Abstract
Verticillium longisporum is a soil-borne vascular pathogen of oilseed rape and other Brassica crops. The limited availability of chemical control measures against vascular pathogens calls for the exploration of control alternatives. Cross-protection may be conveyed by the use of non-pathogenic or non-aggressive isolates of potential pathogens as biocontrol agents. V. longisporum consists of three lineages that originated from three independent hybridization events of four haploid Verticillium parents. Previous pathogenicity tests in the greenhouse have shown that lineage A1/D2 is non-pathogenic or non-aggressive on different Brassica and non-Brassica hosts. Thus, the cross-protection potential of the A1/D2 lineage against an aggressive V. longisporum isolate in oilseed rape was tested. With root-dip inoculation, A1/D2 reduced disease symptoms when applied before or at the same time as the aggressive isolate. The induction of salicylic acid, a signal known to play a role in basal and cultivar-related resistance, was not involved in the biocontrol mechanism. The most practical and best feasible method of application as a seed coat failed to confirm the biocontrol effect of A1/D2 observed with root-dip inoculation. Confocal microscopy analysis revealed that seed coating led to insufficient A1/D2 hyphal establishment on the roots compared to root-dip inoculation, which may explain the lack of a biocontrol effect after seed coating and illustrates the importance of the application method for efficacy of a cross-protective biocontrol agent.
Similar content being viewed by others
Introduction
Soil-borne vascular pathogenic fungi are amongst the most destructive plant pathogens. The long survival of their fungal resting structures, microsclerotia, in the soil and the lack of effective curative measures make these pathogens very difficult to control (Deketelaere et al. 2017). Verticillium longisporum is pathogenic in Brassica horticultural crops, as well as in oilseed rape (Depotter et al. 2016). Significant yield losses caused by V. longisporum have been recorded in individual plants under controlled conditions and in the field (Depotter et al. 2019; Dunker et al. 2008; Zheng et al. 2019a).
The limited availability of chemical control measures against vascular pathogens calls for the exploration of alternatives, such as the use of microorganisms as biocontrol agents (Boyetchko 1999; Deketelaere et al. 2017). Cross-protection is a biocontrol approach against vascular pathogens using non-pathogenic isolates or weak (non-aggressive) strains from the same species or genus (Deketelaere et al. 2020, 2017; Qin et al. 2008; Tyvaert et al. 2014). In cross-protection, protective isolates typically share the same ecological niche as the pathogen and typically present several biocontrol modes of action (Deketelaere et al. 2017). The rapid activation of defense responses upon pathogen infection after treatment with certain compounds or organisms is called priming (Conrath et al. 2002). A priming effect has been reported for fungal and bacterial biocontrol agents against various soil-borne pathogens (Begum et al. 2010; El-Mougy and Abdel-Kader 2008; Müller and Berg 2008).
There has been extensive research on the biocontrol potential of non-pathogenic Fusarium isolates against Fusarium wilts in different crops (Elmer 2004; Fravel et al. 2003; Freeman et al. 2002; Kaur et al. 2010; Nel et al. 2006; Sajeena et al. 2020; Silva and Bettiol 2005). Additionally, some reports have shown that non-pathogenic Fusarium isolates conferred protection against Verticillium wilts (Angelopoulou et al. 2014; Pantelides et al. 2009; Veloso and Díaz 2012; Zhang et al. 2015). Similarly, non-pathogenic V. dahliae isolates reduced Verticillium wilt in tomato, cotton, and strawberry (Diehl et al. 2013; Shittu et al. 2009; Zhu et al. 2013) and V. tricorpus protected lettuce against V. dahliae (Qin et al. 2008). So far, three studies have shown significant biocontrol effects of a non-pathogenic V. isaacii isolate against V. longisporum in greenhouse and field conditions (Deketelaere 2020; França et al. 2013; Tyvaert et al. 2014).
The scarce research on non-pathogenic or non-aggressive Verticillium isolates as biocontrol agents against V. longisporum calls for the screening of new biocontrol candidates. V. longisporum consists of three genetically distinct lineages (A1/D1, A1/D2 and A1/D3) that originated from three independent hybridization events of four haploid Verticillium parents. While isolates from the lineage A1/D1 and A1/D3 have a wider geographic distribution and host range, A1/D2 has so far only been found in horseradish in Illinois, USA and Ontario, Canada (Inderbitzin et al. 2011; Yu et al. 2016). Pathogenicity tests in the greenhouse revealed that A1/D1 was the most aggressive lineage on oilseed rape, while A1/D2 was the least aggressive on all tested Brassica and non-Brassica crops (Novakazi et al. 2015). Thus, due to its consistent non-aggressiveness, isolates from the A1/D2 lineage were considered candidates for effective cross-protection.
In the present study, the potential of A1/D2 isolates as biocontrol agents against an aggressive A1/D1 isolate was investigated on oilseed rape. To this end, an in planta assay with root-dip inoculation was used to screen the biocontrol candidates within the lineage A1/D2. The best performing isolate from those assays was tested as a seed coat, with the aim to assess the impact of the application method on its potential suitability in the field. Because salicylic acid (SA) is involved in V. longisporum resistance (Zheng et al. 2019b), its role in the biocontrol effect triggered by A1/D2 was investigated, as well as whether priming is involved in the biocontrol mechanism. Finally, differences in root colonization by A1/D2 upon different application methods were investigated with confocal microscopy.
