Abstract
Adult parasitoids are well known to feed on sugar-rich resources such as floral nectar. Recently, an increasing body of evidence has shown that nectar is ubiquitously colonized by microorganisms and, as a consequence, microbial metabolic activity can affect several traits of floral nectar. Yet, how the fermentation of nectar by yeasts impacts the olfactory responses and performance of parasitoids is largely understudied, especially in the case of egg parasitoids. In this study, we investigated whether fermentation by the nectar yeasts Metschnikowia gruessii and M. reukaufii affects the olfactory responses of Trissolcus basalis and Ooencyrtus telenomicida, two egg parasitoid species associated with the southern green stink bug Nezara viridula. We also investigated how yeast fermentation affects the longevity and survival of the egg parasitoids. Results of static four-chamber olfactometer tests showed that nectar fermented by M. gruessii (but not by M. reukaufii) was attractive to both egg parasitoid species, whereas no significant yeast-mediated effects were found in terms of wasp longevity. Gas chromatography coupled with mass spectrometry (GC-MS) showed a clear separation of the volatile profiles among M. gruessii, M. reukaufii and non-fermented control nectar supporting the results of the insect bioassays. The results of our study highlight the need to consider the role of microbes when studying interactions between flower nectar and egg parasitoids and could have implications from a conservation biological control perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In agroecosystems, flowers represent essential niches that are frequently visited by a multitude of insect species that play different ecological functions (pests and beneficials) (Olesen et al. 2007; Géneau et al. 2012; Vannette 2020). Hymenopteran parasitoids are among the common insect visitors of flowers because they largely feed on nectar as adults (Jervis et al. 1993). In fact, adult parasitoids depend highly on floral nectar to sustain their energetic requirements (Winkler et al. 2009; Sobhy et al. 2018), as nectars are rich in sugars, amino acids, vitamins, and essential minerals (Lievens et al. 2015). Highly energetically demanding activities, such as host location and oviposition, require a continuous provision of sugar-rich resources to support parasitoid fitness and enhance efficiency (Takasu and Lewis 1995; Araj et al. 2011; Nafziger and Fadamiro 2011). In agricultural landscapes, flowers can be scarce, and, therefore, resident parasitoids may be starved (van Rijn and Wäckers 2016; Sentil et al. 2022). Thus, it is a common practice in conservation biological control to incorporate non-crop flowering plants along peripheries or within crop rows in the field enabling easy-access floral nectar for the parasitoids (Foti et al. 2017; Kowalska et al. 2022).
Despite the clear importance of floral nectar from a biological control perspective, we still have a long way to go in order to fully understand floral nectar ecology (Colazza et al. 2023). Floral nectars are rich in resources, and thus they also represent alluring habitats for diverse microorganisms (Pozo et al. 2014; Lievens et al. 2015; Vannette 2020). Nectar-inhabiting microbes are regarded as omnipresent colonizers of flowers, yet their role in conservation biological control is largely overlooked (Klaps et al. 2020). Nectar yeasts and bacteria are among the most frequently reported microbes that colonize floral nectars where they could grow into high densities (Herrera et al. 2009; Lievens et al. 2015). Nectar-inhabiting microbes generally modify various nectar traits including, but not limited to, the nectar volume (Vannette and Fukami 2018), nectar acidity (Vannette et al. 2013), sugar or amino acid ratios and compositions (Herrera et al. 2008; de Vega and Herrera 2013; Vannette et al. 2013), or even the occurrence of secondary metabolites (Vannette and Fukami 2016) and shifts in nectar temperature (Herrera and Pozo 2010). Nectar microbes are also thought to influence the scent profile of flowers due to the production of microbial volatile organic compounds (mVOCs) emitted from the fermented nectar (Golonka et al. 2014; Rering et al. 2018; Sobhy et al. 2018; Cusumano et al. 2022a). All these microbe-mediated effects on flower nectar could alter the foraging behavior of insect parasitoids and also their performance (Sobhy et al. 2018).
To date, there are only a few studies that have illustrated how fermentation by nectar microbes affects the foraging behavior and performance of insect parasitoids. These studies have focused on yeasts of the ascomycete genus Metschnikowia which are specialist nectar-inhabiting microbes documented to be associated with nectar globally (Brysch-Herzberg 2004; de Vega et al. 2009; Pozo et al. 2011; Canto and Herrera 2012), and thus they are of particular interest when studying plant-parasitoid interactions. mVOCs from nectar fermented by M. gruessii and M. reukaufii were found to be attractive to Aphidius ervi, a generalist parasitoid of aphids (Sobhy et al. 2018, 2019). Also, fermentation by these nectar specialist yeasts enhanced adult parasitoid performance compared to the generalist yeast species isolated from floral nectar (Sobhy et al. 2018). However, our current knowledge on the role of microbial fermentation by nectar-inhabiting yeasts in plant-parasitoid interactions is restricted to only this case study with an aphid parasitoid species. Therefore, further research efforts must be conducted to generalize parasitoid responses to microbe-fermented nectar.
