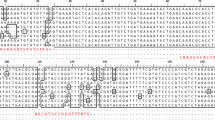
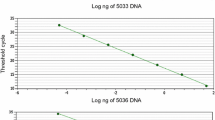
Abstract
Biological control agents (BCAs) play an important role in crop protection. They can improve sustainability, prevent resistance to fungicides in target pathogens and reduce fungicide residues on produce. Bacillus subtilis strain QST713 (Serenade Max; Bayer CropScience, Leverkusen, Germany) and Bacillus amyloliquefaciens subsp. plantarum strain D747 [Amylo-X; CBC Europe S.r.l.—Biogard Division, Nova Milanese (MB), Italy] are two commercially available BCAs that are used against grey mould on table grape and other crops. TaqMan-based quantitative (q)PCR assays were developed and validated for quantitative and specific detection of the two BCAs on grape bunches following field sprays. Specific molecular markers were developed for each BCA, and TaqMan probes were designed and tested in qPCR on DNA extracted from washing water of grape berries. The assay proved specific and sensitive for both BCAs allowing detection at density as low as three colony-forming units (CFU) per gram of berries. The method will be useful to investigate the population dynamics of the two BCAs following their applications in vineyards.


Similar content being viewed by others
References
Antwerpen MH, Zimmermann P, Bewley K, Frangoulidis D, Meyer H (2008) Real-time PCR system targeting a chromosomal marker specific for Bacillus anthracis. Mol Cell Probes 22:313–315
Arrebola E, Sivakumar D, Bacigalupo R, Korsten L (2010) Combined application of antagonist Bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Prot 29:369–377
Berdal KG, Holst-Jensen A (2001) Roundup Ready® soybean event-specific real-time quantitative PCR assay and estimation of the practical detection and quantification limits in GMO analyses. Eur Food Res Technol 213:432–438
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Chen H, Wang L, Su CX, Gong GH, Wang P, Yu ZL (2008) Isolation and characterization of lipopeptide antibiotics produced by Bacillus subtilis. Lett Appl Microbiol 47:180–186
Chen XH, Koumoutsi A, Scholz R, Borriss R (2009) More than anticipated—production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J Mol Microbiol Biotechnol 16:14–24
Danielsson J, Reva O, Meijer J (2007) Protection of oilseed rape (Brassica napus) toward fungal pathogens by strains of plant-associated Bacillus amyloliquefaciens. Microb Ecol 54:134–140
Elmer PAG, Reglinski T (2006) Biosuppression of Botrytis cinerea in grapes. Plant Pathol 55:155–177
EU (2009) Directive 2009/128/EC of the European parliament and of the council of 21 October 2009 establishing a framework for community action to achieve the sustainable use of pesticides. Off J Eur Union L 309/71
Fernández-No IC, Guarddon M, Böhme K, Cepeda A, Calo-Mata P, Barros-Velázquez J (2011) Detection and quantification of spoilage and pathogenic Bacillus cereus, Bacillus subtilis and Bacillus licheniformis by real-time PCR. Food Microbiol 28:605–610
Francis I, Holsters M, Vereecke D (2010) The Gram-positive side of plant-microbe interactions. Environ Microbiol 12:1–12
Fredlund E, Gidlund A, Olsen M, Börjesson T, Spliid NHH, Simonsson M (2008) Method evaluation of Fusarium DNA extraction from mycelia and wheat for down-stream real-time PCR quantification and correlation to mycotoxin levels. J Microbiol Methods 73:33–40
Hu HQ, Li XS, He H (2010) Characterization of an antimicrobial material from a newly isolated Bacillus amyloliquefaciens from mangrove for biocontrol of Capsicum bacterial wilt. Biol Control 54:359–365
Jacometti MA, Wratten SD, Walter M (2010) Review: alternatives to synthetic fungicides for Botrytis cinerea management in vineyards. Aust J Grape Wine Res 16:154–172
Jørgensen C, Leser TD (2007) Estimating amplification efficiency improves multiplex real-time PCR quantification of Bacillus licheniformis and Bacillus subtilis spores in animal feed. J Microbiol Methods 68:588–595
Joshi R, McSpadden Gardener BB (2006) Identification and characterization of novel genetic markers associated with biological control activities in Bacillus subtilis. Phytopathology 96:145–154
Kinsella K, Schulthess CP, Morris TF, Stuart JD (2009) Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol Biochem 41:374–379
Kwon G-H, Lee H-A, Park J-Y, Kim JS, Lim J, Park C-S, Kwon DY, Kim Y-S, Kim JH (2009) Development of a RAPD–PCR method for identification of Bacillus species isolated from Cheonggukjang. Int J Food Microbiol 129:282–287
Leelasuphakul W, Hemmanee P, Chuenchitt S (2008) Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol Technol 48:113–121