Methods
Fungal isolates and inoculum production
V. longisporum isolates were obtained from the fungal isolate collection of the Division of Plant Pathology and Crop Protection of the Georg-August University of Göttingen. To assess the biocontrol potential of the non-aggressive lineage A1/D2 against an aggressive A1/D1 isolate on oilseed rape, three isolates from A1/D2 were selected. The selected A1/D1 isolate (VL43) was collected from a diseased oilseed rape plant in Germany (Zeise and Tiedemann 2001, 2002). The three A1/D2 isolates were obtained from horseradish in the USA and are non-aggressive on oilseed rape (Depotter et al. 2017; Novakazi et al. 2015) (Table 1).
Spore inoculum
For the preparation of spore inoculum, 1 ml of spore suspension, which had previously been stored axenically at − 80 °C in 25% aqueous glycerol, was added to potato dextrose broth (PDB) in Erlenmeyer flasks with 400 ppm streptomycin, 50 ppm chloramphenicol, and 50 ppm rifampicin. PDB flasks were incubated on a shaker (100 rpm) at 22 °C in the dark. After ten days, the culture was filtered through a sterile sieve. The spore concentration was determined using a Thoma counting chamber and diluted to 1 × 106 spores ml−1. The spore suspension was applied either by root-dip inoculation (30 min) or as a seed coat.
Microsclerotia inoculum
Sand-rye flour (SRF) medium (2 kg Quartz sand, 142 g rye flour, and 190 ml water) was prepared to produce microsclerotia inoculum. SRF medium was autoclaved two times in plastic bags (55.0 × 33.5 cm). After cooling, three agar plugs from two-week-old fungal PDA cultures, as well as 20 ml sterile tap water, were added to each plastic bag. Bags were stored in the dark at room temperate for up to eight weeks and kneaded a couple of times per week to promote fungal growth throughout the whole medium. After sufficient production of microsclerotia, visible through the appearance of black grains inside the SRF medium, the bags were opened and their content was scattered onto plastic trays (50.0 × 32.0 × 6.5 cm). The trays were stored in a drying chamber at 25 °C for 3–5 days until moisture evaporated. For separation of the microsclerotia from the SRF medium, the mixture was poured in a sieve shaker and partitioned by particle sizes of 0–100 µm, 100–315 µm, 315–500 µm und > 500 µm. The sieved microsclerotia were stored in the dark at 4 °C until application as a soil amendment or seed coat.
Biocontrol potential of A1/D2 applied with root-dip inoculation
Experimental design
To evaluate the potential in planta biocontrol effect of the non-aggressive V. longisporum lineage A1/D2, a greenhouse screening with oilseed rape seedlings was conducted. Seeds of the susceptible oilseed rape cultivar (cv.) ‘Falcon’ were pre-germinated with a L:D 14:10 photoperiod (Horti-Lux HPS-400 Watt) at 18–24 °C in quartz sand. Ten days after sowing, seedlings were removed from the quartz sand and washed thoroughly under running tap water. Washed seedlings were dipped in a spore suspension prepared as described above. Roots of control seedlings were immersed in water.
Three inoculation methods were used, with which A1/D2 isolates were inoculated before, at the same time or after inoculation with the aggressive isolate VL43 (lineage A1/D1). For pre-inoculation, seedlings were first inoculated with A1/D2 isolates or water. At seven days post-inoculation (dpi), seedlings were removed from the soil, and the roots were gently washed before treatment with water or VL43. For co-inoculation, roots were inoculated with a mixed suspension of each non-aggressive A1/D2 and aggressive VL43. With post-inoculation, VL43 or water were inoculated and, at 7 dpi, seedlings were treated with water or A1/D2. To avoid cross-contamination, seedlings inoculated with different treatments in the first week were treated separately in the second week.
The three treatments (water, VL43, and A1/D2 + VL43) of each inoculation method consisted of 24 plants. Treated plants were transplanted into 7 × 7 × 8 cm pots filled with a soil mixture of sand and steamed compost with a 1:3 volume-based ratio. Each pot had two plants with the same treatment and four pots were placed in one tray to avoid cross-contamination between treatments during irrigation. All trays were organized according to a completely randomized design and kept with the same light and temperature conditions as described for pre-germination. The experiment was performed twice.
Evaluation of disease development
Disease assessment was conducted at 7, 14, 21, and 28 dpi. In the case of the pre- and post-inoculation methods, assessment dates were counted starting after the second inoculation. The evaluation of yellowing and death of leaves was performed according to a 9 score assessment key described by Eynck et al. (2009), and the net area under the disease progress curve (AUDPC) was calculated accordingly. At 28 dpi, plant height was measured from the cotyledons to the tip of the highest leaf to calculate the height reduction caused by disease in relation to the average height of the control treatment.