In order to start filling this gap, we investigated the indirect effects of fermentation by the nectar yeasts M. gruessii and M. reukaufii on the olfactory responses, longevity, and survival of two egg parasitoids, Trissolcus basalis and Ooencyrtus telenomicida, both of which are associated with eggs of the southern green stink bug, Nezara viridula. The southern green stink bug is a cosmopolitan pest species responsible for serious economic losses in a wide variety of vegetable crops. Trissolcus basalis is the main biological control agent of N. viridula worldwide, which co-occurs together with O. telenomicida in southern Italy (Peri et al. 2014). These two egg parasitoids are known to feed on floral nectar and engage in intrinsic and extrinsic interspecific competition for possession of host resources (Peri et al. 2011; Cusumano et al. 2022b). Interestingly, the two egg parasitoid species also display divergent nutritional needs because O. telenomicida performs concurrent host feeding while T. basalis does not (Cusumano et al. 2015). Such behavior, which consists of feeding on host resources before laying an egg (Jervis and Kidd 1986), is displayed by some parasitoid species like O. telenomicida, which produce yolk-rich eggs and are expected to have high nutritional demands, particularly in terms of proteins necessary for egg development and maturation (Cusumano et al. 2015). Thus, we could hypothesize that these two stink bug egg parasitoids would have different responses toward yeast-fermented nectar.
To shed light on whether microbial fermentation by the nectar yeasts M. gruessii and M. reukaufii mediates preference behaviors and performances of egg parasitoids of insect pests, we produced synthetic nectar, inoculated with the individual yeast species, and underwent fermentation. Following removal of the yeast cells, we conducted a two-choice bioassay to assess the indirect yeast-mediated effects on the olfactory responses of the egg parasitoids when exposed simultaneously to yeast-fermented synthetic nectar and unfermented, non-inoculated control nectar. We also carried out a no-choice feeding bioassay to investigate whether consuming yeast-fermented synthetic nectar impacted the longevity and survival of the two stink bug egg parasitoids. The results of these experiments provide an assessment of the impact that microbial fermentation by specialist yeasts may have on the nectar selection behavior and fitness of stink bug egg parasitoids with potential application for biological control of insect pests.
Materials and methods
Study organisms
Nectar yeasts. The nectar yeasts Metschnikowia gruessii (isolate ST12.14/016) and M. reukaufii (ST12.14/017) (Saccharomycetales: Metschnikowiaceae) used throughout this study were isolated from the floral nectar of the wild comfrey plant, Symphytum officinale (Sobhy et al. 2018).
Parasitoids. The egg parasitoids Trissolcus basalis (Hymenoptera: Scelionidae) and Ooencyrtus telenomicida (Hymenoptera: Encyrtidae) were reared on egg masses of the southern green stink bug, Nezara viridula (Hemiptera: Pentatomidae). Southern green stink bug populations were initially established from field-collected adults throughout Palermo, Sicily, Italy (38°03′57″N, 13°28′10″E). Colonies were maintained in insect netted cages (47.5 × 47.5 × 47.5 cm, BugDorm-44545 MegaView Science Co. Ltd, Taichung, Taiwan) inside an experimental chamber (24 ± 2 °C, 70 ± 5% RH, and a L:D 16:8 photoperiod). Nymphs of different instars and adults were reared in separate cages. Fresh seasonal vegetables supplemented with sunflower/soybean seeds were provided ad libitum, and replenished every 2–3 days. Water dispensers in Petri dishes with cotton pads were also provided and crumpled or stripped tissue papers were either hung or scattered inside the adult cages to serve as the substrate for oviposition. The presence of eggs was checked every 2–3 days and collected when present for the maintenance of the stink bug population and the egg parasitoids.
Egg masses of N. viridula were individually exposed to five females of T. basalis or O. telenomicida for two days. Then, parasitized egg masses were isolated and incubated in 55 ml glass tubes (25 × 150 mm) closed with cotton plugs until parasitoid emergence. Drops of diluted organic honey (proportion honey:water 80:20 v:v) on parafilm were used to feed the parasitoids. Female egg parasitoids used in the olfactometer bioassay were between two and five days old and considered naïve (i.e., with no prior exposure to synthetic nectar odors). Female egg parasitoids used for the longevity and survival bioassays were newly emerged (≈ 24 h old) and fed only with water.
Inoculation and fermentation of synthetic nectar
To produce yeast-fermented synthetic nectar (referred to as 'YFSN' hereafter), synthetic nectar solutions were inoculated with either of the nectar yeasts, M. gruessii or M. reukaufii. We used synthetic nectar that mimicked the composition of many natural nectars and was produced by mixing sucrose and casamino acid, as described by Vannette and Fukami (2014). Specifically, synthetic nectar was prepared by mixing filter sterilized 50% w/v sucrose solution (Carlo Erba Reagents S.A.S., Val-de-Reuil, France) and 3.16 mM casamino acid (OmniPur, Merck KGaA, Darmstadt, Germany) (Vannette and Fukami 2014).
