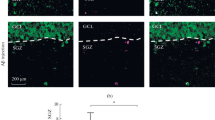
The deposition of beta-amyloid (Aβ) in the brain is detected in Alzheimer’s disease and during ageing. Until now, ultrastructural studies of changes caused by Aβ in the dentate gyrus are very scarce. The effects of Aβ 1-42 injection into the CA1 field of rat hippocampus were studied by electron microscopy. In 2 weeks after injection of aggregated Aβ in low concentrations, destructive changes were seen in the structure of dentate gyrus cells, which consisted in a decrease in the number of dentate gyrus neurons and axo-dendritic synapses. These changes were accompanied by enlargement of the endoplasmic reticulum cisterns and widening of the active zones of synapses. Thus, injection of aggregated Aβ 1-42 into the hippocampus led to irreversible (a decrease in the number of neurons and axo-dendritic synapses, agglutination of synthetic vesicles) and adaptive changes (an increase in the sizes of endoplasmic reticulum cisterns and active zones of synapses) in dentate gyrus neurons aimed at the maintenance of functional activity of the nervous system.
Similar content being viewed by others
References
Bogolepov NN. Ultrastructure of synapses of the human cortex in senile age. Ann. Klin. Eksp. Nevrol. 2008;2(4):22-27. Russian.
D’aychkova LN. Changes in the ultrastructure of the somatosensory cortex in white rats during space flight. Izv. Ross. Akad. Nauk. Ser. Biol. 2007;(3):372-375. Russian.
Pavlik LL, Mikheeva IB, Al’-Mugkhrabi YM, Berest VP, Kirova YI, Germanova EL, Luk’yanova LD, Mironova GD. Specific Features of Immediate Ultrastructural Changes in Brain Cortex Mitochondria of Rats with Different Tolerance to Hypoxia under Various Modes of Hypoxic Exposures. Bull. Exp. Biol. Med. 2018;164(3):376-381. doi: https://doi.org/10.1007/s10517-018-3993-1
Strukov AI, Serov VV. Pathological Anatomy. Moscow, 2010. Russian.
Chen H, Chan DC. Mitochondrial dynamics-fusion, fission, movement and mitophagy in neurodegenerative diseases. Hum. Mol. Genet. 2009;18, R2:R169-R176.
Gomes LC, Scorrano L. Mitochondrial morphology in mitophagy and macroautophagy. Biochem. Biophys. Acta. 2013;1833(1):205-212.
Iaccarino L, Tammewar G, Ayakta N, Baker SL, Bejanin A, Boxer AL, Gorno-Tempini ML, Janabi M, Kramer JH, Lazaris A, Lockhart SN, Miller BL, Miller ZA, O’Neil JP, Ossenkoppele R, Rosen HJ, Schonhaut DR, Jagust WJ, Rabinovici GD. Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer’s disease. Neuroimage Clin. 2018;17:452-464.
Kesner RP. An analysis of the dentate gyrus function. Behav. Brain Res. 2013;254:1-7.
Ramírez E, Mendieta L, Flores G, Limón ID. Neurogenesis and morphological-neural alterations closely related to amyloid β-peptide (25-35)-induced memory impairment in male rats. Neuropeptides. 2018;67:9-19.
Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466-470.
Skulachev VP, Bakeeva LE, Chernyak BV, Domnina LV, Minin AA, Pletjushkina OY, Saprunova VB, Skulachev IV, Tsyplenkova VG, Vasiliev JM, Yaguzhinsky LS, Zorov DB. Tread-grain transition of mitochondrian reticulum as a step of mitoptosis. Mol. Cell Biochem. 2004;256-257(1-2):341-358.
Sohanaki H, Baluchnejadmojarad T, Nikbakht F, Roghani M. Pelargonidin improves memory deficit in amyloid beta25-35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 2016;83:85-91.
Striedter GF. Evolution of the hippocampus in reptiles and birds. J. Comp. Neurol. 2016;524(3):496-517.
Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11(5):578-598.
Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279-282.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 168, No. 12, pp. 767-771, December, 2019
Rights and permissions
About this article
Cite this article
Mikheeva, I.B., Pavlik, L.L., Shubina, L.V. et al. Ultrastructural Alterations in Granular Neurons of the Dentate Fascia Caused by Intrahippocampal Injection of Beta-Amyloid 1-42. Bull Exp Biol Med 168, 802–806 (2020). https://doi.org/10.1007/s10517-020-04806-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-020-04806-9