Biocontrol potential of A1/D2 applied as seed coat
Seed coating
The best performing A1/D2 isolate from the root-dip inoculation assay was selected to test two seed coating methods. Seeds of the spring oilseed rape cv. ‘Licosmos’ were surface sterilized by soaking in 70% ethanol for 2 min, washed with sterile water and stirred in 1% sodium hypochlorite + 1% tween 80 solution for 15 min. Following disinfection, seeds were washed three times with sterilized tap water. Seeds were coated either with microsclerotia or a spore suspension. For the microsclerotia seed coating, preparation of the liquid formulation followed the procedure by Lopisso et al. (2017) with modifications. First, a 1% methylcellulose (MC) sticker solution (MFCD00081763, Sigma-Aldrich; USA) was prepared in distilled boiled water. The solution was mixed on a magnetic stirrer for two hours. When the solution reached room temperature, 60 mg microsclerotia of 0–100 µm in size were dissolved in a 100 ml MC solution. For the seed coating, 2 g of oilseed rape seeds were mixed with 2.5 ml of the microsclerotia-MC solution in 15 ml falcon tubes. Mixing was performed by vigorously shaking the tubes for 1 min by hand and vortexing them five times for 1 min. Afterwards, coated seeds were distributed apart from each other to prevent clumping and dried under a laminar flow cabinet for 12 h. Seeds of the control treatment were coated with MC alone.
For the seed coating with spores, a spore suspension was prepared as described above. The sterilized seeds were left in an A1/D2 spore suspension (10 ml spore suspension per gram of seeds) for 30 min, and the suspension was stirred manually three times during the incubation period. Afterwards, they were dried under a laminar flow cabinet for 4 h. Seeds of the untreated control treatment were coated with water.
Microsclerotia soil amendment
VL43 was applied into the soil as a microsclerotia soil amendment. For that, microsclerotia of size 315–500 µm were added at a concentration of 800 mg microsclerotia per kg soil. Microsclerotia were mixed manually in the soil by adding 50 ml of water per kg of soil.
Experimental design and evaluation of disease development
Treated seeds were directly transplanted into 7 × 7 × 8 cm pots filled with a soil mixture of sand compost in a 1:3 volume-based ratio and with one plant per pot. For this assessment, 32 plants divided in four trays were used per treatment (control, VL43 soil amendment alone, or combined with A1/D2 seed coating). All trays were organized according to a completely randomized design. Each seed coating method was tested in a climate chamber and greenhouse. In the climate chamber, conditions were the same as during the biocontrol assessment of A1/D2 when applied with root inoculation. In the greenhouse, the temperature range was 15–25 °C. Disease evaluation was conducted by weekly assessing the plant height (cm) from 28 to 84 dpi from the hypocotyl to the highest point of the plant (including leaves, flowers, and pods). Necrotic dead plants were denoted as zero. The plant height of the treatments relative to the average plant height of the control treatment was calculated.
Microscopic assessment of different applications of A1/D2 on oilseed rape roots
Experimental design
To assess root colonization ability of A1/D2 following different application methods (seed coating and root-dip inoculation), hyphal colonization growth of the isolate A1/D2b on the external layers of the roots of the oilseed rape cv. Falcon was assessed by confocal microscopy at 14 dpi. Twelve ten-day-old seedlings root-dip inoculated with a spore suspension of A1/D2b in the biocontrol assessment with root-dip inoculation, as well as twelve seeds coated with A1/D2b spores or microsclerotia in the biocontrol assessment with a seed coat, were planted or sown in trays containing quartz sand. Water-treated seeds and seedlings were used as control. Sand was selected as substrate to ensure the harvest of complete roots without any adhered material. Pots were watered every other day and fertilized twice per week with the full nutrient solution “Flory Basisdünger” (EUFLOR GmbH, Schermbeck, Germany).
The experiment was conducted twice with the same conditions as the climate chamber. Two weeks after treatments, six plants per treatment were carefully uprooted and roots were gently washed. The differentiation zone of the main root was investigated by selecting, with light microscopy, two sections that showed presence of hyphae. On these sections, hyphal colonization was investigated with confocal microscopy.
Staining and confocal microscopy
The entire roots were stained with a sequential double staining method. Samples were first immersed in 50 µg ml−1 Wheat Germ Agglutinin, Alexa Fluor® 488 Conjugate (W11261, Molecular probes; USA) and vacuum-infiltrated in the dark at room temperature for 30 min. Subsequently, samples were vacuum-infiltrated for 20 min with 10 µg ml−1 propidium iodide (2,470,810 Carl Roth GmbH, Karlsruhe, Germany). Roots were immediately dipped twice in distilled water, mounted on 50% glycerol and covered with a coverslip (0.16 mm thickness). Microscopic examinations were performed using a Leica TCS SP2 confocal laser scanning microscope (Leica Microsystems, Manheim, Germany). A two-channel analysis was carried out, with a 488 nm wavelength to excite Alexa Fluor and 500–530 nm to receive the emission. In the case of propidium iodide, a 550–560 nm wavelength range was used for excitation and 608–680 nm to receive the emission.
Potential priming effect of A1/D2
Experimental design
To elucidate a potential priming effect of A1/D2 in oilseed rape against VL43, the effect of inoculation with A1/D2 on SA production of oilseed rape cv. Falcon was investigated. The five trays per treatment were arranged in a completely randomized design. Plants were root-dip inoculated following the pre-inoculation method described above. The four treatments were water-water, A1/D2b-water, water-VL43, and A1/D2b-VL43. Aerial parts of the plants were sampled at 6 and 10 dpi. For each treatment and sampling day, five samples from ten pooled plants were harvested. Samples were immersed in liquid nitrogen immediately after collection to halt any metabolic processes and stored in a freezer at − 22 °C. The experiment was performed twice.