For the inoculation and fermentation of synthetic nectar, following the procedures described in Sobhy et al. (2018), yeast isolates were streaked on YPDA (Yeast Potato Dextrose Agar) and incubated for two days at 25 °C. Next, a single colony was inoculated in 10 ml YPDB (Yeast Potato Dextrose Broth) in a sterile 50 ml conical centrifuge tube and incubated overnight in a rotary shaker (150 rpm) with a temperature set at 25 °C. Then, cultures were washed twice with sterile physiological water (sodium chloride solution 0.9%) and suspended in the same buffer to obtain an optical density of 1 (OD600). Twenty milliliters of sterile synthetic nectar in 50 ml centrifuge tubes were then inoculated with 100 µl of cell suspension of either yeast strain and subjected to static incubation at 25 °C for five days. The incubation period was based on the approximate floral lifespan of many plant species (Peay et al. 2012; Vannette and Fukami 2014; Lenaerts et al. 2016, 2017). Non-inoculated sterile synthetic nectar was also included during the fermentation to represent the control treatment. After fermentation, YFSN and the control were centrifuged at 10,000 rpm for 15 min and filtered using a 0.20-µm syringe filter to obtain cell-free cultures. Aliquots of filtered YFSN were streaked on YPDA to check potential yeast growth or contamination. Cell-free YFSN was then aliquoted to 1 ml amber glass vials and stored at − 80 °C until the subsequent bioassays. The freezing method is regarded to have no adverse effects on the general chemistry and quality of the YFSN (Peay et al. 2012).
Impact of yeast-fermented nectars on parasitoid olfactory responses
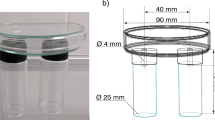
A static olfactometer with four equally divided chambers was used to assess the olfactory response behavior of the egg parasitoids toward the YFSN. The olfactometer was made of acrylic glass formed into a cylinder-shaped enclosure with a height of 4.5 cm and 20 cm in diameter, as described by Steidle and Schoeller (1997). The static four-chamber olfactometer was fitted with a removable gauze cover (0.5 mm mesh) supported by an acrylic glass rim (0.9 mm high), which served as a walking arena for the insects. A netted lid was also laid on the top of the olfactometer to prevent the insects from escaping while ensuring vertical diffusion of odor in order to prevent saturation of volatiles inside the chamber. Two hundred µl of either test solution (YFSN, fermented by either M. gruessii or M. reukaufii) or control solution (non-fermented synthetic nectar) was pipetted on a filter paper disc (3.8 cm diameter, Whatman No. 1) placed in a Petri dish. The same test treatments were placed in two diagonal olfactometer chambers, while the other two chambers were for the control. About 24 h before performing the bioassays, female parasitoids were individually put in small vials (1.5 × 5.0 cm) without food to induce starvation. One insect was released in the center of the walking arena in every run, and the insect was given one minute to be acclimatized. After that, the insect olfactory response was evaluated, and the residence time spent in each chamber was recorded for 5 min with the aid of a video camera (Logitech C920 HD Pro Webcam) using JWatcher V 0.9 software (https://www.jwatcher.ucla.edu/). In each assay, 40 naïve female parasitoids for each species were used, and each of them was only used once and assigned as one independent replicate. The olfactometer was rotated quarterly after one replication to avoid positional bias. The filter paper disc with synthetic nectar was replaced with every replicate. After each bioassay, the olfactometer was cleaned with 90% ethyl alcohol and tap water and left overnight to dry up at room temperature. The experiment was performed in a rack covered with a black curtain with a white fluorescent lamp (Philips, TLD 58 W/640) situated approximately 0.5 m just above the center of the static four-chamber olfactometer. Windows in the olfactometer room were also covered with cardboard to eliminate any visual bias due to light coming from the outside. The experiments were carried out between 09h00 and 17h00 with a room temperature maintained at 21 ± 5 °C and 50 ± 10% RH.
Impact of yeast-fermented nectars on parasitoid longevity and survival
To determine whether the changes in synthetic nectar due to microbial fermentation affect parasitoid fitness, a longevity and survival bioassay was carried out. Longevity was considered as the length of time that the adult parasitoid lived from emergence up until the point of death, while survival was considered as the percentage of parasitoid individuals that are still alive in a given time after being exposed or fed on the different synthetic nectars. Egg parasitoids were isolated individually in 5 ml glass vials. Drops of YFSN (fermented by M. gruessii or M. reukaufii) were provided ad libitum at the glass vial's side and regularly replenished. As a comparison, unfermented synthetic nectar was included in the experiment, while water was included as a negative control. The bioassay was conducted at 24 ± 2 °C, 70 ± 5% RH, and a L:D16:8 photoperiod. For each treatment, 15 mated female parasitoids were used. Dead insects were scored daily, and the experiments were concluded when all insects had died.
