Abstract
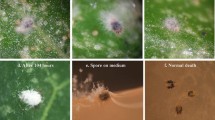
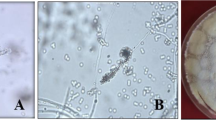
The entomopathogenic fungus, Beauveria bassiana, is capable of infecting pest mites, while the effect on different mite stages has rarely been reported. The present study evaluates the effect of several B. bassiana isolates (GZGY-1-3, LNSZ-26, SDDZ-9, XJWLMQ-32, SCWJ-2 and JXJGS-1) on Tetranychus urticae by a series of assays and observations. A potted bean plant assay indicated the fungal sprays resulted in greater reduction of adult T. urticae populations, but poor suppression in eggs and immature stages. During the one-month experiment, the different fungal isolates reduced the numbers of T. urticae eggs, immatures and adults by 38.7–55.2, 3.7–18.7 and 61.0–72.1%, respectively. Laboratory bioassays showed their corrected mortalities were 2.7–3.8, 17.5–25.8 and 63.2–71.2%, respectively, at seven days days post-fungal treatment. Fecundity of female mites was significantly reduced after fungal spray due to the lethal effect on females, while egg hatchability was not affected. Light microscopy observations indicated fungal outgrowths were evident in mite cadavers, but were not visible on eggs. Scanning electronic microscopy observations demonstrated fungal mycelia grew prolifically from the adult mite 60 h following fungal spray, although no symptoms of fungal infection were exhibited in most immature cadavers. Despite the fact that fungal conidia were able to adhere to and germinate on eggshells, and the germ tubes elongated on the shell surface, they were never observed to penetrate the eggs. Our results demonstrating that the failure to control T. urticae on bean plants using B. bassiana, which we attributed to its poor infectivity to mite eggs and immature stages, may provide useful information in future attempts to develop effective mite control strategies.




Similar content being viewed by others
References
Abbott WS (1925) A method for computing the effectiveness of insecticides. J Econ Entomol 18:265–267
Alves SB, Rossi LS, Lopes RB, Tamai MA, Pereira RM (2002) Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and Tetranychus urticae (Acari: Tetranychidae). J Invertebr Pathol 81:70–77
Bostanian NJ, Hardman JM, Racette G, Franklin JL (2007) The relationship between winter egg counts of the European red mite Panonychus ulmi (Acari: Tetranychidae) and its summer abundance in a reduced spray orchard. Exp Appl Acarol 42:185–195
Butt TM, Jackson CW, Magan N (eds) (2001) Fungi as biocontrol agents: progress, problems and potential. CABI Publishing, Wallingford
Dagli F, Tunc I (2001) Dicofol resistance in Tetranychus cinnabarinus: resistance and stability of resistance in populations from Antalya, Turkey. Pest Manag Sci 57:609–614
Farazmand A, Amir-Maafi M (2018) A population growth model of Tetranychus urticae Koch (Acari: Tetranychidae). Persian J Acarol 7:193–201
Feng M, Poprawski T, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol 4:3–34
Ferron P (1985) Fungal control. In: Kerkut GA, Gilbert LI (eds) Comparative insect physiology, biochemistry and pharmacology, vol 12. Pergamon Press, Oxford, pp 313–346
Gatarayiha MC, Laing MD, Miller RM (2011) Field evaluation of Beauveria bassiana efficacy for the control of Tetranychus urticae Koch (Acari: Tetranychidae). J Appl Entomol 135:582–592
Gindin G, Samish M, Zangi G, Mishoutchenko A, Glazer I (2002) The susceptibility of different species and stages of ticks to entomopathogenic fungi. Exp Appl Acarol 28:283–288
Goettel MS, Inglis DG (1997) Fungi: Hyphomycetes. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, London, pp 213–249
