Abstract
Root-knot nematodes cause global economic losses in a wide range of crops. We investigated the potential of seed coatings of the cover crop Phacelia tanacetifolia (Boraginaceae) when inoculated with the nematophagous fungus Pochonia chlamydosporia (Hypocreales: Clavicipitaceae) to protect subsequently grown tomato plants from root galling caused by the root-knot nematode Meloidogyne hapla (Tylenchida: Meloidogynidae). Therefore, seeds of P. tanacetifolia were coated with P. chlamydosporia blastospores and planted in M. hapla-infested pots. After 50 days of growth in infested soil, M. hapla eggs were extracted from P. tanacetifolia roots and quantified. Tomato plants grown in the remaining soil served as bioindicator of M. hapla infestation as expressed by the gall index. Results showed that seed coating of P. tanacetifolia with P. chlamydosporia (290 ± 51 CFU per seed) reduced the number of M. hapla eggs up to 95.6% in comparison to untreated controls. Pochonia chlamydosporia as blastospore suspension (5·108 blastospores per 600 ml soil) reduced the number of M. hapla eggs by up to 75.5%. Additionally, tomato plants grown for 50 days in substrates previously planted with P. tanacetifolia seeds coated with P. chlamydosporia showed a significantly lower gall index than plants grown in untreated pots. In conclusion, biological enhancement of P. tanacetifolia by seed coating with P. chlamydosporia successfully reduced M. hapla and thus provides an additional tool in the management of this nematode. The method still has potential for further improvement such as increasing blastospore viability within the seed coating by optimized formulation technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant-parasitic nematodes can reduce crop yields by feeding on plant tissues, thus causing substantial economic losses of agronomically important crops (Nicol et al. 2011; Jones et al. 2013). In particular, root-knot nematodes Meloidogyne spp. are among the most harmful plant-parasitic nematodes, being polyphagous and distributed worldwide (Carter 1985; Jones et al. 2013). In temperate regions, Meloidogyne hapla (Tylenchida: Meloidogynidae) is one of the most damaging root-knot nematodes, mainly infecting vegetable crops. A survey carried out in Germany detected Meloidogyne in 51% of soil samples of vegetable fields, with M. hapla being the most prominent species (Hallmann et al. 2007). Maximum densities of M. hapla reached up to 3,300 juveniles in 100 ml soil. Damage by M. hapla is most severe in sandy soils and on susceptible crops such as carrots and onions (Hallmann et al. 2007; Wesemael et al. 2011). Control of M. hapla can be achieved, among other means, by crop rotation with non-host plants (e.g., cereals) or resistant cultivars (e.g., fodder radish). However, these methods are often insufficient at high infestation densities (Moens et al. 2009; Wesemael et al. 2011; Vestergård 2019). The introduction of a sanitation year as suggested by Hallmann (2021) successfully controls M. hapla and is cost-effective. Here, crops were exclusively grown for the purpose of reducing M. hapla below the economic threshold level, i.e., the farmer does not generate any income during this period. Chemical control is, in most cases, not a viable option due to increasing requirements regarding human and ecosystem safety and costs. At the same time, cover crops and biological control are of increasing interest in nematode management, raising the question whether both strategies can be used concomitantly to further improve nematode control.
Cover crops are an indispensable tool in sustainable cropping practices. One such cover crop is Phacelia tanacetifolia (Hydrophylloideae), which is widely grown in temperate climates (Scavo et al. 2022), e.g., in the Czech Republic and Germany. The beneficial effect of P. tanacetifolia as a cover crop is mainly due to its suppression of volunteer plants and weeds (Brant et al. 2009; Brust et al. 2014; Handlířová et al. 2017), but also its low susceptibility to plant-parasitic nematodes. The following early spring, plant residues are incorporated into the soil and the main crop is sown. Being the only cultivated species in the subfamily Hydrophylloideae (Boraginaceae), P. tanacetifolia has its own unique spectrum of pests that in most cases is different from that of other crops grown within a rotation. For M. hapla, P. tanacetifolia is a maintenance host, meaning that the nematode population will neither increase nor decrease during the cropping season (Viaene and Abawi 1998). This makes P. tanacetifolia an ideal candidate for biological enhancement, especially in cases where the succeeding main crop is a good host for M. hapla such as tomato or potato.
The potential of antagonistic microorganisms to control plant-parasitic nematodes is well documented (Hallmann et al. 2009; Stirling 2014; Peiris et al. 2020). However, only few commercial products are available for control of Meloidogyne spp. (Hallmann et al. 2009; Wesemael et al. 2011; Flores Francisco et al. 2021; Darling et al. 2023). One such biocontrol agent is the nematophagous fungus Pochonia chlamydosporia (Hypocreales: Clavicipitaceae). P. chlamydosporia is known to parasitize eggs and adults of various nematode taxa like Heterodera spp., Meloidogyne spp., Globodera spp. and Nacobbus spp. (Kerry et al. 1993; Tobin et al. 2008; Manzanilla-López et al. 2011). Therefore, P. chlamydosporia is a promising candidate for plant-parasitic nematode biocontrol, and commercial products are available such as Rizotec® (Rizoflora Biotecnologia S.A., Brazil) and KlamiC® (Centro Nacional de Sanidad Agropecuaria, Cuba). In both products, P. chlamydosporia chlamydospores are formulated as granular nematicides. Chlamydospores are thick-walled aerial cell structures that lead to high persistence during periods of unfavourable conditions associated with formulation and application. Production of chlamydospores is usually performed on solid media. Application is done via drip irrigation, spraying or soil drenching, which requires high amounts of product and water resulting in overall high costs. One option for reducing application rates and thus costs is fungal delivery through seed coating. This would also make it possible to place the biocontrol agent directly at the root-soil interface, where protection of the young seedling is needed.