Chemical analysis of yeast-fermented nectars
To assess whether yeast fermentation affected the VOC profiles, nectars were analyzed by headspace solid phase micro extraction gas chromatography followed by mass spectrometry detection (HS–-SPME-GC-MS) as described previously (Goelen et al. 2020). Briefly, GC-MS analyses were performed with a Thermo Trace 1300 GC system equipped with a MXT-5 column (30 m length × 0.18 mm inner diameter × 0.18 μm film thickness; Restek Bellefonte, Pennsylvania, USA) and compounds were subsequently detected by a ISQ mass spectrometer (Thermo Fisher Scientific). The HS-SPME volatile collection was conducted using a 50/30 μm DVB/CAR/PDMS coating fiber (Supelco, Bellefonte, Pennsylvania, USA). After five min of equilibration, the SPME fiber was exposed to the headspace sample for 30 min. Splitless injection was used with an inlet temperature of 320 °C, a split flow of 9 ml min-1, a purge flow of 5 ml min-1 and an open valve time of 3 min. To obtain a pulsed injection, a programmed gas flow was used whereby the helium gas flow was set at 2.7 ml min-1 for 0.1 min, followed by a decrease in flow of 20 ml min-1 to the normal 0.9 ml min-1. The GC oven was programmed as follows: the temperature was initiated at 30 °C, held for 3 min and then raised to 80 °C at 7 °C min-1. Next, the temperature was raised to 125 °C at 2 °C min-1, and finally the temperature was raised to 270 °C at 8 °C min-1 for 15 min to thermally desorb the compounds that were trapped on the fiber in the injection port of the chromatograph. Mass spectra were recorded in centroid mode using a mass acquisition range of 33–550 atomic mass units, a scan rate of 5 scans s-1 and an electron impact ionization energy of 70 eV. A mix of linear n-alkanes (from C7 to C23; Supelco) was injected into the GC-MS under identical conditions to serve as external retention index markers. Compounds were identified and quantified as described in Reher et al. (2019). Chromatograms were analyzed with AMDIS 32 to deconvolute overlapping peaks. Obtained spectra were subsequently annotated using the NIST MS Search v2.0 g software, using the NIST2011, FFNSC and Adams libraries. Peak areas were compared to the background signal, which was identified by running a GC-MS with a sample obtained from demineralized water. The background signal was then subtracted from the peak areas of the corresponding tentatively identified compounds. When the difference in peak area fell below a set threshold of 1,000, compounds were eliminated from further analysis. To further extract and integrate the compound elution profiles, a file was used with all our target compounds containing the expected retention times and spectrum profiles. Extraction was performed for every compound in every chromatogram over a time-restricted window using weighted non-negative least square analysis (Lawson and Hanson 1995), and for every compound, the peak areas were computed from the extracted profiles.
Statistical analysis
Olfactometer, longevity and survival data were analyzed with the R software, version R 4.1.3 (R Core Team 2022). Initially, the normality assumption of the data on parasitoid residence time above the static olfactometer chambers was assessed using the Shapiro-Wilk test. The analysis revealed that the normality assumption was not violated (p > 0.05). Consequently, a parametric analysis was performed using a t-test for dependent samples.
Longevity data were analyzed using Generalized Linear Models (GLMs), fitting a gamma error distribution and a reciprocal link function (Crawley 2007). Tukey’s test was used for post-hoc comparisons of means, given that means among different treatments were significantly different. The survival of the adult egg parasitoids fed on the different nectars was analyzed using the Kaplan-Meier estimator with the log-rank test of the Benjamini-Hochberg procedure for pairwise comparisons. Consequently, survival probability curves were generated for both egg parasitoids.
Multivariate data analysis (Principal Component Analysis—PCA) was used to analyze peak areas of the VOCs detected. Therefore, peak areas were first log-transformed, mean-centered and subsequently scaled to unit variance before they were subjected to the analysis using MetaboAnalyst (Xia et al. 2009). The results of the analysis were visualized in score plots, which reveal the sample structure according to model components, and loading plots, which display the contribution of the variables to these components.
Results
Impact of yeast-fermented nectars on parasitoid olfactory responses
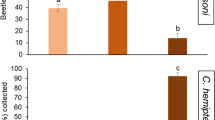
Trissolcus basalis females preferred the odors emitted by M. gruessii-fermented synthetic nectar to unfermented synthetic nectar (t = − 3.112, df = 39, p = 0.003). However, no differences in terms of residence time were found when wasps were given a choice between M. reukaufii-fermented synthetic nectar and unfermented synthetic nectar (t = − 0.083, df = 39, p = 0.934) (Fig. 1a). The same trend was found in the response of O. telenomicida to YFSN. Parasitoids showed a preference for odors emitted by M. gruessii-fermented synthetic nectar over unfermented synthetic nectar (t = − 3.068, df = 39, p = 0.004), whereas no preferences were displayed between M. reukaufii-fermented synthetic nectar and the control treatment (t = − 0.284, df = 39, p = 0.778) (Fig. 1b).
Olfactory responses of the female egg parasitoids a Trissolcus basalis and b Ooencyrtus telenomicida assessed in terms of residence time in two-choice assays over an observation time of 300 s. Test treatments represent synthetic nectars fermented by the yeasts Metschnikowia gruessii (black bars) and Metschnikowia reukaufii (light gray bars). Unfermented synthetic nectar was used in all the pairwise comparisons as a control (white bars). Each treatment was replicated 40 times (paired t-tests for dependent samples, *p < 0.05; ns = not significant). Error bars represent SE
Impact of yeast-fermented nectars on parasitoid longevity and survival
When testing the effects of the different YFSNs, non-fermented synthetic nectar and water (control) on the longevity of T. basalis, only water had a statistically significant effect among all treatments (F = 112.25, df = 3,56 p < 0.001) (Fig. 2a). Parasitoids provided only with water died after 3–5 days. No differences were found when wasps were fed with unfermented synthetic nectar compared with wasps fed with synthetic nectar fermented by the two Metschnikowia strains. Longevity of wasps was above 90 days in the three treatments. The same pattern was found for the survival curves of T. basalis (χ2 = 78.1, df = 3, p < 0.0001) (Fig. 2b). Likewise, only the water treatment had a significant effect on the longevity of O. telenomicida when compared with the synthetic nectars (F = 72.064, df = 3,56 p < 0.001). No differences were detected between parasitoid wasps fed on synthetic nectar fermented by the two Metschnikowia strains and wasps fed on unfermented synthetic nectar (Fig. 3a). The same trend was found for the survival curves of O. telenomicida (χ2 = 70.4, df = 3, p < 0.0001) (Fig. 3b).