Grbić M, van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, Osborne EJ, Dermauw W, Ngoc PCT, Ortego F, Hernández-Crespo P, Diaz I, Martinez M, Navajas M, Sucena É, Magalhães S, Nagy L, Pace RM, Djuranović S, Smagghe G, Iga M, Christiaens O, Veenstra JA, Ewer J, Villalobos RM, Hutter JL, Hudson SD, Velez M, Yi SV, Zeng J, Pires-daSilva A, Roch F, Cazaux M, Navarro M, Zhurov V, Acevedo G, Bjelica A, Fawcett JA, Bonnet E, Martens C, Baele G, Wissler L, Sanchez-Rodriguez A, Tirry L, Blais C, Demeestere K, Henz SR, Gregory TR, Mathieu J, Verdon L, Farinelli L, Schmutz J, Lindquist E, Feyereisen R, van de Peer Y (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492
Guo FY, Zhang ZQ, Zhao ZM (1998) Pesticide resistance of Tetranychus cinnabarinus (Acari: Tetranychidae) in China: a review. Syst Appl Acarol 3:3–7
Herron GA, Rophail J, Wilson LJ (2004) Chlorfenapyr resistance in two-spotted spider mite (Acari: Tetranychidae) from Australian cotton. Exp Appl Acarol 34:315–321
Hoque MF, Islam W, Khalequzzaman M (2008) Life tables of two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) and its predator Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). J Biosci 16:1–10
Irigaray FJS, Marco-Mancebón V, Pérez-Moreno I (2003) The entomopathogenic fungus Beauveria bassiana and its compatibility with triflumuron: effect on the two spotted spider mite, Tetranychus urticae. Biol Control 26:168–173
Kakoki S, Kamimuro T, Ikenoue Y, Inokuchi M, Tsuda K, Sakamaki Y (2019) The response of three species of phytoseiid mite (Acari: Phytoseiidae) to synthetic pyrethroid pesticides in the laboratory and the field. Exp Appl Acarol 77:27–41
Littell RC, Pendergast J, Natarajan R (2000) Tutorial in biostatistics: modelling covariance structure in the analysis of repeated measures data. Stat Med 19:1793–1819
Maniania NK, Bugeme DM, Wekesa VW, Delalibera I Jr, Knapp M (2008) Role of entomopathogenic fungi in the control of Tetranychus evansi and Tetranychus urticae (Acari: Tetranychidae), pests of horticultural crops. Exp Appl Acarol 46:259–274
Mondel M, Ara N (2006) Biology and fecundity of the two-spotted spider mite, Tetranychus urticae Koch. (Acari: Tetranychidae) under laboratory conditions. J Life Earth Sci 1:43–47
Oduor GI (1995) Abiotic factors and the epizootiology of Neozygites cf. floridana, a fungus pathogenic to the cassava green mite. PhD Thesis, University of Amsterdam, The Netherlands
Prischmann DA, James DG, Wright LC, Teneyck RD, Snyder WE (2005) Effects of chlorpyrifos and sulfur on spider mites (Acari: Tetranychidae) and their natural enemies. Biol Control 33:324–334
Samish M, Gindin G, Alekseev E, Glazer I (2001) Pathogenicity of entomopathogenic fungi to different developmental stages of Rhipicephalus sanguineus (Acari: Ixodidae). J Parasitol 87:1355–1359
SAS Institute (2009) Base SAS® 9.2 Procedures Guide. SAS Institute, Cary
Shi WB, Feng MG (2004) Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol Control 30:165–173
Shi WB, Feng MG, Liu SS (2008a) Sprays of emulsifiable Beauveria bassiana formulation are ovicidal towards Tetranychus urticae (Acari: Tetranychidae) at various regimes of temperature and humidity. Exp Appl Acarol 46:247–257
Shi WB, Zhang L, Feng MG (2008b) Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to three hypocrealean fungi as biocontrol agents. Biol Control 46:495–501
Shin TY, Bae SM, Kim DJ, Yun HG, Woo SD (2017) Evaluation of virulence, tolerance to environmental factors and antimicrobial activities of entomopathogenic fungi against two-spotted spider mite, Tetranychus urticae. Mycoscience 58:204–212
Ullah MS, Lim UT (2017) Synergism of Beauveria bassiana and Phytoseiulus persimilis in control of Tetranychus urticae on bean plants. Syst Appl Acarol 22:1924–1935
Ullah MS, Haque MA, Nachman G, Gotoh T (2012) Temperature-dependent development and reproductive traits of Tetranychus macfarlanei (Acari: Tetranychidae). Exp Appl Acarol 56:327–344
Vestergaard S, Gillespie AT, Butt TM, Schreiter G, Eilenberg J (1995) Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips, Frankliniella occidentalis. Biocontrol Sci Technol 5:185–192
Wang Z, Cang T, Wu S, Wang X, Qi P, Wang X, Zhao X (2018) Screening for suitable chemical acaricides against two-spotted spider mites, Tetranychus urticae, on greenhouse strawberries in China. Ecotoxicol Environ Saf 163:63–68
