Abstract
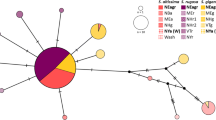
The snail, Cochlicella acuta (Müller), is an introduced pest of grain crops in southern Australia, and native to Europe and north-west Africa. Parasitoid flies (Sarcophaga villeneuveana (Enderlein)) have previously been sourced from France to attempt biological control of the pest in Australia, but have failed. Cochlicella acuta has three mitochondrial lineages (CO1 & 16S based) within its native distribution. The snails now found in Australia belong to just one of these lineages. Their origins most likely lie in the southern Iberian Peninsula or Morocco, not in France. We collected S. villeneuveana attacking C. acuta in France, Spain, Portugal, Morocco and Australia. Genetic structure (CO1) was not found within the fly that aligned with the lineages recorded for the snail host in the same regions. However, geographical patterns were detected amongst haplotypes of S. villeneuveana which encourage further, better targeted searches in Morocco and the southern Iberian Peninsula for a more effective biological control agent(s).




Similar content being viewed by others
References
Baker GH (2002) Helicidae and Hygromiidae as pests in cereal crops and pastures in southern Australia. In: Barker GM (ed) Molluscs as crop pests. CABI Publishing, Wallingford, pp 193–215
Baker GH (2008) The population dynamics of the Mediterranean snails, Cernuella virgata, Cochlicella acuta (Hygromiidae) and Theba pisana (Helicidae) in pasture-cereal rotations in South Australia: a 20-year study. Aust J Exp Agric 48:1514–1522
Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Ostell J, Pruitt KD, Sayers EW (2018) GenBank. Nucleic Acids Res 46:D41–D47
Buenaventura E, Whitmore D, Pape T (2017) Molecular phylogeny of the hyperdiverse genus Sarcophaga (Diptera: Sarcophagidae), and comparison between algorithms for identification of rogue taxa. Cladistics 33:109–133
Clement M, Posada D, Crandall K (2000) TCS : a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660
Coupland JB (1994) Diptera associated with snails collected in south-western and west-Mediterranean Europe. Vertigo 3:19–24
Coupland JB (1995) Susceptibility of helicid snails to isolates of the nematode Phasmarhabditis hermaphrodita from southern France. J Invert Path 66:207–208
Coupland JB (1996a) The efficacy of metaldehyde formulations against helicid snails: the effect of concentration, formulation and species. In: Henderson IF (ed) Slug and snail pests in agriculture. BCPC Monograph No. 66. British Crop Protection Council, Farnham, pp 151–156
Coupland JB (1996b) The biological control of helicid snail pests in Australia: surveys, screening and potential agents. In: Henderson IF (ed) Slug and snail pests in agriculture. BCPC Monograph No. 66. British Crop Protection Council, Farnham, pp 255–261
Coupland JB, Baker GH (1994) Host distribution, larviposition behaviour and generation time of Sarcophaga penicillata (Diptera: Sarcophagidae), a parasitoid of conical snails. Bull Entomol Res 84:185–189
Coupland JB, Baker GH (1995) The potential of several species of terrestrial Sciomyzidae as biological control agents of pest helicid snails in Australia. Crop Prot 14:573–576
Coupland JB, Barker GM (2004) Diptera as predators and parasitoids of terrestrial gastropods, with emphasis on Phoridae, Calliphoridae, Sarcophagidae, Muscidae and Fanniidae. In: Barker GM (ed) Natural enemies of molluscs. CABI Publishing, Wallingford, pp 85–158
Coupland JB, Baker GH (2007) Search for biological control agents of invasive Mediterranean snails. In: Vincent C, Goettel MS, Lazarovits G (eds) Biological control: a global perspective. CABI Publishing, Wallingford, pp 7–12
Coupland JB, Espiau A, Baker GH (1994) Seasonality, longevity, host choice, and infection efficiency of Salticella fasciata (Diptera: Sciomyzidae), a candidate for the biological control of pest helicid snails. Biol Control 4:32–37
Fendane Y, Richet R, Thomann T, Jourdan M, Baker G, Ghamizi M, Sheppard A (2018) First records of flesh flies (Diptera: Sarcophagidae) emerging from terrestrial snails in Morocco. Afr Entomol 26:124–130
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit 1 from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Huelsenbeck JP, Ronquist FR (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Jordaens K, Sonet G, Richet R, Dupont E, Braet Y, Desmyter S (2013) Identification of forensically important Sarcophaga species (Diptera: Sarcophagidae) using the mitochondrial CO1 gene. Int J Leg Med 127:491–504
Jourdan M, Thomann T, Kriticos D, Bon M-C, Sheppard A, Baker GH (2019) Sourcing effective biological control agents of conical snails, Cochlicella acuta, in Europe and north Africa for release in southern Australia. Biol Control 134:1–14
Kerney MP, Cameron RAD (1979) A field guide to the land snails of Britain and north-west Europe. Collins, London
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Leigh JW, Bryant D (2015) PopART: full feature software for haplotype network construction. Meth Ecol Evol 6:1110–1116
Leonard E, Baker G, Hopkins D (2003) Bash ‘em, burn ‘em, bait ‘em. SARDI, Adelaide, Australia
Lewis G (1975) Shell polymorphism in the snail Cochlicella acuta (Müller) and some data on its genetics. Biol J Linn Soc 7:147–160
Lewis G (1977) Polymorphism and selection in Cochlicella acuta. Phil Trans R Soc Lond B 276:399–451
Leyson M, Hopkins DC, Charwat S, Baker GH (2003) Release and establishment in South Australia of Sarcophaga penicillata (Diptera: Sarcophagidae), a biological control agent for Cochlicella acuta (Mollusca: Hygromiidae). In: Dussart GBJ (ed) Slugs and snail: agricultural, veterinary & environmental perspectives. BCPC Symposium Proceedings No. 80. British Crop Protection Council, Thornton Heath, pp 295–300.