a Boxplot illustrating the distribution of longevity for the adult female egg parasitoid Trissolcus basalis fed on synthetic nectar fermented by Metschnikowia gruessii (M.g.), synthetic nectar fermented by Metschnikowia reukaufii (M.r.), unfermented/uninoculated synthetic nectar (SN, reference) and water (negative control). The box represents the interquartile range (IQR), with the median indicated by the bolded horizontal line inside the box. Whiskers extend to the minimum and maximum values within 1.5 times the IQR. Different letters indicate significant differences among treatments (GLM, p < 0.05). b Kaplan-Meier survival curves for Trissolcus basalis. Different letters indicate significant differences among parasitoid survival curves according to the pairwise log-rank post-hoc tests with the Benjamini-Hochberg correction (p < 0.05)
a Boxplot illustrating the distribution of longevity for the adult female egg parasitoid Ooencyrtus telenomicida fed on synthetic nectar fermented by Metschnikowia gruessii (M.g.), synthetic nectar fermented by Metschnikowia reukaufii (M.r.), unfermented/uninoculated synthetic nectar (SN, reference) and water (negative control). The box represents the interquartile range (IQR), with the median indicated by the bolded horizontal line inside the box. Whiskers extend to the minimum and maximum values within 1.5 times the IQR. Different letters indicate significant differences among treatments (GLM, p < 0.05). b Kaplan-Meier survival curves for Ooencyrtus telenomicida. Different letters indicate significant differences among parasitoid survival curves according to the pairwise log-rank post-hoc tests with the Benjamini-Hochberg correction (p < 0.05)
Volatile analysis of yeast-fermented nectars
Analysis of the VOCs that were collected from the different yeast-fermented nectars and the unfermented synthetic nectar revealed large differences in qualitative and quantitative chemical composition of the VOC blends (Fig. 4a). In total, 38 VOCs were consistently detected across the three biological replicates for M. gruessii, 33 VOCs were detected for M. reukaufii and 21 compounds were found in the unfermented synthetic nectar (Table 1). A PCA of the VOCs showed that the first component accounted for 58.8% of the total variation in volatile profiles whereas the second component accounted for 18.9% of the variation (Fig. 4a). The five greatest loadings of PC1, in descending order of absolute values, were for ethyl-(E)-cinnamate (ID 37: 0.1975), 2-butanone (ID 4: − 0.1964), 3-methyl-2-hexanol (ID 15: 0.1948), propyl acetate (ID 9: 0.1947) and pentyl-octanoate (ID 35: 0.1940), whereas the greatest loadings of PC2 were for 2-ethyl-1-hexanol (ID 23: 0.3259), benzyl alcohol (ID 21: 0.3211), isoborneol (ID 31: − 0.3080), prenyl isobutyrate (ID 25: − 0.2788) and γ-terpinene (ID 26: 0.2676) (Fig. 4b).
Principal component analysis (PCA) of synthetic nectar fermented by Metschnikowia gruessii (M.g.) and Metschnikowia reukaufii (M.r.) and non-fermented synthetic nectar (Control, reference). All biological replicates (N = 3) indicate cell-free nectars. a Score plot visualize the grouping pattern of the samples according to the first two principal components (PCs) with the explained variance in parenthesis. Ellipses indicate 95% confidence interval. b Loading plot of the first two PCs showing the contribution of each compound to the two PCA components. See Table 1 for the full list of compound IDs
Discussion
In this study, we investigated the indirect effect of two nectar specialist yeasts in the genus Metschnikowia on the olfactory responses and longevity and survival of two egg parasitoids, T. basalis and O. telenomicida. Results revealed that both parasitoids responded positively to the odors of the synthetic nectars fermented by the yeast M. gruessii, while no significant differences were found in the case of M. reukaufii in the two-choice bioassays. Despite the differential yeast-mediated effects on the olfactory responses of the two egg parasitoids, continuous consumption of M. gruessii-fermented nectar did not result in differences in adult longevity and survival compared to M. reukaufii-fermented nectar. To date, this is the first evidence documenting the relevant—yet overlooked—role of nectar-inhabiting yeasts on egg parasitoids.
