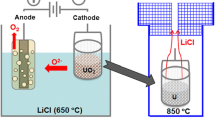
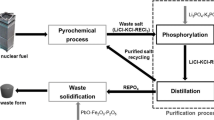
The possibility of purifying the metalized products of pyrochemical operations by removing the electrolyte by distilling off lithium chloride is checked experimentally. This study is performed in connection with the development of promising variants of an industrial method of metalizing uranium dioxide, which is the main component of the spent nuclear fuel after its voloxidation. The electrolyte was distilled from metalized pellets and powders based on uranium dioxide with their continuous evacuation at 700–900°C. It was found that the main component of the sublimates is lithium chloride; the content of rare-earth elements and uranium is very low. The distillation regimes where 98.8–99.9% of the LiCl can be removed from the metalized products were determined.
Similar content being viewed by others
References
E.-Y. Choi and S. Jeong, “Electrochemical processing of spent nuclear fuels: an overview of oxide reduction in pyroprocessing technology,” Progr. Nucl. Energy Mater. Intern., 25, 572–582 (2015).
I. Kim, S. Oh, H. Im, et al., “Distillation of LiCl from the LiCl–Li2O molten salt of the electrolytic reduction process,” J. Radioanal. Nucl. Chem., 295, 1413– 1417 (2013).
B. Westphal, K. Marsden, J. Price, and D. Laug, “On the development of a distillation process for electrometalurgical treatment of irradiated spent nuclear fuel,” Nucl. Eng. Technol., 40, 163–174 (2008).
E.-Y. Choi, C. Won, and D.-S. Kanga, et al., “Production of uranium metal via electrolytic reduction of uranium oxide in molten LiCl and salt distillation,” J. Radioanal. Nucl. Chem., 304, 535–546 (2015).
A. Roine, HSC Chemistry 7.0 Thermochemical Database, Outokumpu Research Oy, Finland (2009).
C. Yaws, Thermophysical Properties of Chemicals and Hydrocarbons, William Andrew, Norwich, New York (2008).
H. Kang, E.-Y. Choi, S.-W. Kim, et al., “Distillation characteristics of LiCl–Li2O electrolyte for UO2 electrolytic reduction process,” J. Radioanal. Nucl. Chem., 310, 1165–1171 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Atomnaya Énergiya, Vol. 126, No. 4, pp. 199–202, April, 2019.
Rights and permissions
About this article
Cite this article
Salyulev, A.B., Shishkin, A.V., Shishkin, V.Y. et al. Distillation of Lithium Chloride From the Products of Uranium Dioxide Metalization. At Energy 126, 226–229 (2019). https://doi.org/10.1007/s10512-019-00541-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10512-019-00541-1