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
McKillup SC, McKillup RV (2002) Flies that attack polymorphic snails on coloured backgrounds: selection for crypsis by a sarcophagid parasitoid of Littoraria filosa. Biol J Linn Soc 77:367–377
McKillup SC, McKillup RV, Pape T (2000) Flies that are parasitoids of a marine snail: the larviposition behaviour and life cycles of Sarcophaga megafilosia and Sarcophaga meiofilosia. Hydrobiologia 439:141–149
Meiklejohn KA, Wallman JF, Dowton M (2011) DNA-based identification of forensically important Australian Sarcophagidae (Diptera). Int J Leg Med 125:27–32
Moran S (1987) Insect enemies of the landsnail Theba pisana in Israel. Isr J Ent 21:129–130
Richet R, Blackith RM, Pape T (2011) Sarcophaga of France (Diptera: Sarcophagidae). Pensoft, Sofia, Bulgaria
Ronquist FR, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Srivathsan A, Meier R (2012) On the inappropriate use of Kimura-2-parameter (K2P) divergences in the DNA-barcoding literature. Cladistics 28:190–194
Stamper T, Dahlem GA, Cookman C, Debry RW (2013) Phylogenetic relationships of flesh flies in the subfamily Sarcophaginae based on three mtDNA fragments (Diptera: Sarcophagidae). Syst Ent 38:35–44
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan SW, Rizman-Idid M, Mohd-Aris E, Kurahashi H (2010) DNA-based characterisation and classification of forensically important flesh flies (Diptera: Sarcophagidae) in Malaysia. Forensic Sci Int 199:43–49
Thompson JD, Higgins DG, Gibson TJ (1994) W:CLUSTAL. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wells JD, Pape T, Spurling FAH (2001) DNA-based identification and molecular systematics of forensically important Sarcophagidae. J Forensic Sci 46:1098–1102
Welter-Schultes FW (2012) European non-marine molluscs, a guide for species identification. Planet Poster Editions, Göttingen, Germany
Whitmore D, Pape T, Cerretti P (2013) Phylogeny of Heteronychia : the largest lineage of Sarcophaga (Diptera: Sarcophagidae). Zool J Linn Soc 169:604–639
Zehner R, Amendt J, Schütt S, Sauer J, Krettek R, Povolný S (2004) Genetic identification of forensically important flesh flies (Diptera: Sarcophagidae). Int J Leg Med 118:245–247
Acknowledgements
We thank the Grains Research & Development Corporation Australia for funding the research (Projects DAS00095 and DAS00134). Greg Baker and Kelly Hill (SARDI, Australia) kindly provided CO1 sequences for two S. villeneuveana individuals, Daniel Whitmore (Natural History Museum, UK) identified S. longestylata, Fatiha Guermache (EBCL, France) provided technical advice in molecular biology, Elven Kerdellant (EBCL, France) assisted with snail dissections, and Valérie Caron (CSIRO) provided advice on a draft of the manuscript. We particularly thank the La Consejera de Medio Ambiente y Ordenacion del Territorio de la Junta de Andalucia and Generalitat de Valenciana Conselleria de Infraestructures, Territori I Medi Ambient for authorisations of collections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Research involving human participants and/or animals
The article does not contain any studies with human participants or animals other than those detailed for the snails and flies.
Informed consent
Informed consent was obtained from all individual participants named in the study.
Additional information
Handling Editor: Stefano Colazza
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jourdan, M., Thomann, T., Richet, R. et al. Genetic variability in the parasitic fly, Sarcophaga villeneuveana, in south-western Europe and Morocco. BioControl 65, 59–70 (2020). https://doi.org/10.1007/s10526-019-09985-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09985-7