Abstract
Objective
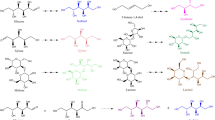
To synthesize octyl β-d-glucopyranoside (OG) and decyl β-d-glucopyranoside (DG) in three non-aqueous reaction systems, namely organic solvents, ionic liquids and co-solvent mixtures, via reverse hydrolysis reactions catalyzed by the N189F dalcochinase mutant.
Results
The highest yield of OG (67 mol%) was obtained in the reaction containing 0.5 M glucose, 3 unit ml−1 enzyme in 20% (v/v) octanol and 70% (v/v) [BMIm][PF6] at 30 °C. On the other hand, the highest yield of DG (64 mol%) was obtained in the reaction containing 0.5 M glucose, 3 unit ml−1 enzyme in 20% (v/v) decanol, 20% (v/v) acetone and 50% (v/v) [BMIm][PF6] at 30 °C. The identities of OG and DG products were confirmed by HRMS and NMR.
Conclusion
This is the first report of enzymatic synthesis of OG and DG via reverse hydrolysis reactions in ionic liquids and co-solvent mixtures. The N189F dalcochinase mutant and the non-aqueous reaction systems described here show great potential for future commercial production of long-chain alkyl glucosides.

Similar content being viewed by others
References
Adasch V, Hoffmann B, Milius W, Platz G, Voss G (1998) Preparation of alkyl α- and β-D-glucopyranosides, thermotropic properties and X-ray analysis. Carbohydr Res 314:177–187
Adlercreutz P (2008) Fundamentals of biocatalysis in neat organic solvents. In: Carrea G, Riva S (eds) Organic synthesis with enzymes in non-aqueous media. Wiley, Weinheim, pp 3–24
Alves LA, Almeida e Silva JB, Giulietti M (2007) Solubility of D-glucose in water and ethanol/water mixtures. J Chem Eng Data 52:2166–2170
Chung KH, Kim H, Park Y-K, Kim B-H, Kim S-J, Jung S-C (2019) Decyl glucoside synthesized by direct glucosidation of D-glucose over zeolite catalysts and its estrogenicity as non-endocrine disruptive surfactant. J Nanosci Nanotechnol 19:1172–1175
Czjzek M, Cicek M, Zamboni V, Bevan DR, Henrissat B, Esen A (2000) The mechanism of substrate (aglycone) specificity in β-glucosidases is revealed by crystal structures of mutant maize β-glucosidase-DIMBOA, -DIMBOAGlc, and -dhurrin complexes. Proc Natl Acad Sci USA 97:13555–13560
de Roode BM, Oliehoek L, van der Padt A, Franssen MCR, Boom RM (2001a) Downstream processing of enzymatically produced geranyl glucoside. Biotechnol Prog 17:881–886
de Roode BM, van Beek J, van der Padt A, Franssen MCR, Boom RM (2001b) The integrated enzymatic production and downstream processing of hexyl glucoside. Enzyme Microb Technol 29:513–520
Ducret A, Carrière J-F, Trani M, Lortie R (2002) Enzymatic synthesis of octyl glucoside catalyzed by almond β-glucosidase in organic media. Can J Chem 80:653–656
Ferrières V, Bertho J-N, Plusquellec D (1998) A convenient synthesis of alkyl D-glycofuranosiduronic acids and alkyl D-glycofuranosides from unprotected carbohydrates. Carbohydr Res 311:25–35
Kaftzik N, Wasserscheid P, Kragl U (2002) Use of ionic liquids to increase the yield and enzyme stability in the β-galactosidase catalyzed synthesis of N-acetyllactosamine. Org Process Res Dev 6:553–557
Ketudat Cairns JR, Esen A (2010) β-Glucosidases. Cell Mol Life Sci 67:3389–3405
Kim Y-M, Kim B-H, Ahn J-S, Kim G-E, Jin S-D, Nguyen T-H, Kim D (2009) Enzymatic synthesis of alkyl glucosides using Leuconostoc mesenteroides dextransucrase. Biotechnol Lett 31:1433–1438
Kongsaeree PT, Ratananikom K, Choengpanya K, Tongtubtim N, Sujiwattanarat P, Porncharoennop C, Onpium A, Svasti J (2010) Substrate specificity in hydrolysis and transglucosylation by family 1 β-glucosidases from cassava and Thai rosewood. J Mol Catal B Enzym 67:257–265
Lang M, Kamrat T, Nidetzky B (2006) Influence of ionic liquid cosolvent on transgalactosylation reactions catalyzed by thermostable β-glycosylhydrolase CelB from Pyrococcus furiosus. Biotechnol Bioeng 95:1093–1100
Lirdprapamongkol K, Svasti J (2000) Alkyl glucoside synthesis using Thai rosewood β-glucosidase. Biotechnol Lett 22:1889–1894
Pan S, Liu X, Xie Y, Yi Y, Li C, Yan Y, Liu Y (2010) Esterification activity and conformation studies of Burkholderia cepacia lipase in concentional organic solvents, ionic liquids and their co-solvent mixture media. Bioresour Technol 101:9822–9824
Schmidt-Lassen J, Lindhorst TK (2014) Exploring the meaning of sugar configuration in a supramolecular environment: comparison of six octyl glycoside micelles by ITC and NMR spectroscopy. Med Chem Commun 5:1218–1226
