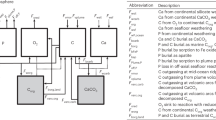
Abstract
Marine chemistry of the coastal environment starts with principles of rock weathering that use carbonic acid to mobilize elements, only some of which comprise the majority of sea salt. The principle reason is reverse weathering, extensively represented in coastal waters, and returns most elements to newly formed colloids or minerals while recycling carbon dioxide to the atmosphere. This includes the deeper ocean expanse of sediment diagenesis, plus hydrothermal plumes and attendant low-temperature basalt alteration. Within the estuarine and extended shelf regimes, both conservative and non-conservative processes can be distinguished and modeled to determine proportions of weathered elements transmitted to the sea or consumed by reverse weathering. Conceptually, the steady-state processes that lead to the composition of seawater can be viewed as heterogeneous equilibria between dissolved constituents and solid mineral products taking hundreds of millennia. However, initial processes in the estuarine and coastal environment are characterized by shorter term scavenging associated with inorganic and organic colloids. These recycle both carbon and trace elements on timescales commensurate with estuarine flushing and coastal exchange with the ocean. The natural uranium and thorium decay series provide powerful tools for quantifying the rates of estuarine processes, including those within groundwater and the subterranean estuary. In the future, new mass spectrometric and nuclear magnetic resonance techniques will help to define the molecular nature of newly formed estuarine colloids as has been done for dissolved organic matter. As the coastal environment undergoes the forces of climate change in the form of warming and sea level rise, future research should address how these will impact chemistry of the coastal environment as a net source or sink of carbon dioxide and associated organic material.



Similar content being viewed by others
References
Aller RC (2014) Sedimentary diagenesis, depositional environments, and benthic fluxes. In: Holland HD, Turekian KK (eds) Treatise on geochemistry, Chap 11, vol 8, 2nd edn. Elsevier, Amsterdam, pp 293–334. doi:10.1016/B978-0-08-095975-7.00611-2
Bauer JE, Cai W-J, Raymond PA, Bianchi TS, Hopkinson CS, Regnier PAG (2013) The changing carbon cycle of the coastal ocean. Nature 504:61–70. doi:10.1038/nature12857
Boyle EA, Collier R, Dengler AT, Edmond JM, Ng AC, Stallard RF (1974) On the chemical mass balance in estuaries. Geochim Cosmochim Acta 38:1719–1728. doi:10.1016/0016-7037(74)90188-4
Boyle EA, Edmond JM, Sholkovitz ER (1977) The mechanism of iron removal in estuaries. Geochim Cosmochim Acta 41:1313–1324. doi:10.1016/0016-7037(77)90075-8
Broecker WS (1971) A kinetic model for the chemical composition of sea water. Quat Res 1:188–207. doi:10.1016/0033-5894(71)90041-X
Bruland KW, Coale KH (1986) Surface water 234Th/238U disequilibria: spatial and temporal variations of scavenging rates within the Pacific Ocean. In: Burton JD et al (eds) Dynamic processes in the chemistry of the upper ocean. Plenum, New York, pp 159–172. doi:10.1007/978-1-4684-5215-0_13
Buesseler KO, Benitez-Nelson CR, Moran SB, Burd A, Charette M, Cochran JK, Coppola L, Fisher NS, Fowler SW, Gardner WD, Guo LD, Gustafsson O, Lamborg C, Masque P, Miquel JC, Passow U, Santschi PH, Savoye N, Stewart G, Trull T (2006) An assessment of particulate organic carbon to thorium-234 ratios in the ocean and their impact on the application of 234 Th as a POC flux proxy. Mar Chem 100:213–233. doi:10.1016/j.marchem.2005.10.013
Charette MA, Cho Y-K (2014) Global estimate of submarine groundwater discharge based on an observationally constrained radium isotope model. Geophys Res Lett. doi:10.1002/2014GL061574