Therefore, this study aimed to enhance the effectiveness of the cover crop P. tanacetifolia by combining it with a biological control agent to target M. hapla. This was done by coating seeds of P. tanacetifolia with P. chlamydosporia. In contrast to commercial products based on fungal chlamydospores, we used blastospores of P. chlamydosporia as active ingredient. Blastospores are yeast-like vegetative cells produced by budding of the hyphae within their host or are formed during in vitro growth at nutrient-rich and oxygen-rich culture conditions in liquid media as shown for Beauveria bassiana (Mascarin et al. 2015). Blastospores have several advantages over aerial conidia, such as a higher infectivity, as shown for the endoparasitic fungus Esteya vermicola controlling the pinewood nematode Bursaphelenchus xylophilus (Wang et al. 2013). Additionally, the fermentation process for producing blastospores appears cheaper and more efficient than that of chlamydospores, as shown for various filamentous and dimorphic fungi (Jaronski and Mascarin 2017; Dietsch et al. 2021; Silva et al. 2022). Conversely, P. chlamydosporia blastospores have a thinner cell wall than chlamydospores and are therefore be more sensitive to the drying processes (Dietsch et al. 2021). We did not compare the survival rate of chlamydospores and blastospores after drying, but blastospores were found to successfully survive the drying process and are thus considered a suitable delivery system for this fungus. Here, we hypothesize, that (1) application of P. chlamydosporia as a seed coating on a cover crop will reduce the number of M. hapla eggs on a succeeding tomato plant compared to treatments without P. chlamydosporia, and (2) the resulting lower nematode inoculum level will limit gall formation on roots of subsequently grown tomato plants.
Material and methods
Pochonia chlamydosporia blastospore production
Pochonia chlamydosporia strain Pc001, deposited in the fungal culture collection at the Julius Kühn Institute, Germany, was originally isolated from surface-sterilized cysts of Heterodera schachtii collected from sugar beet fields located in North Rhine-Westphalia, Germany (Nuaima et al. 2021). Chlamydospores were cultivated on potato dextrose (PDA, 29 g l-1, Carl Roth GmbH & Co. KG, Karlsruhe, Germany). Petri plates with 14-day-old fungal cultures were washed several times with 0.1% Tween 80 to collect the chlamydospores. The chlamydospores were counted using a Thoma cell counting chamber (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany). Chlamydospores were used as inoculum to produce blastospores. To cultivate blastospores, sterile 250 ml flasks with baffled base were filled with 100 ml sterile potato dextrose broth (PDB, 26.5 g l-1, Carl Roth GmbH & Co. KG, Karlsruhe, Germany), inoculated with 106 P. chlamydosporia chlamydospores and incubated at 26 °C on a rotary shaker at 150 rpm (IKA KS 4000 ic, Staufen, Germany) (Wang et al. 2015, modified). After seven days, the PDB liquid culture was filtered through a 5–10-µm Whatman filter (VWR, Darmstadt, Germany) under sterile conditions to separate blastospores from mycelium. The blastospores were washed twice with 0.9% sterile NaCl solution by centrifugation (3600 g, 20 °C, 10 min) and resuspended in a 0.9% sterile NaCl solution. The number of blastospores was counted under a light microscope (Axiostar plus; Carl Zeiss MicroImaging GmbH, Göttingen, Germany) in a Thoma cell counting chamber at 200 × magnification.
Seed coating
Seed coating was performed in two ways: a custom-made formulation and a commercial formulation. The custom-made seed coating of P. tanacetifolia was performed in a self-constructed drum dryer. Seeds were surface-sterilized in 70% ethanol, 3% (v/v) NaClO and 70% ethanol for 3 min each, and washed thrice in sterile ddH2O. 25 g surface-sterilized P. tanacetifolia seeds (approx. 12,500 seeds) were mixed with 30 g potato starch superior (Emslandstärken, Emlichheim, Germany) and 12 g bentonite (Tonsil 510ff, Clariant AG, Muttenz, Switzerland) in the drum under constant rotation of 33 rpm. The biopolymer suspension for seed coating was prepared by mixing the blastospores with 0.9% NaCl and 0.5% gellan gum (AppliChem GmbH, Darmstadt, Germany). This resulted in 109 (FH Bi, Experiment I) or 5·107 (FH Bi + , Experiment II) blastospores in 20 ml of 0.5% gellan gum that were finally added to the P. tanacetifolia seeds. After thorough mixing of all components, the coated seeds were dried by warm air. Maximum temperature on the seed surface was 30 °C, measured with an infrared sensor. The drying process was finished when the water activity (aw) of the treated seeds was below 0.55 (LabMaster-aw, Novasina, Lachen, Switzerland), which was reached after 30–45 min. The target density of vital blastospores was at least 200 CFU per seed in Experiment I and 50 CFU per seed in Experiment II. The commercial seed coating Mantelsaat® (MS− and MS +) used in Experiment II was produced and provided by Feldsaaten Freudenberger GmbH & Co. KG (Krefeld, Germany). To produce MS-, fertiliser (carbonic acid lime), rock flour (mixture of feldspar, mica, and kaolinite), clay mineral (calcium bentonite), clay, perlite, humic acids, cellulose, and adhesives were coated onto 50 kg seeds (approx. 25,000,000 seeds) of P. tanacetifolia. Pochonia chlamydosporia was incorporated into MS + as a biopolymer suspension prepared by mixing the blastospores with 0.9% NaCl and 0.5% gellan gum. The seed coating process was also performed in a rotating drum. The targeted spore density of MS + was 50 CFU per seed, the same as for FH Bi + in Experiment II. To investigate the coating efficiency and survival of the P. chlamydosporia blastospores within the process, three replicates of ten seeds each of freshly coated and dried seeds were washed in 0.9% NaCl and plated on PDA (39 g l-1 PDA) containing 0.1 g l-1 streptomycin, 0.05 g l-1 tetracycline, 0.1 g l-1 dodine, and 0.05 g l-1 cycloheximide (Strasser et al. 1996). Plates were incubated for five days at 23 °C in the dark and CFU were counted.
Meloidogyne hapla inoculum
The M. hapla inoculum was obtained from galled tomato roots (Solanum lycopersicum cv. Moneymaker) grown under greenhouse conditions. The nematode is part of the living nematode collection hosted at the Julius Kühn Institute, Germany. The M. hapla juveniles are routinely checked for purity using the protocol of Adam et al. (2007) and using the species-specific IGS primers JMV1 and JMVhapla. For the extraction of nematode eggs, the tomato shoot was cut off and discarded and the substrate with roots soaked in tap water for a few minutes. Next, the roots were carefully separated from the substrate and rinsed under tap water to remove adhering soil particles. The roots were then cut into 1–2 cm pieces and transferred to a 500 ml polyethylene bottle. The bottles were filled with 250 ml of 1% NaClO solution (Dan Klorix household bleach diluted 1:1.8 with tap water) and shaken at 450 rpm for 3 min. Finally, the suspension was passed through a 250 µm sieve to remove the roots and placed over a 20 µm sieve to collect the nematode eggs. The egg suspension on the 20 µm sieve was rinsed with tap water for a few seconds to remove residual NaClO. Eggs on the 20 µm sieve were transferred to a beaker and adjusted to 600 eggs ml-1. The ratio of embryonal and juvenile egg stages was 1:1.