Free SA extraction
Frozen samples of plant tissue were homogenized with a 5 mm tungsten ball by using a MM400 mill (Retsch GmbH, Haan, Germany) at 25 Hz for 1 min. Free SA was extracted according to Kamble and Bhargava (2007) with modifications. Ground plant tissue (100 mg) was suspended in 1.5 ml of acetone, shaken vigorously and centrifuged (5500 rpm) at 4 °C for 45 min. This step was repeated twice and the two supernatants were merged and evaporated in a speed vacuum centrifuge at 30 °C. The residue was dissolved in 1 ml demineralized water, and 1 ml ethyl acetate was subsequently added. The upper phase was transferred and evaporated at 35 °C. The residue was dissolved again in 200 µl of high-performance liquid chromatography (HPLC) grade methanol (1481.2500, CHEMSOLUTE).
High performance liquid chromatography
Samples were centrifuged for 2 min at 5000 rpm to precipitate unsolvable particles before loading them into HPLC vials. An HPLC-fluorescence system consisting of a ProStar 410 autosampler, a Prostar 210 binary pump system (Varian, Darmstadt, Germany) with a 10 W SS head, a LiChrospher ® 100 RP18 reverse phase column (particle size 5 µm) inside a LiChroCART ® 125–1 HPLC cartridge (Merck KGaA, Darmstadt, Germany) at 30 °C, and a Varian ProStar 363 fluorescence detector was used. For fluorescence detection, an excitation wavelength of 315 nm and emission wavelength of 405 nm were used. Each sample was analyzed for 27 min under a bi-mobile phase with (A) 20 mM sodium acetate (CHEMSOLUTE) pH 5.0 and (B) methanol with a flow rate of 1 ml min−1 with the following protocol: 0.0–2.0 min 10% B, 2.0–10.0 min from 10 to 30% B, 10.0–13.0 min from 30 to 98% B, 13.0–18.0 min 98% B, 18.0–21.0 min from 98 to 10% B, and 21.0–25.0 min 10% B.
A dilution series from 100 to 20 µM of SA dissolved in HPLC-grade methanol was used as a standard. The free SA was quantified using the Galaxie chromatography workstation software (Varian, Darmstadt, Germany).
Statistical analysis
The statistical analysis was carried out with R (version 4.0.2.; R Core Team 2021). For the analysis of biocontrol potential of A1/D2 with root-dip inoculation, the data from the two experimental repetitions was merged for each inoculation method (pre-, co- and post-inoculation). One experimental repetition had three replicates per treatment, each consisting of the average of eight plants in one tray. The data was analyzed by a multi-factor ANOVA where the significance of V. longisporum treatments on the relative plant height and net AUDPC was assessed for each inoculation method. In the case of the net AUDPC, a prior box cox transformation was necessary.
For the assessment of the potential biocontrol method of A1/D2 as a seed coat, a box cox transformation of the data was required. The significance of the V. longisporum treatments on the relative plant height was assessed for each seed coat method with a linear model. Because the experiment was carried out in two different environmental conditions, the effect of the greenhouse and climate chamber was also considered in the multi-factor ANOVA. For each environment there were four replicates per treatment, each consisting of the average height of six plants in one tray.
For the evaluation of the potential priming effect of A1/D2 by the assessment of free salicylic acid, the data from two experimental replicates was merged. One experimental repetition had five replicates per treatment, each consisting of ten plants. After a box cox transformation, the interaction of the V. longisporum treatments and day of harvest on the free salicylic acid in the plant tissue was assessed by multi-factor ANOVA. The homogeneity of variance of the residues of all models was assessed visually. For the multiple pos-hoc comparisons, a Sidak test was used.
Results
Biocontrol potential of A1/D2 with root-dip inoculation
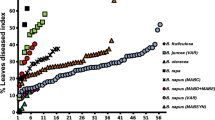
The analysis revealed differences on relative height between the treatments with a pre- (ANOVA, F(3, 20) = 4.25, P < 0.05), co- (ANOVA, F(3, 20) = 16.24, P < 0.05) and post-inoculation (ANOVA, F(3, 20) = 5.45, P < 0.05). The pre-inoculation of A1/D2b led to a relative height of seedlings significantly increased by up to 15% compared to single treatment with VL43 (Fig. 1a). Pre-inoculation with A1/D2a and A1/D2c did not show a significant biocontrol effect with regard to plant height. Co-inoculation of VL43 with the A1/D2 isolates increased relative plant height by up to 30% compared to seedlings only treated with VL43 (Fig. 1b). The analysis revealed differences on AUDPC between the treatments with a pre- (ANOVA, F(3, 20) = 8.35, P < 0.05), co- (ANOVA, F(3, 20) = 8.34, P < 0.05) and post-inoculation (ANOVA, F(3, 20) = 5.54, P < 0.05). With a pre- and co-inoculation of the A1/D2 isolates there was a significant symptom reduction of up to 50%. The post-inoculation assay had the highest AUDPC symptoms and no biocontrol effect was observed (Fig. 1c).
Assessment of biocontrol effect of the non-aggressive Verticillium longisporum lineage A1/D2 (isolates A1/D2a, A1/D2b, and A1/D2c) against the aggressive isolate VL43 after root-dip inoculation (1 × 106 spores ml−1) on oilseed rape seedlings (cultivar Falcon). Disease symptoms are expressed as net area under the disease progress curve (AUDPC) and plant height relative to the plant height of the control treatment at 28 dpi. A1/D2 isolates were pre- (a), co- (b), or post-inoculated (c) in relation to VL43. Data points represent the mean of six biological replicates, each consisting of merged data from eight plants. Error bars refer to SD. Different letters indicate significant differences (Sidak test, P < 0.05)
Biocontrol potential of A1/D2 applied as seed coat
The biocontrol potential of A1/D2b against VL43 was tested on spring oilseed rape by applying A1/D2b as a microsclerotia or spore seed coat. There was no effect of environment, timepoints of disease assement, and biocontrol treatment on the disease development, both when the biocontrol was applied as a microsclerotia seed coat (ANOVA, F(4, 60) = 0.52, P > 0.05) or a spore seed coat (ANOVA, F(4, 60) = 0.098, P > 0.05) (Supplementary Tables S1, S2).