Charette MA, Sholkovitz ER (2002) Oxidative precipitation of groundwater-derived ferrous iron in the subterranean estuary of a coastal bay. Geophys Res Lett. doi:10.1029/2001GL014512 (cover article)
Chen JH, Edwards RL, Wasserburg GJ (1986) 238U, 234U and 232Th in seawater. Earth Planet Sci Lett 80:241–251. doi:10.1016/0012-821X(86)90108-1
Church TM (ed) (1975) Marine chemistry in the coastal environment. American Chemical Society, Symposium Series, vol 18
Church TM (1986) Biogeochemical factors influencing the residence time of microconstituents in a large tidal estuary, Delaware Bay. Mar Chem 18:393–406. doi:10.1016/0304-4203(86)90020-4
Church TM, Sarin MM (1995) Natural U–Th series radionuclide studies of estuarine processes. In: Dyer KR, Orth RJ (eds) Changes in fluxes in Estuaries: implications from science to management, proceedings of the ECSA22/ERF symposium, Plymouth, England, September 13–18, 1992, pp 91–95
Church TM, Scudlark JR (1998) Trace metals in estuaries: a Delaware Bay synthesis. In: Allen HE, Garrison AW, Luther GW III (eds) Metal speciation and contamination of surface water, Chapter 1. Ann Arbor Press, Inc, pp 1–20
Church TM, Sarin MM, Fleisher MQ, Ferdelman TG (1996) Salt marshes: an important coastal sink for dissolved uranium. Geochim Cosmochim Acta 60(20):3879–3887. doi:10.1016/0016-7037(96)00211-6
Church TM, Sommerfield CK, Velinsky DJ, Point D, Benoit C, Amouroux D, Plaa D, Donard OFX (2006) Marsh sediments as records of sedimentation, eutrophication and metal pollution in the urban Delaware Estuary. Mar Chem 102:72–95. doi:10.1016/j.marchem.2005.10.26
Elsinger RJ, Moore WS (1983) Ra-224, Ra-228 and Ra-226 in Winyah Bay and Delaware Bay. Earth Planet Sci Lett 64:430–436. doi:10.1016/0012-821X(83)90103-6
Garrels RM (1965) Silica: role in the buffering of natural waters. Science 148(3666):69. doi:10.1126/science.148.3666.69
Garrels RM, Mackenzie FT (1972) Silica: a quantitative model for the sedimentary rock cycle. Mar Chem 1:27–41. doi:10.1016/0304-4203(72)90004-7
Gartman A, Findlay AJ, Luther GW III (2014) Nanoparticulate pyrite and other nanoparticles are a ubiquitous component of hydrothermal vent black smoker emissions. Chem Geol 366:32–41. doi:10.1016/j.chemgeo.2013.12.013
Gledhill M, Achterberg EP, Li K, Mohamed KN, Rijkenberg MJA (2015) Influence of ocean acidification on the complexation of iron and copper by organic ligands in estuarine waters. Mar Chem 177:421–433. doi:10.1016/j.marchem.2015.03.016
Goldschmidt VM (1937) The principles of distribution of elements in minerals and rocks. J Chem Soc. doi:10.1039/JR370000655
Guo LD, Santschi PH (1997a) Composition and cycling of colloids in marine environments. Rev Geophys 35:17–40. doi:10.1029/96RG03195
Guo LD, Santschi PH (1997b) Isotopic and elemental characterization of colloidal organic matter from the Chesapeake Bay and Galveston Bay. Mar Chem 59:1–15. doi:10.1016/S0304-4203(97)00072-8
Guo L, Santschi PH (2000) Sedimentary source of old high molecular weight dissolved organic carbon from the ocean margin benthic nepheloid layer. Geochim Cosmochim Acta 64:651–660. doi:10.1016/S0016-7037(99)00335-X
Guo L, Santschi PH, Baskaran M (1997) Interactions of thorium isotopes with colloidal organic matter in oceanic environments. Colloids Surf A 120:255–271. doi:10.1016/S0927-7757(96)03723-5
Hussain N, Church TM, Kim G (1999) Use of 222Rn and 226Ra to trace groundwater discharge into the Chesapeake Bay. Mar Chem 65:127–134. doi:10.1016/S0304-4203(99)00015-8
Kaufman A, Li Y-H, Turekian KK (1981) The removal rates of 234Th and 228Th from waters of the New York Bight. Earth Planet Sci Lett 54:385–392. doi:10.1016/0012-821X(81)90054-6