Greenhouse experimental set up
Two greenhouse experiments with either three (Experiment I) or six (Experiment II) treatments were set up to study the biocontrol potential of P. chlamydosporia towards M. hapla. Each of the two experiments was performed once and consisted of two stages: first, the “cover crop” stage with P. chlamydosporia-treated or non-treated P. tanacetifolia (cv. Balo, provided by Feldsaaten Freudenberger GmbH & Co.KG, Krefeld, Germany) grown in M. hapla infested substrate and, second, the “host plant” stage with tomato plants as bioindicator for nematode infestation.
Experiment I consisted of three treatments: (1) uncoated seeds without P. chlamydosporia (control), (2) uncoated seeds with P. chlamydosporia as blastospores suspension (BS), (3) coated seeds P. chlamydosporia blastospores (FH Bi) (Table 1). Seeds were sown in 600 ml pots containing a sterile sand/vermiculite substrate (4:1, v/v) amended with 0.125% slow-release osmocote fertilizer. One half of the treatments was inoculated with 6000 eggs of M. hapla per pot after sowing via the same 2 cm deep holes that were made for sowing. The other half received no nematode inoculation. Directly afterwards, P. chlamydosporia was added as blastospore suspension with 5·108 spores per pot in 0.9% NaCl via the same holes (BS). Each treatment consisted of ten replicates. Pots were randomly set up in the greenhouse at 22 °C:19 °C day:night temperature and watered as required. The total number of germinated seeds per pot was counted after 14 days.
The first stage of Experiment I was terminated and evaluated 50 days after inoculation, when about 50% of the newly formed M. hapla eggs had reached juvenile stages. The shoots of P. tanacetifolia were harvested and fresh and dry weights were determined. Next, the roots were separated from the substrate by gentle shaking and root fresh weight was recorded. The M. hapla eggs were then extracted from roots with 1% NaClO as described above. The number of healthy as well as parasitized eggs, identifiable by fungal outgrowths, was counted in 1 ml aliquots with an inverted microscope at 100 × magnification. The number of M. hapla eggs per root system for single pots was analysed, but for better legibility the unit eggs per pot was chosen. Finally, the reproduction factor (RF) of M. hapla was calculated as the quotient of the final number (Pf) of extracted M. hapla eggs 50 days after inoculation divided by the inoculated number of M. hapla eggs (Pi = 6000).
For the second stage of Experiment I, the substrate of each pot was mixed by hand and a 200 ml aliquot filled into folded plastic boxes (4 × 4 × 12 cm, Seufert, Rodgau, Germany) in which a 10-day-old tomato seedling (cv. Moneymaker) was transplanted. The folded boxes were set up in the greenhouse at equal conditions as described above. After another 50 days, the above-ground plant parts were discarded and roots separated from the substrate. The boxes were soaked for 5 min in water and the soil was then gently washed from roots. Tomato root galling was assessed using the 0 (no galls visible) to 10 (all roots severely galled, plant usually dead) index according to Bridge and Page (1980).
Experiment II was performed as described above, but with modifications of the seed coating (Table 1). The experiment consisted of six treatments: (1) uncoated P. tanacetifolia seeds (control), (2) uncoated seeds with P. chlamydosporia blastospore suspension (BS), (3) custom-made coated seeds without P. chlamydosporia (FH Bi-), (4) custom-made coated seeds with P. chlamydosporia blastospores (FH Bi +), (5) commercially coated seeds without P. chlamydosporia (MS− ), and (6) commercially coated seeds with P. chlamydosporia blastospores (MS +). In the first stage of the experiment, seeds were sown and inoculated with M. hapla as described above. Treatments FH Bi− and MS− without P. chlamydosporia were employed to investigate the effect of seed coating itself. Each treatment consisted of ten replicates. The termination and evaluation of the first and second stages of Experiment II were performed as described above for Experiment I.
Statistical analysis
Data were statistically analysed with R version 7.2 (R Core Team 2022). Figures were created using the ggplot2 package (Wickham, 2009). First-stage data (“cover crop”) were analysed with (generalized) linear models. Linear models were fitted to predict above-ground and root dry weight with P. chlamydosporia inoculum, M. hapla inoculum and seed treatment. Standardized parameters were obtained by fitting the model on a standardized version of the dataset. 95% confidence intervals and p-values were computed using a Wald t-distribution approximation. A generalized linear model using Poisson distribution with a log link function was fitted to test differences between treatments with P. chlamydosporia and control in the total number of M. hapla eggs. A generalized linear model using binomial distribution with a logit link function was fitted to model the percentage of parasitized M. hapla eggs with P. chlamydosporia inoculum and seed treatment. Standardized parameters were obtained by fitting the generalized linear models on a standardized version of the dataset. 95% confidence intervals and p-values were computed using a Wald test. Estimated marginal means (EMM, R package emmeans, by Lenth 2022) were calculated based on these models. EMM and contrasts were calculated with emmeans and the trt.vs.ctrl() function. Germination of P. tanacetifolia and gall indices of tomato roots (second stage data “host plant”) were compared using Kruskal–Wallis test with pairwise Wilcoxon rank sum tests with Benjamini & Hochberg (1995) correction as post-hoc tests.
Results
The P. chlamydosporia blastospores remained viable after coating and drying in both Experiments I and II (Table 1). The survival rates, as compared to the initial blastospore concentration during the seed coating process, were 0.72 ± 0.13% for FH Bi and 1.64 ± 0.51% for FH Bi + , respectively. For MS + , the CFU per seed indicated a survival rate of 17.40 ± 4.90% in relation to the initial number of blastospores. The number of CFU per seed in the seed coatings FH Bi + and MS were not significantly different from each other. Therefore, the effects of these two seed coatings in Experiment II could be directly compared.