Microscopic assessment of root colonization of A1/D2 on oilseed rape
Root colonization ability by A1/D2b applied as a root-dip inoculation or seed treatment (microsclerotia and spore suspension) was assessed with confocal microscopy. This assay confirmed that the three application methods allowed hyphal colonization of the root surface at 14 dpi (Fig. 2). A1/D2b hyphae were clearly observed after root-dip inoculation and spore seed coating (Fig. 2a, b, f), but the microsclerotia seed coat led to fungal colonization without any typical hyphal morphology (Fig. 2c, d). Instead, fungal structures presented a crumbly morphology, which was also observed in the spore seed coating (Fig. 2e). Overall, root-dip inoculation resulted in the most intense hyphal coverage of the roots.
Confocal microscopic analysis of the external root colonization pattern of the non-aggressive Verticillium longisporum lineage A1/D2 (isolate A1/D2b) on oilseed rape seedlings cultivar Falcon at 14 days post inoculation. A1/D2b was applied with a root-dip inoculation (1 × 106 spores ml−1) (a, b), as a microsclerotia seed coat (800 mg microsclerotia per kg of soil") (c, d) and as a spore seed coat (1 × 106 spores ml−1) (e, f). Root-dip inoculation led to intense hyphal growth (a, b). With the microsclerotia seed treatment, fungal structures lacked the typical hyphal morphology and, instead, had a crumbly appearance (c, d). This morphology was also seen with the spore seed coating treatment (e), but this treatment also resulted in typical looking hyphae (f). Hyphae were stained green-yellow with Alexa Fluor and roots red with propidium iodide. Bar a–b = 100 µm, c–d = 40 and 35 µm, e–f = 60 and 80 µm
Analysis of the potential priming effect caused by A1/D2
The potential priming effect of A1/D2 against VL43 in oilseed rape was assessed by quantifying the free SA production at 6 and 10 dpi. In both replicated experiments, there were no significant differences in the levels of SA between the treatments (ANOVA, F(3, 60) = 1.17, P > 0.05) (Supplementary Fig. S1).
Discussion
Biocontrol potential of A1/D2 applied by root-dip inoculation
In the present study, a cross-protection potential of the A1/D2 lineage against an aggressive V. longisporum isolate in oilseed rape was demonstrated for the first time. Applied with root-dip inoculation, A1/D2 isolates reduced symptoms of oilseed rape seedlings when they were pre- or co-inoculated with VL43 (Fig. 1a, b). In former studies, co-inoculation of the biocontrol agent with the aggressive isolate typically did not result in biocontrol, as reported for non-aggressive isolates of V. tricorpus and V. albo-atrum against V. dahliae on potato (Robinson et al. 2007). This suggested that the biocontrol effect of protective isolates against vascular diseases requires pre-inoculation (Alabouvette et al. 2009; Deketelaere et al. 2020; Tyvaert et al. 2014). However, a successful biocontrol effect against V. dahliae with both inoculation strategies has been reported for V. tricorpus in lettuce (Qin et al. 2008), as well as for non-aggressive isolates of V. dahliae and Gibelullopsis nigrescens in cotton and tomato (Shittu et al. 2009; Zhu et al. 2013). The reduction of symptoms observed with co-inoculation of VL43 with A1/D2 isolates indicates that A1/D2 is a potent competitor for infection sites and space on the root. This mode of action has been confirmed for a non-pathogenic Fusarium isolate against V. dahliae in pepper (Pantelides et al. 2009). In contrast to the observations of Zhu et al. (2013) and Qin et al. (2008), the present study revealed a higher disease reduction with co-inoculation than with pre-inoculation when applying A1/D2 isolates (Fig. 1b). This might be related to the higher expression of VL43 symptoms in the co-inoculation assay. With pre-inoculation, the seedlings were one week older when inoculated with the aggressive isolate. Consequently, they were bigger and more robust, which might have made them less susceptible to the pathogen (Sharabani et al. 2013).
By the time of post-inoculation with A1/D2b, VL43 was already well established in the root vascular system, as aggressive isolates start invading the vascular system 60 h after inoculation (Eynck et al. 2007). There was no biocontrol effect observed with post-inoculation application (Fig. 1c), which indicates that cross-protection by the lineage A1/D2 is more effective before the systemic colonization of the pathogen.
Seed coating and microscopic assessment
Biocontrol assessments should preferably mimic natural conditions as much as possible (Deketelaere et al. 2020, 2017; Stadler and Tiedemann 2014). Consequently, the biocontrol effect of A1/D2b was also tested on a spring type oilseed rape cultivar in unsterile greenhouse conditions. In addition, treatment as a seed coat is a particularly suitable method under practical terms. However, in this study, both seed coating treatments (spore suspension and microsclerotia) did not result in a significant biocontrol, neither in the greenhouse nor in the climate chamber (Supplementary Tables S1, S2). This result is similar to previous studies that reported no biocontrol effect against V. dahliae when protective isolates were applied as a seed coat (Angelopoulou et al. 2014; Lopisso et al. 2017).