Interestingly, it has been demonstrated that a variety of insects prefer flowers colonized by Metschnikowia yeasts and that they forage and remove more nectar from yeast-colonized flowers than from uncolonized flowers (Herrera et al. 2013; Schaeffer et al. 2014; Schaeffer and Irwin 2014; 2017). Specialized nectar microbes, such as Metschnikowia yeasts, are thought to alter nectar traits to lure flower-visiting insects, which then serve as vectors for fungal dispersion to new environments (Christiaens et al. 2014; Rering et al. 2018). So far, it is only widely known that nectar-inhabiting yeasts attract pollinators (Pozo et al. 2014), but interestingly, a few recent studies also suggest that carnivorous insects are similarly attracted to the volatiles emitted by specialist yeasts. In fact, we found that synthetic nectar fermented by M. gruessii attracted both T. basalis and O. telenomicida, and the mVOCs from the same Metschnikowia strains were also found to attract the aphid parasitoid A. ervi (Sobhy et al. 2018, 2019). The fact that only fermentation by M. gruessii (but not M. reukaufii) elicited a behavioral response in both egg parasitoids suggests that the nectar odors between the two closely related yeasts would be different although it is not clear, from an evolutionary perspective, why the egg parasitoids displayed such preferences. The GC-MS analyses showed that nectar fermentation strongly affected the chemical composition of the nectar odors and the PCA clearly separated the two different yeast treatments, corroborating our findings in the olfactometer. According to the PCA model, the compounds that are mostly responsible for the chemical differences are ethyl-(E)-cinnamate, 2-butanone, 3-methyl-2-hexanol, propyl acetate and pentyl-octanoate. Further experiments with the aid of synthetic standards are required to confirm the role of these compounds in mediating egg parasitoid attraction towards odors from yeast-fermented nectars.
Up to now, it is not clear how microbes affect the nectar quality for parasitoids (Cusumano and Lievens 2023). On one hand, it is reasonable to assume that microbial fermentation may remove, or reduce the amount of key sugars/amino acids (Vannette and Fukami 2018) and thus reduce nectar quality for parasitoids (Colazza et al. 2023). On the other hand, microbial fermentation cannot simply be considered a mere removal of key sugars/amino acids because microbes can also produce compounds, including vitamins, in the nectar media, and there are studies showing that microbial fermentation can positively affect parasitoid longevity (Lenaerts et al. 2017). Our study supports the latter hypothesis since we found that fermentation by Metschnikowia spp. did not reduce the longevity and survival of T. basalis and O. telenomicida compared with unfermented synthetic nectar.
Surprisingly, the two egg parasitoid species performed in a way contrary to our expectations, despite their differential dietary requirements. Given that female O. telenomicida must produce yolk-rich eggs and therefore have high protein requirements (Cusumano et al. 2015), we hypothesized that O. telenomicida females would perform poorly when fed on YFSN. However, results revealed otherwise, suggesting that yeast fermentation could yield nectar that is capable of fulfilling diverse nutritional needs across parasitoid species. It is worth noting that our study aimed at assessing the indirect effects that microbial fermentation per se may have on egg parasitoids. Future research efforts should be directed towards understanding the role played by the yeast cells themselves since microbes may have a direct contribution to insect nutrition and well-being (Crotti et al. 2009; Sobhy et al. 2018). This could be particularly important in the case of O. telenomicida which requires more protein and microbes can act as an additional protein source to floral visitors.
Our results corroborate the findings of previous reports, showing that nectar specialist yeasts are unlikely to affect the performance of floral visitors and may provide honest signals for food resource location. For example, A. ervi parasitoids fed on synthetic nectar fermented by M. gruessii and M. reukaufii did not reduce their longevity and survival compared with unfermented synthetic nectar (Sobhy et al. 2018). While we found no effects of yeast fermentation on the longevity and survival of egg parasitoids, we cannot exclude that other fitness-related traits, such as egg-laying capacity, can be negatively affected, because there are parasitoid species that may require more proteins for egg development, such as the O. telenomicida. Although it has been reported that M. reukaufii did not negatively affect egg laying and the number of eggs produced by pollinators (Schaeffer et al. 2017), to date, no studies have looked at the effect of microbial fermentation on the reproductive capacity of egg parasitoid species.
In summary, we have demonstrated that fermentation by nectar yeasts can positively influence the olfaction of two stink bug egg parasitoids without any detectable negative effect on their longevity and survival. Our findings indicate that specialist nectar yeasts hold the potential to develop ecological strategies that can attract parasitoids in the field or help in locating high-quality resources through honest microbial signaling which could lead to improved parasitoid fitness and effectiveness in pest control. However, in order to fully understand the potential applications that nectar-inhabiting microbes such as Metschnikowia spp. may have for biological control, future research should be carried out to evaluate not only the indirect effects due to microbial fermentation, but also the direct effects due to microbial cells naturally thriving in floral nectar. Once both direct and indirect effects on parasitoids will be unraveled, we believe that microbe-mediated approaches targeting floral nectar could represent additional tools that biological control practitioners may use to improve insect pest management programs based on conservation biological control.