Experiment I
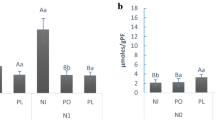
Seed germination was significantly lower for FH Bi in comparison to the control without P. chlamydosporia and BS treatment (χ2 = 33.74, df = 2, p < 0.001). M. hapla inoculum had no effect on seed germination (χ2 = 0.11, df = 1, p = 0.74) (Table 2). In contrast, above-ground dry weight of FH Bi-treated plants was significantly higher than in control plants with a difference of 1.0 g (β = 1.00, 95% CI [0.65, 1.34], t56 = 5.82, p < 0.001) regardless of M. hapla inoculation (β = − 0.07, 95% CI [− 0.35, 0.21], t56 = − 0.49, p = 0.63). The model explained a statistically significant and substantial proportion of variance (R2 = 0.47, F3,56 = 16.42, p < 0.001). The model's intercept, corresponding to no M. hapla inoculation and no treatment with P. chlamydosporia was at 1.07 g (95% CI [0.79, 1.35], t56 = 7.62, p < 0.001). Root dry weight was not significantly affected by M. hapla inoculation (β = − 0.09, 95% CI [− 0.20, 0.03], t56 = − 1.52, p = 0.14), but root dry weight was significantly higher in FH Bi compared to the control with a difference of 0.25 g (β = 0.25, 95% CI [0.11, 0.39], t56 = 3.51, p < 0.001). The same trend was observed in the presence of M. hapla, but differences of 0.09 g were not statistically significant (β = 0.09, 95% CI [− 0.05, 0.23], t56 = 1.29, p = 0.20). The model explained a statistically significant and moderate proportion of variance (R2 = 0.21, F3,56 = 4.96, p = 0.004). The model’s intercept, corresponding to no M. hapla inoculation and no treatment with P. chlamydosporia was at 0.33 g (95% CI [0.21, 0.44], t56 = 5.73, p < 0.001). The average RF of M. hapla in P. tanacetifolia without P. chlamydosporia was 1.83 ± 0.53. The RF of M. hapla on tomato plants without P. chlamydosporia was 33.60 ± 8.04. Direct inoculation of P. chlamydosporia blastospores (BS) and application of blastospores as seed coating (FH Bi) significantly reduced the total number of M. hapla eggs on P. tanacetifolia by 75.50% (2683 ± 1194 eggs per pot; β = − 1.41, 95% CI [− 1.42, − 1.39], p < 0.001; EMM: t27 = − 2.81, p = 0.02) and 95.60% (485 ± 122 eggs per pot; β = − 3.12, 95% CI [− 3.15, -3.09], p < 0.001; EMM: t27 = − 3.56, p = 0.003), respectively, in comparison to the control without P. chlamydosporia (Fig. 1a). The model's intercept, corresponding to no P. chlamydosporia inoculation was at 10,962 ± 3201 eggs per pot (β = 9.30, 95% CI [9.30, 9.31], p < 0.001). The parasitization rate of M. hapla eggs after coating the seeds with P. chlamydosporia was more than fourfold higher (114 ± 25 parasitized eggs per pot, 37.20% ± 10.58%, RF: 0.11 ± 0.02; β = 0.67, 95% CI [0.63, 0.71], p < 0.001; EMM: F2 = 932.51, p < 0.0001) than in the control (1017 ± 349 parasitized eggs per pot, 8.67% ± 0.85%; Fig. 1b). For BS, the parasitization rate was more than two-fold higher (447 ± 192 parasitized eggs per pot, 20.53% ± 6.29%; RF: 0.45 ± 0.20, β = 0.67, 95% CI [0.63, 0.71], p < 0.001) than in the control (Fig. 1b). The model's explanatory power was substantial (Nagelkerke's R2 = 0.39). The model's intercept, corresponding to no P. chlamydosporia inoculation was at − 2.28% (95% CI [− 2.30, − 2.26], p < 0.001).
Effect of cover crop Phacelia tanacetifolia seed coating with Pochonia chlamydosporia on Meloidogyne hapla population density, parasitism, and gall index of subsequently planted tomato plants (Experiment I). Boxplots show the extremes, the upper (75%) and lower (25%) quartiles, the median (line), mean (X), and outliers (dots). Abbreviations: + MH: with M. hapla, -PC: without P. chlamydosporia, BS: blastospore suspension, FH Bi: seed coating FH Bi. a Total number of M. hapla eggs per plant 50 days after inoculation. Dotted line indicates number of inoculated eggs at experimental start (n = 10). b Percentage of parasitized M. hapla eggs 50 days after inoculation (n = 10). a and b: ‘*’ indicates significant differences of treatments in comparison to the control (− PC + MH) at the p < 0.05 level according to generalized linear models and estimated marginal means. c Gall index of tomato plants 50 days after sowing in previously inoculated substrate (n = 10). Values with no letter in common are significantly different (Kruskal–Wallis test with pairwise Wilcoxon rank sum tests with Benjamini & Hochberg (1995) correction, as post-hoc tests, p < 0.05)
In the subsequently grown tomato plants, the root gall index of individual plants ranged from 0 (no galls visible) to 7 (galls appear on main roots—majority of main roots galled). Average gall indices were 3 in the control, 1.5 in treatment BS and 1 in treatment FH Bi (Fig. 1c). The lower gall index compared to the control was significant for FH Bi (χ2 = 6.87, df = 2, p = 0.025; Fig. 1c), but not for BS (χ2 = 6.87, df = 2, p = 0.44). Percentage of parasitized M. hapla eggs and gall index were negatively correlated (Spearman’s ρ = − 0.58, p < 0.05, data not shown).