For successful biocontrol with seed coating, the protective isolate must colonize the root surface during seed germination (El-Mougy and Abdel-Kader 2008). With confocal microscopy, we confirmed that A1/D2b was able to colonize the root surface after seed coating (Fig. 2). Nevertheless, A1/D2b root colonization was significantly lower when A1/D2b was applied as a seed treatment than by root-dip inoculation. The less intense hyphal colonization of the roots may explain the lower competitive effects and poorer induction of plant defense when non-aggressive strains are applied via seed coating. This is in agreement with the results from Bao and Lazarovits (2001), who showed that physical plant defense on the roots occurred only in the root section where the biocontrol agent was present.
The study by Deketelaere et al. (2020) revealed a biocontrol effect of V. isaacii against V. longisporum in cauliflower when using a high-precision application technology for seed treatment. In contrast, Lopisso et al. (2017) reported no biocontrol effect against V. dahliae in sugar beet when F. oxysporum F2 and V. tricorpus 1808 microsclerotia were applied as a seed coat with a methylcellulose formulation. This indicates that the application technique of microsclerotia seed coating is crucial to ensure sufficient colonization of plant roots with the biocontrol agent for efficient disease reduction.
Less intense hyphal colonization of the roots after seed coating with spores may be attributed to a low survival rate of spores when applied with this method. Crumbled fungal structures on the root might indicate dead spores or only partially germinated microsclerotia. Thus, further research should focus on coating materials that can improve the survival rate of spores and extend their shelf-life, as well as on nourishment for the biocontrol agent (Alaboubette et al. 2009; Rocha et al. 2019).
Potential priming effect of A1/D2
Biocontrol mechanisms that could be involved after the vascular establishment of the non-aggressive Verticillium isolates include plant growth promotion, antibiosis, or induction of plant defense. At this stage of colonization, a direct antagonistic mechanism through antibiosis would only be possible if A1/D2b was able to invade the vascular system (Deketelaere et al. 2017). Shittu et al. (2009) reported that a non-aggressive V. dahliae isolate was able to colonize the xylem as an endophyte. However, a previous study indicated that A1/D2c does not colonize above-ground parts of oilseed rape (Depotter et al. 2017). Thus, intense vascular systemic colonization in oilseed rape by A1/D2 is not likely to occur. Consequently, induction of plant defense is the most plausible mechanism of A1/D2b when post-inoculated after VL43. A similar biocontrol mechanism has been reported for two non-pathogenic F. oxysporum isolates, which upregulate pathogenesis-related proteins (PR) upon inoculation in eggplant and Arabidopsis (Angelopoulou et al. 2014; Veloso and Díaz 2012).
The A1/D2b biocontrol effect via root-dip inoculation suggests that induction of plant defense might be involved. SA is assumed to be the translocated signal in systemic acquired resistance (Conrath 2006). Moreover, Zheng et al. (2019b) showed that induction of SA at early stages of infection is required for basal and cultivar-related resistance in oilseed rape against V. longisporum. Priming has been reported as the biocontrol mechanism of non-pathogenic F. oxysporum isolates against F. oxysporum f. sp. lycopersici in tomato (Aimé et al. 2008) and Verticillium wilt in pepper (Veloso et al. 2016). In the present study, free SA was quantified to assess whether A1/D2b protects seedlings against VL43 by directly inducing defense reactions or through priming. Our results indicate that neither VL43 nor A1/D2b have an effect on SA production in the susceptible cultivar Falcon. Similarly, Zheng et al. (2019b) reported a lack of early induction of SA by VL43 in Falcon and this was not altered by applying the protective isolates. These results indicate that the induction of SA is probably not involved in the cross-protective biocontrol mechanism of A1/D2b and suggest that other pathways of plant defense rather than components of basal resistance are activated or enhanced by A1/D2b. Among these are jasmonate-dependent defense pathways, the production of defense-related enzymes, or accumulation of lignin (Deketelaere et al. 2017).
To the authors’ best knowledge, this is the first report of biocontrol effects of a non-aggressive isolate against a pathogenic strain of V. longisporum in oilseed rape applied by root-dip inoculation. The non-aggressive lineage A1/D2 demonstrated to be a potential biocontrol candidate when applied in a pre- or co-inoculation manner. Altogether, the results from this study suggest that this isolate might induce plant defense and compete with the pathogen for infection sites on the root surface. The induction of SA, a signal of basal and cultivar-related resistance to V. longisporum, does not appear to be involved in this biocontrol mechanism. No biocontrol effect was observed when the biocontrol agent was applied as a seed coat, which illustrates the importance of the application method for the efficacy of a biocontrol agent.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Code availability
Not applicable.