Llop P, Caruso P, Cubero J, Morente C, Lopez MM (1999) A simple extraction procedure for efficient routine detection of pathogenic bacteria in plant material by polymerase reaction. J Microbiol Methods 37:23–31
Ludwig W, Schleifer KH (2000) How quantitative is quantitative PCR with respect to cell counts? Syst Appl Microbiol 23:556–562
Martínez MA, Delgado OD, Breccia JD, Baigorí MD, Siñeriz F (2002) Revision of the taxonomic position of the xylanolytic Bacillus sp. MIR32 reidentified as Bacillus halodurans and plasmid-mediated transformation of B. halodurans. Extremophiles 6:391–395
Martínez-Blanch JF, Sánchez G, Garay E, Aznar R (2009) Development of a real-time PCR assay for detection and quantification of enterotoxigenic members of Bacillus cereus group in food samples. Int J Food Microbiol 135:15–21
Martínez-Blanch JF, Sánchez G, Garay E, Aznar R (2011) Detection and quantification of viable Bacillus cereus in food by RT–qPCR. Eur Food Res Technol 232:951–955
Niu D, Liu H, Jiang C, Wang Y, Wang Q, Jin H, Guo J (2011) The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol Plant Microbe Interact 24:533–542
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Osman MS, Sivakumar D, Korsten L (2011) Effect of biocontrol agent Bacillus amyloliquefaciens and 1-methyl cyclopropene on the control of postharvest diseases and maintenance of fruit quality. Crop Prot 30:173–178
Pérez-García A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22:187–193
Raymaekers M, Smets R, Maes B, Cartuyvels R (2009) Checklist for optimization and validation of real-time PCR Assays. J Clin Lab Anal 23:145–151
Rodriguez A, Luque MI, Andrade MJ, Rodriguez M, Asensio MA, Cordoba JJ (2011) Development of real-time PCR methods to quantify patulin-producing molds in food products. Food Microbiol 28:1190–1199
Romanazzi G, Lichter A, Gabler FM, Smilanick JL (2012) Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol Technol 63:141–147
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Rueckert A, Ronimus RS, Morgan HW (2006) Development of a real-time PCR assay targeting the sporulation gene, spo0A, for the enumeration of thermophilic bacilli in milk powder. Food Microbiol 23:220–230
Schena L, Ippolito A (2003) Rapid and sensitive detection of Rosellinia necatrix in roots and soils by real time Scorpion-PCR. J Plant Pathol 85:15–25
Seiichi F, Mai M, Yoshinao A, Hironori K, Tsutomu T, Masafumi S, Shungji S (2011) Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Sci Technol 21:705–720
Senthil R, Prabakar K, Rajendran L, Karthikeyan G (2011) Efficacy of different biological control agents against major postharvest pathogens of grapes under room temperature storage conditions. Phytopathol Mediterr 50:55–65
Shu-Bin L, Mao F, Ren-Chao Z, Juan H, Xiao L (2012) Characterization and evaluation of the endophyte Bacillus B014 as a potential biocontrol agent for the control of Xanthomonas axonopodis pv. dieffenbachiae—induced blight of Anthurium. Biol Control 63:9–16
Wattiau P, Renard M, Ledent P, Debois V, Blackman G, Agathos S (2001) A PCR test to identify Bacillus subtilis and closely related species and its application to the monitoring of wastewater biotreatment. Appl Microbiol Biotechnol 56:816–819
Yang IC, Shih DY, Wang JY, Pani TM (2007) Development of rapid real-time PCR and most-probable-number real-time PCR assays to quantify enterotoxigenic strains of the species in the Bacillus cereus group. J Food Prot 70:2774–2781
Yang D, Wang B, Wang J, Chen Y, Zhou M (2009) Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and Sclerotinia stem rot of rape. Biol Control 51:61–65
Yong X, Zhang R, Zhang N, Chen Y, Huang X, Zhao J, Shen Q (2013) Development of a specific real-time PCR assay targeting the poly-c-glutamic acid synthesis gene, pgsB, for the quantification of Bacillus amyloliquefaciens in solid-state fermentation. Bioresour Technol 129:477–484
Acknowledgments
This research was carried out within the framework of the project ‘Promotion of ECO-friendly processes for the enhancement of quality of apulian food productions’ Granted by the “Distretto Agroalimentare Regionale–D.A.Re. s.c.ar.l.”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Monica Hofte.
Rights and permissions
About this article
Cite this article
Rotolo, C., De Miccolis Angelini, R.M., Pollastro, S. et al. A TaqMan-based qPCR assay for quantitative detection of the biocontrol agents Bacillus subtilis strain QST713 and Bacillus amyloliquefaciens subsp. plantarum strain D747. BioControl 61, 91–101 (2016). https://doi.org/10.1007/s10526-015-9701-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9701-4