Abstract
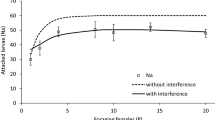
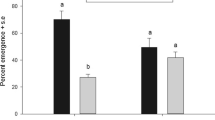
This study shows the effectiveness of deliberately selecting for Coptera haywardi individuals to increase a population’s capacity to discriminate against parasitised hosts. In the ‘selected colony’ (F1–F4), females were selected based on their ability to discriminate parasitised fruit fly pupae, determined by their host searching, foraging and oviposition behaviour. Female parasitoids of successive generations of the selected colony (F1–F4) showed an increasing discriminatory ability, including reduced host searching and foraging time. The last selected generation, i.e. F4 showed an increase in fecundity compared to the standard colony. In F4 individuals from the selected colony, antennae length increased but the hind tibia size did not, compared to individuals from the control colony. Flight ability and survival remained unchanged across all generations. This selection process could be an effective method of recuperating the discriminatory capacity of a C. haywardi colony under mass rearing conditions.



Similar content being viewed by others
References
Aguiar-Menezes E, Menezes E, Loiàcono MS (2003) First record of Coptera haywardi Loiàcono (Hymenoptera: Diapriidae) as a parasitoid of fruit-infesting Tephritidae (Diptera) in Brazil. Neotrop Entomol 32:355–358
Aluja M, Sivinski J, Ovruski S, Guillén L, López M, Cancino J, Torres-Anaya A, Gallegos-Chan G, Ruíz L (2009) Colonization and domestication of seven species of native New World hymenopterous larval-prepupal and pupal fruit fly (Diptera: Tephritidae) parasitoids. Biocontrol Sci Technol 19:49–79
Ardeh MJ, de Jong PW, van Lenteren JC (2005) Intra- and interspecific host discrimination in arrhenotokous and thelytokous Eretmocerus. Biol Control 33:74–80
Baeshen R, Ekechukwu NE, Toure M, Paton D, Coulibaly M, Traoré SF, Tripet F (2014) Differential effects of inbreeding and selection on male reproductive phenotype associated with the colonization and laboratory maintenance of Anopheles gambiae. Malaria J 13:9. https://doi.org/10.1186/1475-2875-13-19
Bautista RC, Harris EJ (1997) Effect of insectary varying on host preference and oviposition behavior of the fruit fly parasitoid Diachasmimorpha longicaudata. Entomol Exp Appl 83:213–218
Beukeboom LW, Koevoets TM, Morales HE, Ferber S, van de Zande L (2015) Hybrid incompatibilities are affected by dominance and dosage in the haplodiploid wasp Nasonia. Front Genet 6:140. https://doi.org/10.3389/fgene.2015.00140
Bodino N, Ferracini C, Tavella L (2016) Is host selection influenced by natal and adult experience in the parasitoid Necremmus tutae (Hymenoptera: Eulophidae). Anim Behav 112:221–228
Boivin G (2010) Phenotypic plasticity and fitness in egg parasitoids. Neotrop Entomol 39:457–463
Boller EF (1972) Behavioral aspects of mass-rearing of insects. Entomophaga 17:9–25
Boller EF, Chambers DL (1977) Quality aspects of mass-reared insects. In: Ridway RL, Vinson SB (eds) Biological control by augmentation of natural enemies. Enviromental Science Research, Springer, Boston, pp 219–235
Cancino J, Liedo P, Ruiz L, López G, Montoya P, Barrera JF, Sivinski J, Aluja M (2012) Discrimination by Coptera haywardi (Hymenoptera: Diapriidae) of hosts previously attacked by conspecifics or by the larval parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Biocontrol Sci Technol 22:899–914
Coelho A Jr, Rugman-Jones PF, Reigada C, Stouthamer R, Parra JRP (2016) Laboratory performance predicts the success of field releases in inbred lines of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). PLoS ONE 11(1):e0146153. https://doi.org/10.1371/journal.pone.0146153
Darrouzet E, Bignon L, Chevrier C (2007) Impact of mating status on egg-laying and superparasitism behaviour in a parasitoids wasp. Entomol Exp Appl 123:279–285
De Moraes CM, Mescher MC (2005) Intrinsic competition between larval parasitoids with different degrees of host specificity. Ecol Entomol 30:564–570
De Moraes CM, Lewis WJ, Tumlinson JH (2000) Examining plant-parasitoid interactions in tritrophic systems. An Soc Ent Bras 29:189–203
Evangelou VI, Bouga M, Emmanouel NG, Perdikis DCh, Papadoulis GT (2013) Discrimination of two natural biocontrol agents in the Mediterranean region based on mitocondrial DNA sequency data. Biochem Genet 51:825–840
FAO/IAEA/USDA (2014) Product quality control for sterile mass-reared and released Tephritid fruit flies, Version 6.0. International Atomic Energy Agency, Vienna, Austria