Experiment II
This experiment compared two different seed coatings for P. chlamydosporia blastospores with direct blastospore application. Seed germination was similar in all treatments, including the control (χ2 = 3.73, df = 3, p = 0.29), regardless if P. chlamydosporia (χ2 = 0.95, df = 1, p = 0.33) or M. hapla (χ2 = 2.03, df = 1, p = 0.15, Table 2) was inoculated or not. In contrast to Experiment I, for above-ground dry weight the linear model explained a statistically not significant and weak proportion of variance (R2 = 0.04, F5,113 = 0.83, p = 0.53). The model's intercept, corresponding to no P. chlamydosporia inoculation, no M. hapla inoculation, and no seed treatment was at 0.85 g (95% CI [0.72, 0.98], t113 = 12.94, p < 0.001). For root dry weight the linear model explained a statistically significant and weak proportion of variance (R2 = 0.10, F5,113 = 2.48, p = 0.036). The model's intercept, corresponding to no P. chlamydosporia inoculation, no M. hapla inoculation, and no treatment was at 0.10 g (95% CI [0.07, 0.12], t113 = 7.31, p < 0.001). Adding P. chlamydosporia increased the root dry weight statistically significant by 0.03 g (β = 0.03, 95% CI [0.01, 0.05], t113 = 2.08, p = 0.04, EMM: F1,113 = 4.318, p = 0.04). The number of M. hapla eggs was reduced in all treatments with P. chlamydosporia compared to the P. tanacetifolia control without P. chlamydosporia (15,074 ± 2,270 eggs/pot, RF: 2.51 ± 0.28; β = − 0.35 ± 0.003, z55 = − 114.51, p < 0.001). The model's intercept, corresponding to no P. chlamydosporia inoculation and no seed treatment was at 15,074 eggs per pot (β = 9.62, 95% CI [9.62, 9.63], p < 0.001): for treatment BS by 26% (11,206 ± 3,151 eggs per pot, RF: 1.87 ± 0.53; β = 0.05 ± 0.005, z55 = 10.59, p < 0.001), for seed coating FH Bi + by 29% (10,658 ± 3464 eggs per pot, RF: 1.78 ± 0.58; β = − 0.16 ± 0.004, z55 = − 43.6, p < 0.001), and in MS + by 49% (7669 ± 1809 eggs per pot, RF: 1.28 ± 0.30; β = − 0.14 ± 0.004, z55 = − 39.58, p < 0.001) (Fig. 2a). The percentage of parasitized M. hapla eggs in Experiment II (max. 9%) was generally lower than in Experiment I (up to 100% in some replicates). The model's intercept, corresponding to no P. chlamydosporia inoculation and no seed treatment was at − 4.54% (95% CI [− 5.60, − 3.75], p < 0.001). Inoculation of P. chlamydosporia increased the proportion of parasitized eggs, but not significantly (β = 0.04, 95% CI [− 0.69, 0.78], p = 0.91). The same trend was calculated for treatments BS (β = 0.78, 95% CI [− 0.49, 2.16], p = 0.24), FH Bi (β = 0.62, 95%CI [− 0.41, 1.81], p = 0.25), and MS (β = 0.20, 95% CI [− 0.89, 1.42], p = 0.73).
Effect of cover crop Phacelia tanacetifolia seed coating with Pochonia chlamydosporia on Meloidogyne hapla population density, parasitism, and gall index of subsequently planted tomato plants (Experiment II). Boxplots show the extremes, the upper (75%) and lower (25%) quartiles, the median (line), mean (X), and outliers (dots). Abbreviations: + MH: with M. hapla, − PC: without P. chlamydosporia, BS: blastospore suspension, FH Bi− : seed coating FH Bi without P. chlamydosporia, FH Bi + : seed coating FH Bi with P. chlamydosporia, MS− : Mantelsaat® without P. chlamydosporia, MS + : Mantelsaat® with P. chlamydosporia. a Total number of M. hapla eggs per plant 50 days after inoculation. Dotted line indicates number of inoculated eggs at experimental start (n = 10). ‘*’ indicates significant differences of treatments in comparison to the control (− PC + MH) at the p < 0.05 level according to generalized linear models and estimated marginal means. b Percentage of parasitized M. hapla eggs 50 days after inoculation (n = 10). No significant differences for treatments in comparison to control (− PC + MH) for all treatments were found (generalized linear models and estimated marginal means p > 0.05). c Gall index of tomato plants 50 days after sowing in previously inoculated substrate (n = 10). Central tendency is not significantly different (p > 0.05) for all treatments (Kruskal–Wallis test with pairwise Wilcoxon rank sum tests with Benjamini & Hochberg (1995) correction, as post-hoc tests)
The gall index of tomato roots was not significantly affected in any of the treatments compared to the control, regardless of P. chlamydosporia inoculation (χ2 = 1.09, df = 1, p = 0.29) or seed coatings (χ2 = 3.57, df = 3, p = 0.31) (Fig. 2c). However, in all treatments with P. chlamydosporia, the median gall index was reduced 1.5-fold compared to the control. The negative correlation of parasitized M. hapla eggs in P. tanacetifolia roots with the gall index of subsequently grown tomato plants was weaker compared to Experiment I (Spearman’s ρ =− 0.09, p < 0.05, data not shown).
Discussion
This study tested the hypothesis whether P. chlamydosporia blastospores, either applied as suspension to the soil or by coating seeds of P. tanacetifolia, could reduce the number of newly produced M. hapla eggs and increase egg parasitism. Unfortunately, our results did not provide a clear picture. While Experiment I clearly supported our hypothesis with up to 95% reduction in M. hapla eggs and up to 49% egg parasitism, Experiment II only resulted in up to 49% reduction in M. hapla eggs and a maximum of 9% egg parasitism. Thus, reduction in M. hapla eggs was confirmed in both experiments, whereas egg parasitism was not or only partly confirmed. Regarding nematode reduction, the results are in line with previously published studies. For example, de Leij et al. (1993) reported over 90% reduction of M. hapla on tomato following application of P. chlamydosporia.
Pochonia chlamydosporia’s efficiency and biocontrol potential
The status of the host plant, P. tanacetifolia, appears important as it is considered a maintenance host and M. hapla is less able to reproduce on P. tanacetifolia than on other hosts such as tomato or potato (Viaene and Abawi 1998). Average RF of M. hapla on P. tanacetifolia was between 1.8 and 2.5 in the absence of P. chlamydosporia, which was far less than for tomato (RF = 33.6—93.6) used as an internal control (see Fig. 2a). Here, we were able to show that even on a maintenance host like P. tanacetifolia, seed enhancement with P. chlamydosporia blastospores can effectively reduce M. hapla reproduction below the initial inoculation level. In the present study, control of M. hapla was shown for one initial nematode density. As reproduction of nematodes is inversely related to their initial densities (Seinhorst 1967), future experiments need to test different M. hapla densities to confirm the biocontrol potential of P. chlamydosporia over a broad spectrum of infestation scenarios.