References
Araj SE, Wratten S, Lister A, Buckley H, Ghabeish I (2011) Searching behavior of an aphid parasitoid and its hyperparasitoid with and without floral nectar. Biol Control 57(2):79–84
Brysch-Herzberg M (2004) Ecology of yeasts in plant bumblebee mutualism in Central Europe. FEMS Microbiol Ecol 50(2):87–100
Canto A, Herrera CM (2012) Micro-organisms behind the pollination scenes: microbial imprint on floral nectar sugar variation in a tropical plant community. Ann Bot 110(6):1173–1183
Christiaens JF, Franco LM, Cools TL, de Meester L, Michiels J, Wenseleers T, Hassan BA, Yaksi E, Verstrepen KJ (2014) The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep 9(2):425–432
Colazza S, Peri E, Cusumano A (2023) Chemical ecology of floral resources in conservation biological control. Annu Rev Entomol 68:13–29
Crawley MJ (2007) The R book. Wiley, New York
Crotti E, Damiani C, Pajoro M, Gonella E, Rizzi A, Ricci I, Negri I, Scuppa P, Rossi P, Ballarini P, Raddadi N, Marzorati M, Sacchi L, Clementi E, Genchi M, Mandrioli M, Bandi C, Favia G, Alma A, Daffonchio D (2009) Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ Microbiol 11(12):3252–3264
Cusumano A, Lievens B (2023) Microbe-mediated alterations in floral nectar: consequences for insect parasitoids. Curr Opin Insect Sci 21:101116
Cusumano A, Peri E, Boivin G, Colazza S (2015) Fitness costs of intrinsic competition in two egg parasitoids of a true bug. J Insect Physiol 81:52–59
Cusumano A, Bella P, Peri E, Rostás M, Guarino S, Lievens B, Colazza S (2022a) Nectar-inhabiting bacteria affect olfactory responses of an insect parasitoid by altering nectar odors. Microb Ecol 86(1):364–376
Cusumano A, Peri E, Alinç T, Colazza S (2022b) Contrasting reproductive traits of competing parasitoids facilitate coexistence on a shared host pest in a biological control perspective. Pest Manag Sci 78(8):3376–3383
de Vega C, Herrera CM (2013) Microorganisms transported by ants induce changes in floral nectar composition of an ant-pollinated plant. Am J Bot 100(4):792–800
de Vega C, Herrera CM, Johnson SD (2009) Yeasts in floral nectar of some South African plants: quantification and associations with pollinator type and sugar concentration. S Afr J Bot 75(4):798–806
Foti MC, Rostás M, Peri E, Park KC, Slimani T, Wratten SD, Colazza S (2017) Chemical ecology meets conservation biological control: identifying plant volatiles as predictors of floral resource suitability for an egg parasitoid of stink bugs. J Pest Sci 90(1):299–310
Géneau CE, Wäckers FL, Luka H, Daniel C, Balmer O (2012) Selective flowers to enhance biological control of cabbage pests by parasitoids. Basic Appl Ecol 13(1):85–93
Goelen T, Sobhy IS, Vanderaa C, Boer JG, Delvigne F, Francis F, Wäckers F, Rediers H, Verstrepen KJ, Wenseleers T, Jacquemyn H, Lievens B (2020) Volatiles of bacteria associated with parasitoid habitats elicit distinct olfactory responses in an aphid parasitoid and its hyperparasitoid. Funct Ecol 34(2):507–520
Golonka A, Johnson B, Freeman J, Hinson DW (2014) Impact of nectarivorous yeasts on Silene caroliniana’s scent. East Biol 3:1–26
Herrera CM, Pozo MI (2010) Nectar yeasts warm the flowers of a winter-blooming plant. Proc R Soc B 277(1689):1827–1834
Herrera CM, García IM, Pérez R (2008) Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89(9):2369–2376
Herrera CM, de Vega C, Canto A, Pozo MI (2009) Yeasts in floral nectar: a quantitative survey. Ann Bot 103(9):1415–1423
Herrera CM, Pozo MI, Medrano M (2013) Yeasts in nectar of an early-blooming herb: sought by bumble bees, detrimental to plant fecundity. Ecology 94(2):273–279
Jervis MA, Kidd NAC (1986) Host feeding strategies in hymenopteran parasitoids. Biol Rev 61(4):395–434
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 27(1):67–105
Klaps J, Lievens B, Álvarez-Pérez S (2020) Towards a better understanding of the role of nectar-inhabiting yeasts in plant–animal interactions. Fungal Biol Biotechnol 7:1
Kowalska J, Antkowiak M, Sienkiewicz P (2022) Flower strips and their ecological multifunctionality in agricultural fields. Agriculture 12(9):1470
Lawson C, Hanson RJ (1995) Solving least squares problems. Society for Industrial and Applied Mathematics, Philadelphia
Lenaerts M, Pozo MI, Wäckers F, van den Ende W, Jacquemyn H, Lievens B (2016) Impact of microbial communities on floral nectar chemistry: potential implications for biological control of pest insects. Basic Appl Ecol 17(3):189–198
Lenaerts M, Goelen T, Paulussen C, Herrera-Malaver B, Steensels J, van den Ende W, Verstrepen KJ, Wäckers F, Jacquemyn H, Lievens B (2017) Nectar bacteria affect life history of a generalist aphid parasitoid by altering nectar chemistry. Funct Ecol 31(11):2061–2069
Lievens B, Hallsworth JE, Pozo MI, Belgacem ZB, Stevenson A, Willems KA, Jacquemyn H (2015) Microbiology of sugar-rich environments: diversity, ecology and system constraints. Environ Microbiol 17(2):278–298
Nafziger TD, Fadamiro HY (2011) Suitability of some farmscaping plants as nectar sources for the parasitoid wasp, Microplitis croceipes (Hymenoptera: Braconidae): effects on longevity and body nutrients. Biol Control 56(3):225–229
