidental de Colombia. Herpetotropicos 4:65–70
Weygoldt P (1989) Changes in the composition of mountain stream frog communities in the Atlantic mountains of Brazil: frogs as indicators of environmental deteriorations? Stud Neotrop Fauna Environ 24(4):249–255
Acknowledgements
This research was supported by Projects CGL2011-30393 and CGL2014-56160-P of the Spanish Government (PI, Ignacio De la Riva). We are thankful to J. Aparicio (CBF), A. Muñoz (MHNC), L. Aguirre (CBG), L. Gonzáles (MNK), M. Calvo (MNCN), J. Cabot and T. García-Díez (EBD), D. Frost and D. Kizirian (AMNH), R. M. Brown (KU), and J. M. Padial (CM), for allowing us to sample amphibians in the Museum collections that they curate. We also thank O. Jiménez-Robles (MNCN) for drawing the map of Bolivia, and former University of Puerto Rico students, A. R. González J. López and M. C. Martes for their help with laboratory work and in entering the data in a perfectly organized spreadsheet. A. Pessier kindly checked our histology preparations of Telmatobius culeus skin.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burrowes, P.A., De la Riva, I. Unraveling the historical prevalence of the invasive chytrid fungus in the Bolivian Andes: implications in recent amphibian declines. Biol Invasions 19, 1781–1794 (2017). https://doi.org/10.1007/s10530-017-1390-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1390-8