Srisomsap C, Subhasitanont P, Techasakul S, Surarit R, Svasti J (1999) Synthesis of homo- and hetero-oligosaccharides by Thai rosewood β-glucosidase. Biotechnol Lett 21:947–951
Svasti J, Phongsak T, Sarnthima R (2003) Transglucosylation of tertiary alcohols using cassava β-glucosidase. Biochem Biophys Res Commun 305:470–475
Thenchartanan P, Pitchayatanakorn P, Wattana-Amorn P, Ardá A, Svasti J, Jiménez-Barbero J, Kongsaeree PT (2020) Synthesis of long-chain alkyl glucosides via reverse hydrolysis reactions catalyzed by an engineered β-glucosidase. Enzyme Microb Technol. In Press https://doi.org/10.1016/j.enzmictec.2020.109591
Tongtubtim N, Thenchartanan P, Ratananikom K, Choengpanya K, Svasti J, Kongsaeree PT (2018) Multiple mutations in the aglycone binding pocket to convert the substrate specificity of dalcochinase to linamarase. Biochem Biophys Res Commun 504:647–653
van Rantwijk F, Woudenberg-van Oosterom M, Sheldon RA (1999) Glycosidase-catalysed synthesis of alkyl glycosides. J Mol Catal B 6:511–532
van Rantwijk F, Sheldon RA (2007) Biocatalysis in ionic liquids. Chem Rev 107:2757–2785
Verdoucq L, Morinière J, Bevan DR, Esen A, Vasella A, Henrissat B, Czjzek M (2004) Structural determinants of substrate specificity in family 1 β-glucosidases. J Biol Chem 279:31796–31803
Vic G, Thomas D, Crout DHG (1997) Solvent effect on enzyme-catalyzed synthesis of β-D-glucosides using the reverse hydrolysis method: application to the preparative-scale synthesis of 2-hydroxybenzyl and octyl β-D-glucopyranosides. Enzyme Microb Technol 20:597–603
Vijayakumar GR, George C, Divakar S (2007) Synthesis of n-alkyl glucosides by amyloglucosidase. Indian J Chem 46:314–319
Wang F, Ma Y, Liu Y-H, Zhang X, Zhang F, Linhardt RJ (2017) Improved octyl glucoside synthesis using immobilized β-glucosidase on PA-M with reduced glucose surplus inhibition. Biocatal Biotransfor 35:349–362
Yang R-L, Li N, Zong M-H (2012) Using ionic liquid cosolvents to improve enzymatic synthesis of arylalkyl β-D-glucopyranosides. J Mol Catal B 74:24–28
Yanhong B, Zhaoyu W, Yanyong M, Shangyong Z, Haijiang Z, Hao S (2012) Ionic liquid effects on the activity of β-glycosidase for the synthesis of salidroside in co-solvent systems. Chin J Catal 33:1161–1165
Acknowledgements
This work was financially supported by the Thailand Research Fund and the Commission on Higher Education [RMU5480004]; and the Higher Education Research Promotion and National Research University Project of Thailand. P.T. is a recipient of the Royal Golden Jubilee Ph.D. scholarship from the Thailand Research Fund.
Supporting information
Supplementary Fig. 1—Production of OG (a) and DG (b) via reverse hydrolysis reactions in various percentage ratios of alcochol:organic solvent.
Supplementary Fig. 2—Production of OG (a) and DG (b) via reverse hydrolysis reactions in various percentage ratios of alcohol:acetone.
Supplementary Fig. 3—Production of OG (a) and DG (b) via reverse hydrolysis reactions in various percentage ratios of alcohol:[BMIm][PF6].
Supplementary Fig. 4—Production of OG (a) and DG (b) via reverse hydrolysis reactions in various percentage ratios of alcohol:acetone:[BMIm][PF6].
Supplementary Fig. 5—The 1H-NMR spectra of OG (a) and DG (b), and the 1H-1H COSY spectra of OG (c) and DG (d) using CD3OD as solvent.
Supplementary Table 1—Production of OG and DG via reverse hydrolysis reactions in various percentage ratios of alcohol:[BMIm][PF6].
Supplementary Table 2—Production of OG and DG via reverse hydrolysis reactions in various percentage ratios of alcohol:acetone:[BMIm][PF6 ].
Supplementary Table 3—Mass determination of OG and DG by HRMS
Supplementary Table 4—Synthesis of OG by β-glucosidases via reverse hydrolysis reactions in organic solvent systems.
Supplementary Table 5—Synthesis of alkyl and arylalkyl glycosides by β-glycosidases via reverse hydrolysis reactions in ionic liquids systems.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thenchartanan, P., Wattana-Amorn, P., Svasti, J. et al. Improved synthesis of long-chain alkyl glucosides catalyzed by an engineered β-glucosidase in organic solvents and ionic liquids. Biotechnol Lett 42, 2379–2387 (2020). https://doi.org/10.1007/s10529-020-02960-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02960-8