Kim G, Alleman LY, Church TM (2004) Accumulation records of radionuclides and trace metals in two contrasting Delaware salt marshes. Mar Chem 87:87–96. doi:10.1016/j.marchem.2004.02.002
Li Y-H, Feely HW, Santschi PH (1979) 228Th-22SRa radioactive disequilibrium in the New York Bight and its implications for coastal pollution. Earth Planet Sci Lett 42:13–26. doi:10.1016/0012-821X(79)90186-9
Li Y-H, Santschi PH, Kaufman A, Benninger LR, Feely HW (1981) Natural radionuclides in waters of the New York Bight. Earth Planet Sci Lett 5:217–228. doi:10.1016/0012-821X(81)90101-1
Mackenzie FT, Garrels RM (1966) Chemical mass balance between rivers and oceans. Am J Sci 261:507–525
Mackenzie FT, Kump LR (1995) Reverse weathering, clay mineral formation, and oceanic element cycles. Science 270:586–587. doi:10.1126/science.270.5235.586
Mackenzie FT, Ristvet BL, Thorstenson DC, Lerman A, Leeper RH (1981) Reverse weathering and chemical mass balance in a coastal environment. In: Marten JM, Burton JD, Eisma D (eds) River inputs to ocean systems. UNEP, Paris, pp 152–187
Mackenzie FT, Lerman A, Andersson AJ (2004) Past and present of sediment and carbon biogeochemical cycling models. Biogeosciences 1:11–32
Mackin JE, Aller RC (1989) The nearshore marine and estuarine chemistry of dissolved aluminum and rapid authigenic mineral precipitation. Rev Aquat Sci 1:537–554
Manheim FT (1967) Evidence for submarine discharge of water on the Atlantic continental slope of the southern United States, and suggestions for further research. Trans N Y Acad Sci Ser 2(29):839–852. doi:10.1111/2164.0947.1967.tb02825.x
Marsan DW, Rigaud S, Church TM (2014) Natural radionuclides 210Po and 210Pb in the Delaware and Chesapeake Estuaries: modeling scavenging rates and residence times. J Environ Radioact 138:447–455. doi:10.1016/j.envrad.2014.08.014
Mayer LM, Wells ML (2011) Aggregation of colloids in estuaries. In: Wolanski E, McLusky DS (eds) Treatise on estuarine and coastal science, vol 4. Elsevier, pp. 143–160. doi:10.1016/B978-0-12-374711-2.00407-1
Maynard JB (1976) The long term buffering of the oceans. Geochim Cosmochim Acta 40:1523–1530. doi:10.1016/0016-7037(76)90091-0
Meyers T, Sickles J, Dennis R, Russell K, Galloway J, Church TM (2000) Atmospheric nitrogen deposition of coastal estuaries and their watersheds. Chapter 3. In: Valigura RA, Alexander RB, Castro MS, Meyers TP, Pearl HW, Stacey PE, Turner RE (eds) Nitrogen loading in coastal water bodies: an atmospheric perspective. AGU Coastal and Estuarine Studies Series vol 57, pp 53–57. doi:10.1029/CE057.p0053
Michalopoulos P, Aller RC (1995) Rapid clay mineral formation in Amazon delta sediments: reverse weathering and oceanic elemental cycles. Science 270:614. doi:10.1126/science.270.5236.614
Michalopoulos P, Aller RC (2004) Early diagenesis of biogenic silica in the Amazon delta: alteration, authigenic clay formation, and storage. Geochim Cosmochim Acta 68(5):1061–1085. doi:10.1016/j.gca.2003.07.018
Moore WS (1999) The subterranean estuary: a reaction zone of ground water and sea water. Mar Chem 65:111–125. doi:10.1016/S0304-4203(99)00014-6
Moore WS (2010) The effect of submarine groundwater discharge on the ocean. Annu Rev Mar Sci 2:345–374. doi:10.1146/annurev-marine-120308-081019
Moore WS, Wilson AM (2005) Advective flow through the upper continental shelf driven by storms, buoyancy, and submarine groundwater discharge. Earth Planet Sci Lett 235:564–576. doi:10.1016/j.epsl.2005.04.043
Mopper K, Stubbins A, Ritchie JD, Bialk HM, Hatcher PG (2007) Advanced instrumental approaches for characterization of marine dissolved organic matter: extraction Techniques, mass spectrometry, and nuclear magnetic resonance spectroscopy. Chem Rev 107:419–442. doi:10.1002/chin.200719276