Olesen JM, Dupont YL, Ehlers BK, Hansen DM (2007) The openness of a flower and its number of flower visitor species. Taxon 56(3):729–736
Peay KG, Belisle M, Fukami T (2012) Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proc R Soc B 279(1729):749–758
Peri E, Cusumano A, Agrò A, Colazza S (2011) Behavioral response of the egg parasitoid Ooencyrtus telenomicida to host-related chemical cues in a tritrophic perspective. BioControl 56(2):163–171
Peri E, Cusumano A, Amodeo V, Wajnberg E, Colazza S (2014) Intraguild interactions between two egg parasitoids of a true bug in semi-field and field conditions. PLoS ONE 9(6):e99876
Pozo MI, Herrera CM, Bazaga P (2011) Species richness of yeast communities in floral nectar of southern Spanish plants. Microb Ecol 61(1):82–91
Pozo M, Lievens B, Jacquemyn H (2014) Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In: Peck RL (ed) Nectar: production, chemical composition and benefits to animals and plants. Nova Science Publishers Inc, New York, pp 1–45
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/
Reher T, van Kerckvoorde V, Verheyden L, Wenseleers T, Beliën T, Bylemans D, Martens JA (2019) Evaluation of hop (Humulus lupulus) as a repellent for the management of Drosophila suzukii. J Crop Prot 124:104839
Rering CC, Beck JJ, Hall GW, McCartney MM, Vannette RL (2018) Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol 220(3):750–759
Schaeffer RN, Irwin RE (2014) Yeasts in nectar enhance male fitness in a montane perennial herb. Ecology 95(7):1792–1798
Schaeffer RN, Phillips CR, Duryea MC, Andicoechea J, Irwin RE (2014) Nectar yeasts in the tall larkspur Delphinium barbeyi (Ranunculaceae) and effects on components of pollinator foraging behavior. PLoS ONE 9(10):e108214
Schaeffer RN, Mei YZ, Andicoechea J, Manson JS, Irwin RE (2017) Consequences of a nectar yeast for pollinator preference and performance. Funct Ecol 31(3):613–621
Sentil A, Wood TJ, Lhomme P, Hamroud L, el Abdouni I, Ihsane O, Bencharki Y, Rasmont P, Christmann S, Michez D (2022) Impact of the “farming with alternative pollinators” approach on crop pollinator pollen diet. Front Ecol Evol 10:824474
Sobhy IS, Baets D, Goelen T, Herrera-Malaver B, Bosmans L, van den Ende W, Verstrepen KJ, Wäckers F, Jacquemyn H, Lievens B (2018) Sweet scents: nectar specialist yeasts enhance nectar attraction of a generalist aphid parasitoid without affecting survival. Front Plant Sci 9:1009
Sobhy IS, Goelen T, Herrera-Malaver B, Verstrepen KJ, Wäckers F, Jacquemyn H, Lievens B (2019) Associative learning and memory retention of nectar yeast volatiles in a generalist parasitoid. Anim Behav 153:137–146
Steidle JLM, Schoeller M (1997) Olfactory host location and learning in the granary weevil parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). J Insect Behav 10(3):331–342
Takasu K, Lewis WJ (1995) Importance of adult food sources to host searching of the larval parasitoid Microplitis croceipes. Biol Control 5:25–30
van Rijn PCJ, Wäckers FL (2016) Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J Appl Ecol 53(3):925–933
Vannette RL (2020) The floral microbiome: plant, pollinator, and microbial perspectives. Annu Rev Ecol Evol Syst 51:363–386
Vannette RL, Fukami T (2014) Historical contingency in species interactions: towards niche-based predictions. Ecol Lett 17(1):115–124
Vannette RL, Fukami T (2016) Nectar microbes can reduce secondary metabolites in nectar and alter effects on nectar consumption by pollinators. Ecology 97(6):1410–1419
Vannette RL, Fukami T (2018) Contrasting effects of yeasts and bacteria on floral nectar traits. Ann Bot 121(7):1343–1349
Vannette RL, Gauthier MPL, Fukami T (2013) Nectar bacteria, but not yeast, weaken a plant—pollinator mutualism. Proc R Soc B 280:20122601
Winkler K, Wäckers F, Pinto DM (2009) Nectar-providing plants enhance the energetic state of herbivores as well as their parasitoids under field conditions. Ecol Entomol 34(2):221–227
Xia J, Psychogios N, Young N, Wishart DS (2009) MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 37:W652–W660
Acknowledgements
This research was partially supported by the PRIMA_MIUR—Partnership for Research and Innovation in the Mediterranean Area with the project "Boosting functional biodiversity to maximize ecosystem services for Mediterranean crop production” (ECOBOOST).
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
There are no ethical concerns regarding the organisms and the topic of this research.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals (vertebrates) performed by any of the authors.
Additional information
Handling Editor: Raul Laumann
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ermio, J.D.L., Peri, E., Bella, P. et al. The indirect effect of nectar-inhabiting yeasts on olfactory responses and longevity of two stink bug egg parasitoids. BioControl (2024). https://doi.org/10.1007/s10526-023-10237-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10526-023-10237-y