Fischer S, Samietz J, Dorn S (2004) Host location of a pupal parasitoid in tritrophic system compared to a model offering mechanosensory cues only. J Insect Behav 17:191–199
Forsman A (2015) Rethinking phenotypic plasticity and its consequences for individuals, population and species. Heredity 115:276–284
Gilchrist AS, Meats AW (2014) An evaluation of outcrossing to improve mass-reared strains of the Queensland fruit fly Bactrocera tryoni. Int J Trop Insect Sci 34:35–44
Giunti G, Canale A, Meesing RH, Donati E, Stefanini C, Michaud JP, Benelli G (2015) Parasitoid learning: current knowledge and implications for biological control. Biol Control 90:208–219
Goibin G (2010) Phenotypic plasticity and fitness in egg parasitoids. Neotrop Entomol 39:457–463
Goubault M, Corsetero AM, Paty C, Fourrier J, Dourlot S, Le Ralec A (2011) Abdominal sensory equipment involved in external host discrimination in a solitary parasitoid wasp. Micros Res Tech 74:1145–1153
Harvey JA, Gols R, Strand MR (2009) Intrinsic competition and its effects on the survival and development of three species of endoparasitoid wasps. Entomol Exp Appl 130:238–248
Hassell MP (2000) Host-parasitoid population dynamics. J Anim Ecol 69:543–566
Henry LM, May N, Acheampong S, Gillespie DR, Roitberg BD (2010) Host-adapted parasitoids in biological control: Does source matter? Ecol Appl 20:242–250
Hernández-Ortiz V, Delfin-González H, Esalante A, Manrique-Saide P (2006) Hymenopteran parasitoids of Anastrepha fruit flies (Diptera: Tephritidae) reared from different hosts in Yucatán, México. Fla Entomol 89:508–515
Hoffmeister TS (2000) Marking decisions and host discrimination in a parasitoid attacking concealed host. Can J Zool 78:1494–1499
Hughes K, Sokolowski MB (1996) Natural selection in the laboratory for a change in resistance by Drosophila melanogaster to the parasitoid wasp Asobara tabida. J Insect Behav 9:477–491
Krivan V, Sirat E (1997) Searching for food and hosts: the influence of parasitoid behavior on host parasitoid dynamics. Theor Popul Biol 51:201–209
Lebreton S, Labarussias M, Chevrier C, Darrouzet E (2008) Discrimination of the age of conspecific eggs by an ovipositing ectoparasitoid wasp. Entomol Exp Appl 130:28–34
Lewis WJ, Martin WR Jr (1990) Semiochemicals for use with parasitoids: status and future. J Chem Ecol 16:3067–3089
Lewis WJ, Vet LEM, Tumlinson JH, van Lenteren JC, Papaj DR (2003) Variations in natural enemy foraging behaviour: essential element of a sound biological control theory. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CABI Publishing, Wallingford, pp 41–58
López G (2009) Capacidad de discriminación del parasitoide de pupas de moscas de la fruta Coptera haywardi (Hymenoptera: Diapriidae) en hospederos parasitados. Tesis Ingeniero Agrónomo Tropical UNACH, Huehuetán, p 51
López M, Aluja M, Sivinski J (1999) Hymenopterous larval-pupal and pupal parasitoids of Anastrepha flies (Diptera: Tephritidae) in Mexico. Biol Control 15:119–129
Machtinger ET, Geden CJ, Teal PE, Leppla NC (2015) Comparison of host-seeking behavior of the filth fly pupal parasitoid, Sapalangia cameroni and Muscidifurax raptor (Hymenoptera: Pteromalidae). Environ Entomol 44:330–337
Mackaueer M, Michaud JP, Völk W (1996) Host choice by Aphidiid parasitoids (Hymenoptera: Aphidiidae): host recognition, host quality, and host value. Can Entomol 128:959–980
Mackauer M (1976) Genetic problems in the production of biological control agents. Annu Rev Entomol 21:369–385
McKay T, Broce AB (2004) Discrimination of self-parasitized hosts by the pupal parasitoid Muscidifurax zaraptor (Hymenoptera: Pteromalidae). Ann Entomol Soc Am 97:592–594
Messina FJ, Karren ME (2003) Adaptation to a novel host modifies host discrimination by seed beetle Callosobruchus maculatus. Anim Behav 65:501–507
Ovruski S (1995) Pupal and larval-pupal parasitoids (Hymenoptera) obtained from Anastrepha spp. and Ceratitis capitata (Dip.: Tephritidae) pupae collected in four localities of Tucumán Province Argentina. Entomophaga 40:367–370
Ovruski S, Aluja M, Sivinski J, Wharton R (2000) Hymenopteran parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the Southern United States: Diversity, distribution, taxonomic states and their use in fruit fly biological control. Int Pest Manag Rev 5:81–107
Parreño M, Scannapieco AC, Renis MI, Juri M, Vera MT, Segura DF, Cladera JL, Lanzavecchia SB (2014) Dynamics of genetic variability in Anastrepha fraterculus (Diptera: Tephritidae) during adaptation to laboratory varing conditions. BMC Genet 15(Suppl 2):S14
Qi-Fu L, Tong-Xian L (2017) Interspecific host discrimination and intrinsic competition between Aphelinus asichis and Apidus gifuensis in Myzus persicae. Entomol Exp Appl 163:265–271