Wekesa VW, Knapp M, Maniania NK, Boga HI (2006) Effects of Beauveria bassiana and Metarhizium anisopliae on mortality, fecundity and egg fertility of Tetranychus evansi. J Appl Entomol 130:155–159
Wilson LJ, Morton R (1993) Seasonal abundance and distribution of Tetranychus urticae (Acari: Tetranychidae), the two spotted spider mite, on cotton in Australia and implications for management. Bull Entomol Res 83:291–303
Witalinski W (1993) Egg-shells in mites—vitelline envelope and chorion in acaridida (Acari). Exp Appl Acarol 17:321–344
Wu SY, Gao YL, Zhang YP, Wang ED, Xu XN, Lei ZR (2014) An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: evidence from laboratory bioassay and scanning electron microscopic observation. PLoS ONE 9(1):e84732
Wu SY, Xie HC, Li MY, Xu XN, Lei ZR (2016) Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Exp Appl Acarol 70:421–435
Wu M, Adesanya AW, Morales MA, Walsh DB, Lavine LC, Lavine MD, Zhu F (2019) Multiple acaricide resistance and underlying mechanisms in Tetranychus urticae on hops. J Pest Sci 92:543–555
Zhang L, Shi WB, Feng MG (2014) Histopathological and molecular insights into the ovicidal activities of two entomopathogenic fungi against two-spotted spider mite. J Invertebr Pathol 117:73–78
Zhang XN, Jin DC, Zou X, Guo JJ (2016) Laboratory and field evaluation of an entomopathogenic fungus, Isaria cateniannulata strain 08XS-1, against Tetranychus urticae (Koch). Pest Manag Sci 72:1059–1066
Zhang XN, Guo JJ, Zou X, Jin DC (2018) Pathogenic differences of the entomopathogenic fungus Isaria cateniannulata to the spider mite Tetranychus urticae (Trombidiformes: Tetranychidae) and its predator Euseius nicholsi (Mesostigmata: Phytoseiidae). Exp Appl Acarol 75:69–84
Acknowledgements
We thank Dr. Cecil L. Smith (University of Georgia, USA) for helping with the language editing. This work was supported by Natural Science Foundation of China (Grant No. 31501704).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Helen Roy
Electronic supplementary material
Below is the link to the electronic supplementary material.
10526_2019_9970_MOESM1_ESM.tif
Supplementary Fig. S1 Population fluctuations of T. urticae on potted bean plants following fungal sprays. Initial densities of T. urticae were determined immediately prior to treatment on day 0. B. bassiana suspension, was sprayed at 1 × 107 ml−1 fungal conidia/ml. (a). Eggs; (b). immatures (larvae, protonymphs and deutonymphs) of T. urticae; (c). Adult T. urticae. Each data point and bar represent the mean and SE, respectively. Black arrows signify fungal spray (B. bassiana strains: LNSZ-26; SDDZ-9; XJWLMQ-32; SCWJ-2 and JXJGS-1) (TIFF 11344 kb)
10526_2019_9970_MOESM2_ESM.tif
Supplementary Fig. S2 Comparison of mean corrected mortality (± SE) of B. bassiana in different stages of T. urticae at 7 days post-fungal treatment in the laboratory (B. bassiana strains: LNSZ-26; SDDZ-9; XJWLMQ-32; SCWJ-2 and JXJGS-1). Bars with different letters indicate that the corrected mortalities in different stages of T. urticae are significant different (all P’s< 0.05)
10526_2019_9970_MOESM3_ESM.tif
Supplementary Fig. S3 Daily oviposition by female T. urticae and egg hatchability after being separately treated with B. bassiana (strain: LNSZ-26, SDDZ-9, XJWLMQ-32, SCWJ-2 and JXJGS-1). (a). Oviposition; (b). Egg hatch rate. Each data point and bar represent the mean and SE, respectively (B. bassiana strains: LNSZ-26; SDDZ-9; XJWLMQ-32; SCWJ-2 and JXJGS-1) (TIFF 5258 kb)
Rights and permissions
About this article
Cite this article
Wu, S., Sarkar, S.C., Lv, J. et al. Poor infectivity of Beauveria bassiana to eggs and immatures causes the failure of suppression on Tetranychus urticae population. BioControl 65, 81–90 (2020). https://doi.org/10.1007/s10526-019-09970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09970-0