References
Aimé S, Cordier C, Alabouvette C, Olivain C (2008) Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. oxysporum Fo47. Physiol Mol Plant Path 73:9–15
Alabouvette C, Olivain C, Migheli Q, Steinberg C (2009) Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol 184:529–544
Angelopoulou DJ, Naska EJ, Paplomatas EJ, Tjamos SE (2014) Biological control agents (BCAs) of Verticillium wilt: influence of application rates and delivery method on plant protection, triggering of host defense mechanisms and rhizosphere populations of BCAs. Plant Pathol 63:1062–1069
Bao JR, Lazarovits G (2001) Differential colonization of tomato roots by nonpathogenic and pathogenic Fusarium oxysporum strains may influence Fusarium wilt control. Phytopathology 91:449–456
Begum MM, Sariah M, Puteh AB, Zainal Abidin MA, Rahman MA, Siddiqui Y (2010) Field performance of bio-primed seeds to suppress Colletotrichum truncatum causing damping-off and seedling stand of soybean. Biol Control 53:18–23
Boyetchko SM (1999) Biological control agents of canola and rapeseed diseases—status and practical approaches. In: Mukerji KG, Chamola BP, Upadhyay RR (eds) Biotechnological approaches in biocontrol of plant pathogens, vol 65. Springer. US, Boston, MA, pp 51–71
Conrath U (2006) Systemic acquired resistance. Plant Signal Behav 1:179–184
Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant–pathogen interactions. Trends Plant Sci 7:210–216
Deketelaere S, Tyvaert L, França SC, Höfte M (2017) Desirable traits of a good biocontrol agent against Verticillium wilt. Front Microbiol 8:1186
Deketelaere S, Spiessens K, Pollet S, Tyvaert L, Rooster LD, Callens D, França SC, Höfte M (2020) Towards practical application of Verticillium isaacii Vt305 to control Verticillium wilt of cauliflower: exploring complementary biocontrol strategies. Plants 9(11):1469
Depotter JRL, Deketelaere S, Inderbitzin P, von Tiedemann A, Hofte M, Subbarao KV, Wood TA, Thomma BP (2016) Verticillium longisporum, the invisible threat to oilseed rape and other brassicaceous plant hosts. Mol Plant Pathol 17(7):1004–1016
Depotter JRL, Rodriguez-Moreno L, Thomma BPHJ, Wood TA (2017) The emerging British Verticillium longisporum population consists of aggressive Brassica pathogens. Phytopathology 107:1399–1405
Depotter JRL, Thomma BPHJ, Wood TA (2019) Measuring the impact of Verticillium longisporum on oilseed rape (Brassica napus) yield in field trials in the United Kingdom. Eur J Plant Pathol 153:321–326
Diehl K, Rebensburg P, Lentzsch P (2013) Field application of non-pathogenic Verticillium dahliae genotypes for regulation of wilt in strawberry plants. Am J Plant Sci 04:24–32
Dunker S, Keunecke H, Steinbach P, von Tiedemann A (2008) Impact of Verticillium longisporum on yield and morphology of winter oilseed rape (Brassica napus) in relation to systemic spread in the plant. J Phytopathol 156:698–707
Elmer WH (2004) Combining nonpathogenic strains of Fusarium oxysporum with sodium chloride to suppress Fusarium crown rot of asparagus in replanted fields. Plant Pathol 53:751–758
El-Mougy NS, Abdel-Kader MM (2008) Long-term activity of bio-priming seed treatment for biological control of faba bean root rot pathogens. Australas Plant Pathol 37:464–471
Eynck C, Koopmann B, Grunewaldt-Stoecker G, Karlovsky P, von Tiedemann A (2007) Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur J Plant Pathol 118:259–274
Eynck C, Koopmann B, von Tiedemann A (2009) Identification of Brassica accessions with enhanced resistance to Verticillium longisporum under controlled and field conditions. J Plant Dis Protect 116:63–72
França SC, Spiessens K, Pollet S, Debode J, de Rooster L, Callens D, Höfte M (2013) Population dynamics of Verticillium species in cauliflower fields: influence of crop rotation, debris removal and ryegrass incorporation. Crop Prot 54:134–141
Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytol 157:493–502
Freeman S, Zveibil A, Vintal H, Maymon M (2002) Isolation of nonpathogenic mutants of Fusarium oxysporum f. sp. melonis for biological control of Fusarium wilt in cucurbits. Phytopathology 92:164–168
Inderbitzin P, Davis RM, Bostock RM, Subbarao KV (2011) The ascomycete Verticillium longisporum is a hybrid and a plant pathogen with an expanded host range. PLoS ONE 6(3):e18260
Kamble A, Bhargava S (2007) β-Aminobutyric acid-induced resistance in Brassica juncea against the necrotrophic pathogen Alternaria brassicae. J Phytopathol 155:152–158
Kaur R, Kaur J, Singh SR (2010) Nonpathogenic Fusarium as a biological control agent. Plant Pathol J 9:79–91
Lopisso DT, Kühlmann V, Siebold M (2017) Potential of soil-derived fungal biocontrol agents applied as a soil amendment and a seed coating to control Verticillium wilt of sugar beet. Biocontrol Sci Technol 27:1019–1110
Müller H, Berg G (2008) Impact of formulation procedures on the effect of the biocontrol agent Serratia plymuthica HRO-C48 on Verticillium wilt in oilseed rape. BioControl 53:905–916
Nel B, Steinberg C, Labuschagne N, Viljoen A (2006) The potential of nonpathogenic Fusarium oxysporum and other biological control organisms for suppressing Fusarium wilt of banana. Plant Pathol 55:217–223
Novakazi F, Inderbitzin P, Sandoya G, Hayes RJ, von Tiedemann A, Subbarao KV (2015) The three lineages of the diploid hybrid Verticillium longisporum differ in virulence and pathogenicity. Phytopathology 105:662–673