Quicke DL (2015) The braconid and ichneuomid parasitoid wasps: Biology, systematics, evolution and ecology. Wiley, West Sussex
Quintero-Fong L, Toledo J, Ruiz L, Rendon P (2016) Selection by mating competitiveness improves the performance of Anastrepha ludens males of the genetic sexing strain Tapachula-7. B Entomol Res 106:624–632
Rehman A, Powell W (2010) Host selection behavior of aphid parasitoids (Aphidiidae: Hymenoptera). J Plant Breed Crop Sci 2:299–311
Rolff J, Kraaijeveld AR (2001) Host preference and survival in selected lines of a Drosophila parasitoid, Asobara tabida. J Evol Biol 14:742–745
Rosenheim JA, Rosen D (1992) Influence of egg load and host size on host-feeding behavior of the parasitoid Aphytis lingnanensis. Ecol Entomol 17:263–272
Ruschioni S, van Loon JJA, Smid HM, van Lenteren JC (2015) Insects can count: Sensory basis of host discrimination in parasitoid wasp revealed. PLoS ONE 10(10):e0138045
Saul PH, McCombs SD (1993) Genetics and ecology of colonization and mass rearing of Hawaiian fruit flies (Diptera: Tephritidae) for use in sterile insect control programs. P Hawaii Entomol Soc 32:21–37
Scholz D, Höller C (1992) Competition for hosts between two hyperparasitoids of aphids, Dendrocerus laticeps and Dendrocerus carpenteri (Hymenoptera: Megaspilidae): The benefit of interspecific host discrimination. J Insect Behav 5:289–300
Schutze MK, Dammalage T, Jessup A, Vreysen MJB, Wornoayporn V, Clarke AR (2015) Effects of laboratory colonization on Bactrocera dorsalis (Diptera: Tephritidae) mating behaviour: “what a difference a year makes”. ZooKeys 540:369–383
Sivinski J, Vulinec K, Menezes E, Aluja M (1998) The bionomics of Coptera haywardi (Oglobin) (Hymenoptera:Diapridae) and the other pupal parasitoids of tephritid fruit flies (Diptera). Biol Control 11:193–202
Sivinski J, Piñero J, Aluja M (2000) The distribution of parasitoids (Hymenoptera) of Anastrepha fruit flies (Diptera: Tephritidae) along an altitudinal gradient in Veracruz, México. Biol Control 18:258–269
Sørense JG, Addison MF, Terblanche JS (2012) Mass-rearing of insects for pest management and advances from evolutionary physiology. Crop Prot 38:87–94
Tejada MT, Arredondo-Gordillo JA, Orozco-Dávila D, Quintero-Fong L, Díaz-Fleisher F (2017) Directional selection to improve the sterile insect technique: survival and sexual performance of dissecation resistant Anastrepha ludens strains. Evol Appl 10:1020–1030
van Baaren J, Boivin G (1998) Learning affects host discrimination behavior in parasitoid wasp. Behav Ecol Sociobiol 42:9–16
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl 57:1–20
van Lenteren JC, Bigler F (2010) Quality control of mass-reared egg parasitoids. In: Consôli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in Agroecosystems with emphasis on Trichogramma. Springer, Netherlands, pp 315–340
Vet LEM, Meyer M, Bakker K, van Alphen JJM (1984) Intra- and interspecific host discrimination in Asobara (Hymenoptera) larval endoparasitoid of Drosophilidae: comparison between closely related and less closely related species. Anim Behav 32:871–874
Vinson SB (1998) The general host selection behavior of parasitoid Hymenoptera and comparison of initial strategies by a larvaphagous and oophagous species. Biol Control 11:79–96
Visser B, Le Lann C, den Blanken FJ, Harvey JA, van Alphen JJM, Ellers J (2010) Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. P Natl Acad Sci USA 107:8677–8682. https://doi.org/10.1073/pnas.1001744107
Wajnberg E (2004) Measuring genetic variation in natural enemies used for biological control: Why and how? Ehler LE. Mateille T, Genetics, evolution and biological control, CABI Publishing, Wallingford, UK, Sforza R, pp 19–37
Wang D, Lü L, He Y, Shi Q (2016) Mate choice and host discrimination behavior of the parasitoid Trichogramma chilonis. B Entomol Res 106:530–537
Yamada Y (1988) Optimal use of patches by parasitoids with a limited fecundity. Res Popul Ecol 30:235–249
Acknowledgements
We wish to thank the Biological Control Department staff of the Moscafrut Program, Javier Valle for statistical advice and Cesar Perez for his assistance in the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stefano Colazza
Rights and permissions
About this article
Cite this article
Cancino, J., Pérez, B., Johnson, A.C. et al. Parasitoids are choosy: increase in the capacity to discriminate parasitised tephritid pupae by Coptera haywardi. BioControl 64, 357–366 (2019). https://doi.org/10.1007/s10526-019-09941-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09941-5