Another aspect concerning the effectiveness of P. chlamydosporia towards M. hapla might be the fungal isolate itself, or the fungus-nematode interaction. Isolates of P. chlamydosporia as well as populations of M. hapla can vary in their specific characteristics, i.e., some populations of M. hapla might be more suitable for certain P. chlamydosporia isolates than others. The P. chlamydosporia P001 studied here was originally isolated from H. schachtii cysts and selected for its virulence against H. schachtii (Nuaima et al. 2021). Stirling (2014) concluded from several studies that P. chlamydosporia is most pathogenic to the host from which it was originally isolated. Now, we could show that P. chlamydosporia P001 also affects M. hapla eggs indicating a broad effect as biocontrol agent. Nonetheless, further studies with different M. hapla populations tested under different environmental conditions are needed to confirm the biocontrol potential on a broader basis.
The biocontrol potential of P. chlamydosporia has been investigated under various conditions against different plant-parasitic nematode species. Studies with M. incognita tentatively show a much lower reduction by P. chlamydosporia than for M. hapla. For example, De Leij et al. (1992) achieved a reduction of about 40% in number of eggs, juveniles, and galls caused by M. incognita on tomato following treatment with P. chlamydosporia at 50 chlamydospores per gram of soil. On okra, Dhawan and Singh (2010) reported a reduction of M. incognita by P. chlamydosporia ranging from 6.9 to 46.5%, depending on application method and application rate. In general, P. chlamydosporia is considered one of the most important players in root-knot nematode control in natural agroecosystems. Viaene & Abawi (2000) reported colonization rates of M. hapla eggs by P. chlamydosporia, measured as egg mass, between 15.5 and 42.6% in M. hapla infested soil. In their study, egg parasitism was associated with reduced penetration of lettuce roots by M. hapla. Furthermore, when lettuce was replanted into the same soil, up to 69.2% of M. hapla egg masses were found to be colonized by P. chlamydosporia. In the present study, the biocontrol potential of P. chlamydosporia was tested under greenhouse conditions and the effect of the tested isolate in the field still has to be evaluated. Field performance of other P. chlamydosporia isolates was evaluated by van Damme et al. (2005) against M. javanica in lettuce and tomato over two consecutive years, showing that a one-time application of P. chlamydosporia delayed the population build-up of M. javanica by five to seven months.
Blastospores as inoculum may be superior to chlamydospores
All of the references on P. chlamydosporia cited above applied persistent chlamydospores as fungal inoculum, whereas the present study applied fast-growing but non-persistent blastospores. This raises the question whether blastospore application might be a preferred method of application. In our study, blastospore application resulted in up to 49% parasitism of M. hapla eggs. This was in the range of what has been reported by Viaene & Abawi (2000) for parasitism of M. hapla eggs using chlamydospores. Properties relevant for choosing blastospores instead of chlamydospores are faster germination rates, shorter fermentation times, better production scalability, and overall less labour (Morales-Reyes et al. 2018). Additionally, biological factors like establishment and survival of blastospores in soil are relevant. Assuming that blastospores are more virulent than chlamydospores, as previously shown for other nematophagous fungi (Wang et al. 2013), blastospores may be able to establish in the soil as well as or better than chlamydospores in presence of host nematodes due to faster colony formation.
Formulation improves effectiveness of biocontrol
In Experiment I, the custom-made seed coating led to a reduced seed germination compared to uncoated seeds. This was most likely due to the inhomogeneous formulation of P. chlamydosporia to the P. tanacetifolia seeds. The material around the seeds was likely too rigid and firm and thus affected seed germination. Despite the reduced seed germination and the subsequent lower number of P. tanacetifolia plants in the seed coating, the biomass production was greater compared to the other treatments. This can be best explained by higher nutrient availability per plant and less intraspecific competition. In accordance with our second hypothesis, the reduced soil infestation level with M. hapla upon P. chlamydosporia treatment should benefit the subsequent plant. Our results confirmed this with the reduced root gall index of the subsequently grown tomato. These effects were stronger in the first experiment than in the second, which might be explained by the higher number of blastospores applied in the first case. This could indicate that the number of applied blastospores is relevant for the performance of P. chlamydosporia controlling M. hapla. Bontempo et al. (2017) described a dose–response dependence for P. chlamydosporia against M. incognita on carrots under field conditions. According to this, an amount of 3 kg ha-1 (3·108 chlamydospores per gramg, in total 9·1011 chlamydospores per hectare) of the powder formulation Rizotec® with P. chlamydosporia chlamydospores (Rizoflora Biotecnologia S.A., Brazil) is required to reduce the population of M. incognita and increase the quality and yield of carrots. In order to reach this concentration, 2.25 105 to 3.6 105 blastospores of P. chlamydosporia per P. tanacetifolia seed are necessary (extrapolated to a sowing density of 2.5·106 to 4.0·106 seeds ha−1). In this study, under greenhouse conditions and limited competition with other microorganisms, the reduction of M. hapla eggs in the first experiment was already significant at a concentration of 290 blastospores per seed. Those numbers refer to 7.25 108 to 1.16 109 blastospores per hectare, which is less than the application rates mentioned above for chlamydospores. Such lower application rates might be caused by a higher virulence of blastospores over chlamydospores (Wang et al. 2013). However, the viability and virulence of blastospores under field conditions need further attention regarding the effect of heat, UV light, water potential, osmotic stress (Vieira dos Santos et al. 2012; Bernardo et al. 2020), soil environment (Luambano et al. 2015), and microbial competition (Siddiqui et al. 2009).
The comparison of Experiment I and II suggests that the number of blastospores per seed is relevant. One method of increasing the number of viable blastospores in seed coatings is to add a higher amount of blastospores during the production process. Even if the same or a larger amount of blastospores is required compared to chlamydospores, the fermentation process of P. chlamydosporia blastospores in liquid culture is presumably more cost-effective and efficient compared to solid fermentation of chlamydospores.