Pantelides IS, Tjamos SE, Striglis IA, Chatzipavlidis I, Paplomatas EJ (2009) Mode of action of a non-pathogenic Fusarium oxysporum strain against Verticillium dahliae using real time qPCR analysis and biomarker transformation. Biol Control 50:30–36
Qin Q-M, Vallad GE, Subbarao KV (2008) Characterization of Verticillium dahliae and V. tricorpus isolates from lettuce and artichoke. Plant Dis 92:69–77
R Core Team (2021) R: A language and environment for statistical computing. R foundation for statistical computing, Austria. https://www.r-project.org/
Robinson N, Piatt HW, Hale LR (2007) Verticillium dahliae interactions with V. albo-atrum and V. tricorpus and their effects on verticillium wilt disease development in potato. Am Potato J 84:229–235
Rocha I, Ma Y, Souza-Alonso P, Vosátka M, Freitas H, Oliveira RS (2019) Seed coating: a tool for delivering beneficial microbes to agricultural crops. Front Plant Sci 10:1357
Sajeena A, Nair DS, Sreepavan K (2020) Non-pathogenic Fusarium oxysporum as a biocontrol agent. Indian Phytopathol 73:177–183
Sharabani G, Shtienberg D, Borenstein M, Shulhani R, Lofthouse M, Sofer M, Chalupowicz L, Barel V, Manulis-Sasson S (2013) Effects of plant age on disease development and virulence of Clavibacter michiganensis subsp. michiganensis on tomato. Plant Pathol 62:1114–1122
Shittu HO, Castroverde DCM, Nazar RN, Robb J (2009) Plant-endophyte interplay protects tomato against a virulent Verticillium. Planta 229:415–426
Silva JCD, Bettiol W (2005) Potential of non-pathogenic Fusarium oxysporum isolates for control of Fusarium wilt of tomato. Can J Bot 30:409–412
Stadler M, von Tiedemann A (2014) Biocontrol potential of Microsphaeropsis ochracea on microsclerotia of Verticillium longisporum in environments differing in microbial complexity. BioControl 59:449–460
Tyvaert L, França SC, Debode J, Höfte M (2014) The endophyte Verticillium Vt305 protects cauliflower against Verticillium wilt. J Appl Microbiol 116:1563–1571
Veloso J, Díaz J (2012) Fusarium oxysporum Fo47 confers protection to pepper plants against Verticillium dahliae and Phytophthora capsici and induces the expression of defence genes. Plant Pathol 61:281–288
Veloso J, Alabouvette C, Olivain C, Flors V, Pastor V, García T, Díaz J (2016) Modes of action of the protective strain Fo47 in controlling Verticillium wilt of pepper. Plant Pathol 65:997–1007
Yu JM, Cafarov IH, Babadoost M (2016) Morphology, molecular identity, and pathogenicity of Verticillium dahliae and V. longisporum associated with internally discolored horseradish roots. Plant Dis 100:749–757
Zeise K, von Tiedemann A (2001) Morphological and physiological differentiation among vegetative compatibility groups of Verticillium dahliae in relation to V. longisporum. J Phytopathol 149:469–475
Zeise K, von Tiedemann A (2002) Host specialization among vegetative compatibility groups of Verticillium dahliae in relation to Verticillium longisporum. J Phytopathol 150:112–119
Zhang Q, Yang L, Zhang J, Wu M, Chen W, Jiang D, Li G (2015) Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant Soil 392:101–114
Zheng X, Pfordt A, Khatri L, Eseola AB, Wilch A, Koopmann B, von Tiedemann A (2019a) Contrasting patterns of colonization with Verticillium longisporum in winter- and spring-type oilseed rape (Brassica napus) in the field and greenhouse and the role of soil temperature. Plant Dis 103:2090–2099
Zheng X, Koopmann B, von Tiedemann A (2019b) Role of salicylic acid and components of the phenylpropanoid pathway in basal and cultivar-related resistance of oilseed rape (Brassica napus) to Verticillium longisporum. Plants 8:491
Zhu HQ, Feng ZL, Li ZF, Shi YQ, Zhao LH, Yang JR (2013) Characterization of two fungal isolates from cotton and evaluation of their potential for biocontrol of Verticillium wilt of cotton. J Phytopathol 161:70–77
Acknowledgements
This work was financially supported by the German Federal Ministry for Food and Agriculture (BMEL) and the Agency for Renewable Resources (FNR). The authors acknowledge the technical assistance of Isabel Müller, Leonard Sundermann, Virginia Casasnovas, and Shilu Dahal with the phenotypic assessments. Dr. Antonia Wilch, Alice Eseola, and Dima Alnajar provided indispensable guidance and assistance on the confocal microscopy assessment. Finally, we thank Dr. Christian Kluth for the statistical advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was financially supported by the German Federal Ministry for Food and Agriculture (BMEL) and the Agency for Renewable Resources (FNR).
Author information
Authors and Affiliations
Contributions
Marta Vega Marin conducted the experiments, recorded and processed the data, designed tables and graphs and prepared the draft manuscript. Andreas von Tiedemann and Marta Vega Marin developed and designed the overall research project, discussed and interpreted the results, and revised and edited the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors state that there are no conflicts of interest. This article does not contain any studies involving human participants performed by any of the authors.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Handling Editor: Jane Debode
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vega-Marin, M., von Tiedemann, A. Cross-protection of oilseed rape against Verticillium longisporum by the non-aggressive lineage A1/D2. BioControl 67, 419–431 (2022). https://doi.org/10.1007/s10526-022-10147-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10147-5