Abstract
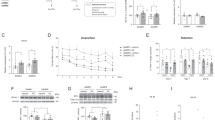
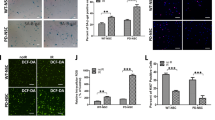
The senescence- accelerated mouse prone 8 (SAMP8) is a well- characterized animal model of senescence that shows early age- related neurodegeneration with impairment in learning and memory skills when compared with control senescence- resistant mice (SAMR1). In the current study, we investigated whether such impairment could be partly due to changes in mitochondrial DNA (mtDNA) repair capacity and mitochondrial DNA damage in the brain of SAMP8 mice. Besides we studied whether these potential changes were related to modifications in two major processes likely involved in aging and neurodegeneration: apoptosis and inflammation. We observed that the specific activity of one of the main mtDNA repair enzymes, the mitochondrial APE1, showed an age- related reduction in SAMP8 animals, while in SAMR1 mice mitochondrial APE1 increased with age. The reduction in mtAPE1 activity in SAMP8 animals was associated with increased levels of the DNA oxidative damage marker 8oxodG in mtDNA. Our results also indicate that these changes were related to a premature increase in apoptotic events and inflammation in the brain of SAMP8 mice when compared to SAMR1 counterparts. We suggest that the premature neurodegenerative phenotype observed in SAMP8 animals might be due, at least in part, to changes in the processing of mtDNA oxidative damage, which would lead to enhancement of apoptotic and inflammatory processes.





Similar content being viewed by others
References
Barja G (2004) Free radicals and aging. Trends Neurosci 27:595–600
Barja G, Herrero A (2000) Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J 14:312–318
Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58:495–505
Butterfield DA, Poon HF (2005) The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp Gerontol 40:774–783
Caballero B, Vega-Naredo I, Sierra V, Huidobro-Fernandez C, Soria-Valles C, De Gonzalo-Calvo D, Tolivia D, Pallas M, Camins A, Rodriguez-Colunga MJ, Coto-Montes A (2009) Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence-accelerated mice. J Pineal Res 46:106–114
Cantuti-Castelvetri I, Lin MT, Zheng K, Keller-McGandy CE, Betensky RA, Johns DR, Beal MF, Standaert DG, Simon DK (2005) Somatic mitochondrial DNA mutations in single neurons and glia. Neurobiol Aging 26:1343–1355
Canugovi C, Shamanna RA, Croteau DL, Bohr VA (2014) Base excision DNA repair levels in mitochondrial lysates of Alzheimer’s disease. Neurobiol Aging 35:1293–1300
Cheng XR, Zhou WX, Zhang YX (2014) The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer’s disease animal model. Ageing Res Rev 13C:13–37
Choi JY, Kim HS, Kang HK, Lee DW, Choi EM, Chung MH (1999) Thermolabile 8-hydroxyguanine DNA glycosylase with low activity in senescence-accelerated mice due to a single-base mutation. Free Radic Biol Med 27:848–854
Cuesta S, Kireev R, Forman K, Garcia C, Escames G, Ariznavarreta C, Vara E, Tresguerres JA (2010) Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8). Exp Gerontol 45:950–956
Cuesta S, Kireev R, Garcia C, Forman K, Escames G, Vara E, Tresguerres JA (2011) Beneficial effect of melatonin treatment on inflammation, apoptosis and oxidative stress on pancreas of a senescence accelerated mice model. Mech Ageing Dev 132:573–582
Edgar D, Shabalina I, Camara Y, Wredenberg A, Calvaruso MA, Nijtmans L, Nedergaard J, Cannon B, Larsson NG, Trifunovic A (2009) Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab 10:131–138
Figueiredo-Pereira ME, Rockwell P, Schmidt-Glenewinkel T, Serrano P (2014) Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front Mol Neurosci 7:104
Flood JF, Morley JE (1993) Age-related changes in footshock avoidance acquisition and retention in senescence accelerated mouse (SAM). Neurobiol Aging 14:153–157
Forman K, Vara E, Garcia C, Kireev R, Cuesta S, Acuna-Castroviejo D, Tresguerres JA (2010) Beneficial effects of melatonin on cardiological alterations in a murine model of accelerated aging. J Pineal Res 49:312–320
Gabbita SP, Lovell MA, Markesbery WR (1998) Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem 71:2034–2040
Garcia-Matas S, Paul RK, Molina-Martinez P, Palacios H, Gutierrez VM, Corpas R, Pallas M, Cristofol R, de Cabo R, Sanfeliu C (2015) In vitro caloric restriction induces protective genes and functional rejuvenation in senescent SAMP8 astrocytes. Aging Cell 14:334–344
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
Gredilla R (2010) DNA damage and base excision repair in mitochondria and their role in aging. J Aging Res 2011:257093
Gredilla R, Stevnsner T (2012) Mitochondrial base excision repair assays. Methods Mol Biol 920:289–304
Gredilla R, Garm C, Holm R, Bohr VA, Stevnsner T (2010) Differential age-related changes in mitochondrial DNA repair activities in mouse brain regions. Neurobiol Aging 31:993–1002
Gredilla R, Weissman L, Yang JL, Bohr VA, Stevnsner T (2012) Mitochondrial base excision repair in mouse synaptosomes during normal aging and in a model of Alzheimer’s disease. Neurobiol Aging 33:694–707
Griffin WS, Sheng JG, Roberts GW, Mrak RE (1995) Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J Neuropathol Exp Neurol 54:276–281
Hegde ML, Hazra TK, Mitra S (2008) Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res 18:27–47
Heneka MT, Golenbock DT, Latz E (2015) Innate immunity in Alzheimer’s disease. Nat Immunol 16:229–236
Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, Someya S, Miyakawa T, Nakayama C, Samhan-Arias AK, Servais S, Barger JL, Portero-Otin M, Tanokura M, Prolla TA, Leeuwenburgh C (2010) Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One 5:e11468
Jeppesen DK, Bohr VA, Stevnsner T (2011) DNA repair deficiency in neurodegeneration. Prog Neurobiol 94:166–200
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309:481–484
Kujoth GC, Bradshaw PC, Haroon S, Prolla TA (2007) The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet 3:e24
Kukreja L, Kujoth GC, Prolla TA, Van Leuven F, Vassar R (2014) Increased mtDNA mutations with aging promotes amyloid accumulation and brain atrophy in the APP/Ld transgenic mouse model of Alzheimer’s disease. Mol Neurodegener 9:16
Leret ML, San Millan JA, Fabre E, Gredilla R, Barja G (2002) Deprenyl protects from MPTP-induced Parkinson-like syndrome and glutathione oxidation in rat striatum. Toxicology 170:165–171
Liu C, Cui G, Zhu M, Kang X, Guo H (2014) Neuroinflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol 7:8342–8355
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Macchi B, Paola DR, Marino-Merlo F, Felice MR, Cuzzocrea S, Mastino A (2015) Inflammatory and Cell Death Pathways in Brain and Peripheral Blood in Parkinson’s disease. CNS Neurolog Disord Drug Targets 14:313–324
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430:631–639
Melov S (2004) Modeling mitochondrial function in aging neurons. Trends Neurosci 27:601–606
Menardo J, Tang Y, Ladrech S, Lenoir M, Casas F, Michel C, Bourien J, Ruel J, Rebillard G, Maurice T, Puel JL, Wang J (2012) Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid Redox Signal 16:263–274
Mitra S, Izumi T, Boldogh I, Bhakat KK, Chattopadhyay R, Szczesny B (2007) Intracellular trafficking and regulation of mammalian AP-endonuclease 1 (APE1), an essential DNA repair protein. DNA Repair (Amst) 6:461–469
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24:1063–1070
Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K (2004) Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab 24:458–466
Riancho JA, Zarrabeitia MT, Amado JA, Olmos JM, Gonzalez-Macias J (1994) Age-related differences in cytokine secretion. Gerontology 40:8–12
Rousseaux MW, Marcogliese PC, Qu D, Hewitt SJ, Seang S, Kim RH, Slack RS, Schlossmacher MG, Lagace DC, Mak TW, Park DS (2012) Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc Natl Acad Sci U S A 109:15918–15923
Sanders LH, McCoy J, Hu X, Mastroberardino PG, Dickinson BC, Chang CJ, Chu CT, Van Houten B, Greenamyre JT (2014) Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol Dis 70:214–223
Sato E, Oda N, Ozaki N, Hashimoto S, Kurokawa T, Ishibashi S (1996) Early and transient increase in oxidative stress in the cerebral cortex of senescence-accelerated mouse. Mech Ageing Dev 86:105–114
Siddiqui A, Rivera-Sanchez S, Castro Mdel R, Acevedo-Torres K, Rane A, Torres-Ramos CA, Nicholls DG, Andersen JK, Ayala-Torres S (2012) Mitochondrial DNA damage is associated with reduced mitochondrial bioenergetics in Huntington’s disease. Free Radic Biol Med 53:1478–1488
Takeda T (2009) Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res 34:639–659
Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T (1981) A new murine model of accelerated senescence. Mech Ageing Dev 17:183–194
Takemura M, Nakamura S, Akiguchi I, Ueno M, Oka N, Ishikawa S, Shimada A, Kimura J, Takeda T (1993) Beta/A4 proteinlike immunoreactive granular structures in the brain of senescence-accelerated mouse. Am J Pathol 142:1887–1897
Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y (2000) Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res 885:25–31
Tian F, Tong TJ, Zhang ZY, McNutt MA, Liu XW (2009) Age-dependent down-regulation of mitochondrial 8-oxoguanine DNA glycosylase in SAM-P/8 mouse brain and its effect on brain aging. Rejuvenation Res 12:209–215
Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA (2007) Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet 39:540–543
Wanagat J, Cao Z, Pathare P, Aiken JM (2001) Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J 15:322–332
Weissman L, de Souza-Pinto NC, Stevnsner T, Bohr VA (2007a) DNA repair, mitochondria, and neurodegeneration. Neuroscience 145:1318–1329
Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA (2007b) Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic Acids Res 35:5545–5555
Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA (2010) Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. J Biol Chem 285:28191–28199
Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP (2014) BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromol Med 16:161–174
Zhu Y, Lee CC, Lam WP, Wai MS, Rudd JA, Yew DT (2007) Cell death in the Purkinje cells of the cerebellum of senescence accelerated mouse (SAMP(8)). Biogerontology 8:537–544
Acknowledgments
This research was supported by a grant from the Complutense University/Community of Madrid to RG (CCG10-UCM/SAL 4798).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Torregrosa-Muñumer, R., Gómez, A., Vara, E. et al. Reduced apurinic/apyrimidinic endonuclease 1 activity and increased DNA damage in mitochondria are related to enhanced apoptosis and inflammation in the brain of senescence- accelerated P8 mice (SAMP8). Biogerontology 17, 325–335 (2016). https://doi.org/10.1007/s10522-015-9612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-015-9612-x