The efficiency of biological control agents depends on environmental factors like temperature, pH, soil structure, UV intensity, and food and water availability (Shields et al. 2019; Peiris et al. 2020). To minimize the influence of these factors, improvement of survival rates and root rhizosphere colonisation of applied biological control organisms through formulations in capsules, granules, or seed coatings is necessary (Vemmer and Patel 2013; Afzal et al. 2020). Drying blastospores is still a challenge and a lot of research will be necessary to identify low-cost and reliable techniques for up-scale production (Dietsch et al. 2021). Here, we showed a new and effective formulation of P. chlamydosporia in which blastospores were dried directly within the (commercial) seed coating.
In conclusion, seed coating with P. chlamydosporia blastospores showed successful control of M. hapla in the cover crop P. tanacetifolia and a subsequent tomato cycle. The commercial Mantelsaat® application proved to be a good delivery system for P. chlamydosporia. In any case, blastospore numbers per seed need to achieve sufficient densities for proper nematode control. To further develop the biocontrol potential of such treatment for optimum performance under field conditions, focus should be given to the improvement of blastospore vitality after drying, shelf life of the coated seeds and fungal establishment in the root zone.
References
Adam MAM, Phillips MS, Blok VC (2007) Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.). Plant Pathol 56:190–197
Afzal I, Javed T, Amirkhani M, Taylor AG (2020) Modern seed technology: seed coating delivery systems for enhancing seed and crop performance. Agriculture 10:526–546
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B 57(1):289–300
Bontempo AF, Lopes EA, Fernandes RH, Freitas L, Dallemole-Giaretta R (2017) Dose-response effect of Pochonia chlamydosporia against Meloidogyne incognita on carrot under field conditions. Rev Caatinga 30:258–262
Brant V, Neckář K, Pivec J, Duchoslav M, Holec J, Fuksa P, Venclová V (2009) Competition of some summer catch crops and volunteer cereals in the areas with limited precipitation. Plant Soil Environ 55:17–24
Bridge J, Page SLJ (1980) Estimation of root-knot nematode infestation levels on roots using a rating chart. Trop Pest Manage 26:296–298
Brust J, Claupein W, Gerhards R (2014) Growth and weed suppression ability of common and new cover crops in Germany. Crop Prot 63:1–8
Carter C (1985) Literature search: host range of Meloidogyne hapla. Int Nematol Netw Newsl 2:16–24
Darling E, Palmisano A, Chung H, Quintanilla-Tornel M (2023) A new biological product shows promising control of the northern root-knot nematode, Meloidogyne hapla, in greenhouse tomatoes. J Nematol 55:20230023
das Bernardo CC, Pereira-Junior RA, Luz C, Mascarin G, Kamp Fernandes É (2020) Differential susceptibility of blastospores and aerial conidia of entomopathogenic fungi to heat and UV-B stresses. Fungal Biol 124:714–722
de Leij FAAM, Kerry BR, Dennehy JA (1992) The effect of fungal application rate and nematode density on effectiveness of Verticillium chlamydospoirum as a biocontrol agent for Meloidogyne incognita. Nematologica 38:112–122
de Leij FAAM, Kerry BR, Dennehy JA (1993) Verticillium chlamydosporium as a biological control agent for Meloidogyne incognita and M. hapla in pot and micro-plot tests. Nematol 39:115–126
Dhawan SC, Singh S (2010) Management of root-knot nematode, Meloidogyne incognita using Pochonia chlamydosporia on okra. Indian J Nematol 40:171–178
Dietsch R, Jakobs-Schönwandt D, Grünberger A, Patel A (2021) Desiccation-tolerant fungal blastospores: from production to application. Curr Res Biotech 3:323–339
Flores Francisco BG, Ponce IM, Plascencia Espinosa MÁ, Mendieta Moctezuma A, López y López VE (2021) Advances in the biological control of phytoparasitic nematodes via the use of nematophagous fungi. World J Microbiol Biotechnol 37:180
Hallmann J (2021) The northern root-knot nematode: a forking problem of carrots in Germany. In: Sikora RA, Desaeger J, Molendijk L (eds) Integrated nematode management: state-of-the-art and visions for the future. CABI pp 277–283
Hallmann J, Frankenberg A, Paffrath A, Schmidt H (2007) Occurrence and importance of plant-parasitic nematodes in organic farming in Germany. Nematol 9:869–879
Hallmann J, Davies KG, Sikora R (2009) Biological control using microbial pathogens, endophytes and antagonists. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI, pp 380–411
Handlířová M, Lukas V, Smutný V (2017) Yield and soil coverage of catch crops and their impact on the yield of spring barley. Plant Soil Environ 63:195–200
Jaronski ST, Mascarin GM (2017) Mass production of fungal entomopathogens. In: Lacey LA (ed) Microbial control of insect and mite pests: from theory to practice. Elsevier, pp 141–155
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WML, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961
Kerry BR, Kirkwood IA, de Leij FAAM, Barba J, Leijdens MB, Brookes PC (1993) Growth and survival of Verticillium chlamydosporium goddard, a parasite of nematodes, in soil. Biocontrol Sci Technol 3:355–365
Lenth R (2022) emmeans: estimated marginal means, aka least-squares means. R package version 1.8.3, https://cran.r-project.org/package=emmeans
Luambano ND, Manzanilla-López RH, Kimenju JW, Powers SJ, Narla RD, Wanjohi WJ, Kerry BR (2015) Effect of temperature, pH, carbon and nitrogen ratios on the parasitic activity of Pochonia chlamydosporia on Meloidogyne incognita. Biol Control 80:23–29
Manzanilla-López RH, Esteves I, Powers SJ, Kerry BR (2011) Effects of crop plants on abundance of Pochonia chlamydosporia and other fungal parasites of root-knot and potato cyst nematodes. Ann Appl Biol 159:118–129
Mascarin GM, Jackson MA, Kobori NN, Behle RW, Dunlap CA, Delalibera Júnior Í (2015) Glucose concentration alters dissolved oxygen levels in liquid cultures of Beauveria bassiana and affects formation and bioefficacy of blastospores. Appl Microbiol Biotechnol 99:6653–6665
Moens M, Perry RN, Starr JL (2009) Meloidogyne species - a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI, Wallingford, pp 1–17
Morales-Reyes C, Mascarin GM, Jackson MA, Hall D, Sánchez-Peña SR, Arthurs SP (2018) Comparison of aerial conidia and blastospores from two entomopathogenic fungi against Diaphorina citri (Hemiptera: Liviidae) under laboratory and greenhouse conditions. Biocontrol Sci Technol 28:737–749
Nicol JM, Turner SJ, Coyne DL, den Nijs L, Hockland S, Maafi ZT (2011) Current nematode threats to world agriculture. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and molecular genetics of plant-nematode interactions. Springer, Netherlands, Dordrecht, pp 21–43
Nuaima RH, Ashrafi S, Maier W, Heuer H (2021) Fungi isolated from cysts of the beet cyst nematode parasitized its eggs and counterbalanced root damages. J Pest Sci 94:563–572
Peiris PUS, Li Y, Brown P, Xu C (2020) Fungal biocontrol against Meloidogyne spp. in agricultural crops: a systematic review and meta-analysis. Biol Control 144:104235
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Scavo A, Fontanazza S, Restuccia A, Pesce PR, Abbate C, Mauromicale G (2022) The role of cover crops in improving soil fertility and plant nutritional status in temperate climates. A review. Agron Sustain Dev 42:93
Seinhorst JW (1967) The relationship between population increase and population density in plant parasitic nematodes. 5. Influence of damage to the host on multiplication. Nematologica 13:481–492
Shields MW, Johnson AC, Pandey S, Cullen R, González- Chang M, Wratten SD, Gurr GM (2019) History, current situation and challenges for conservation biological control. Biol Control 131:25–35
Siddiqui IA, Atkins SD, Kerry BR (2009) Relationship between saprotrophic growth in soil of different biotypes of Pochonia chlamydosporia and the infection of nematode eggs. Ann Appl Biol 155:131–141
Silva DM, de Souza VHM, de Moral R, A, Delalibera Júnior I, Mascarin GM, (2022) Production of Purpureocillium lilacinum and Pochonia chlamydosporia by submerged liquid fermentation and bioactivity against Tetranychus urticae and Heterodera glycines through seed inoculation. J Fungi 8:511
Stirling G (2014) Biological control of plant-parasitic nematodes: soil ecosystem management in sustainable agriculture, 2nd edn. CABI, Wallingford
Strasser H, Forer A, Schinner F (1996) Development of media for the selective isolation and maintenance of virulence of Beauveria brongniartii. Proceedings of the 3rd international workshop on microbial control of soil dwelling pests, Lincoln, New Zealand, pp 125–130
Tobin JD, Haydock PPJ, Hare MC, Woods SR, Crump DH (2008) Effect of the fungus Pochonia chlamydosporia and fosthiazate on the multiplication rate of potato cyst nematodes (Globodera pallida and G. rostochiensis) in potato crops grown under UK field conditions. Biol Control 46:194–201
Van Damme V, Hoedekie A, Viaene NM (2005) Long-term efficacy of Pochonia chlamydosporia for management of Meloidogyne javanica in glasshouse crops. Nematol 7:727–736
Vemmer M, Patel AV (2013) Review of encapsulation methods suitable for microbial biological control agents. Biol Control 67:380–389
Vestergård M (2019) Trap crops for Meloidogyne hapla management and its integration with supplementary strategies. Appl Soil Ecol 134:105–110
Viaene NM, Abawi GS (1998) Management of Meloidogyne hapla on lettuce in organic soil with sudangrass as a cover crop. Plant Dis 82:945–952
Viaene NM, Abawi GS (2000) Hirsutella rhossiliensis and Verticillium chlamydosporium as biocontrol agents of the root-knot nematode Melodiogyne hapla on lettuce. J Nematol 32:85–100
Vieira dos Santos MC, Esteves I, Abrantes I (2012) In vitro water stress bioassays with the nematophagous fungus Pochonia chlamydosporia: effects on growth and parasitism. Biol Control 63:310–319
Wang Y-B, Yang Z-H, Yu J-J, Zhang Y-A, Xue J-J, Li Z, Wang C-Y, Wang Z, Hou J-G, Begum S, Gu L-J, Lee M-R, Sung C-K (2013) Comparison between conidia and blastospores of Esteya vermicola, an endoparasitic fungus of the pinewood nematode, Bursaphelenchus xylophilus. World J Microbiol Biotechnol 29:2429–2436
Wang Y-I, Li L-F, Li D, Wang B, Zhang K, Niu X (2015) Yellow pigment aurovertins mediate interactions between the pathogenic fungus Pochonia chlamydosporia and its nematode host. J Agric Food Chem 63:6577–6587
Wesemael W, Viaene N, Moens M (2011) Root-knot nematodes (Meloidogyne spp.) in Europe. Nematol 13:3–16
Wickham, H (2009) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, ISBN: 978-0-387-98141-3
Acknowledgements
The authors thank Dr. Rasha Haj Nuaima (Julius Kühn-Institut, Braunschweig, Germany) for providing Pochonia chlamydosporia Pc001 and Ute-Christina Mertens for her technical support in carrying out the trials. Furthermore, we would like to thank Feldsaaten Freudenberger GmbH & Co.KG for the provision of seeds as well as the production of Mantelsaat®. This work is part of the FORK project funded by the Federal Ministry of Education and Research (BMBF, FKZ: 13FH118PA8). Article processing charge funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 490988677 – and Hochschule Bielefeld - University of Applied Sciences and Arts.
Funding
Open Access funding enabled and organized by Projekt DEAL. Bundesministerium für Bildung und Forschung, 13FH118PA8, Anant V. Patel
Author information
Authors and Affiliations
Contributions
JU, DJ-S, JHS and JH designed the experiment; JHS and JH conducted the experiment; JU analysed the results; JU prepared a manuscript draft; JHS and JH edited the manuscript; DJ-S, K-JD and AP revised the manuscript for technical and scientific accuracy; DJ-S and AP acquired funding, and supervised the project. All authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose. The authors declare that they have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Ralf-Udo Ehlers
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uthoff, J., Jakobs-Schönwandt, D., Schmidt, J.H. et al. Biological enhancement of the cover crop Phacelia tanacetifolia (Boraginaceae) with the nematophagous fungus Pochonia chlamydosporia to control the root-knot nematode Meloidogyne hapla in a succeeding tomato plant. BioControl 69, 77–90 (2024). https://doi.org/10.1007/s10526-023-10222-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10222-5