Peterson RN, Moore WS, Chappel SL, Viso RF, Libes SM, Peterson LE (2016) A new perspective on coastal hypoxia: the role of saline groundwater. Mar Chem 179:1–11. doi:10.1016/j.marchem.2015.12.005
Quigley MS, Santschi PH, Hung C-C, Guo L, Honeyman BD (2002) Importance of acid polysaccharides for 234Th complexation to marine organic matter. Limnol Oceanogr 47(2):367–377. doi:10.4319/lo.2002.47.2.0367
Regnier P, Friedlingstein P, Ciais P et al (2013) Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat Geosci 6:597–607. doi:10.1038/ngeo1830
Santschi PH, Li Y-H, Bell J (1979) Natural radionuclides in the water of Narragansett Bay. Earth Planet Sci Lett 45:201–213. doi:10.1016/0012-821x(79)90121-3
Santschi PH, Adler D, Amdurer M, Li Y-H, Bell J (1980) Thorium isotopes as analogues for “particle-reactive” pollutants in coastal marine environments. Earth Planet Sci Lett 47:327–335. doi:10.1016/0012-821X(80)90019-9
Santschi PH, Guo L, Baskaran M, Trumbore S, Southon J, Bianchi TS, Honeyman B, Cifuentes L (1995) Isotopic evidence for the contemporary origin of high-molecular weight organic matter in oceanic environments. Geochim Cosmochim Acta 59(3):625–631. doi:10.1016/0016-7037(94)00378-Y
Santschi PH, Lenhart JJ, Honeyman BD (1997) Heterogeneous processes affecting trace contaminant distribution in estuaries: the role of natural organic matter. Mar Chem 58:99–125. doi:10.1016/S0304-4203(97)00029-7
Sarin MM, Church TM (1994) Behaviour of uranium during mixing in the Delaware and Chesapeake estuaries. Estuar Coast Shelf Sci 39:619–631. doi:10.1016/S0272-7714(06)80013-2
Scudlark JR, Church TM (1997) Atmospheric deposition of trace elements to the Mid-Atlantic Bight, Chapter 10. In: Baker JE (ed) Atmospheric deposition of contaminants to the great lakes and coastal waters, SETAC Technical Publication Series, pp 195–208
Scudlark JK, Rice C, Conko KM, Bricker OP, Church TM (2005) Transmission of atmospherically derived trace elements through an undeveloped, forested Maryland watershed. Water Air Soil Pollut 163:53–79. doi:10.1007/s11270-005-8135-5
Sholkovitz ER (1976) Flocculation of dissolved organic and inorganic matter during mixing of river water and seawater. Geochim Cosmochim Acta 40:831–845. doi:10.1016/0016-7037(76)90035-1
Sholkovitz ER, Boyle EA, Price NB (1978) The removal of dissolved humic acids and iron during estuarine mixing. Earth Planet Sci Lett 40:30–136. doi:10.1016/0012-821X(78)90082-1
Sillen LG (1967) The ocean as a chemical system. Science 156:1189–1197
Urey HC (1953) On the concentration of certain elements at the earth’s surface. J Chem Soc 219A:281–292
Velde B, Barré P (2010) The chemistry and mineralogy of plant and soil interactions: plant as manipulators of their environment, Chapter 5. In: Soils, plants and clay minerals; mineral and biologic interactions. Springer, ISBN 978-3-642-03498-5 e-ISBN 978-3-642-03499-2. doi:10.1007/978-3-642-03499-2
Walsh JS, Premuzk ET, Gaffney JS, Macko SA (1985) Organic storage of CO2 on the continental slope off the mid-Atlantic bight, the southeastern Bering Sea, and the Peru coast. Deep Sea Res 32(7):853–883. doi:10.1016/0198-0149(85)90120-7
Walsh JS, Biscoye PE, Csanady GT (1988) The 1983–1984 shelf edge exchange processes (SEEP)-1 experiment: hypotheses and highlights. Cont Shelf Res 8(5–7):435–456
Wen L-S, Santschi PH, Tang DG (1997) Interactions between radioactively labeled colloids and natural particles: evidence for colloidal pumping. Geochim Cosmochim Acta 61(14):2867–2878. doi:10.1016/S0016-7037(97)00139-7
Wollast R (1991) Coastal organic carbon cycle: fluxes, sources, and sinks. In: Mantoura C, Martin J-M, Wollast R (eds) Ocean margin processes in global change, Wiley, Chichester, pp 365–381. doi:10.1007/978-3-642-76064-8-7
Acknowledgments
I thank Greg Cutter for ′ct, and George Luther who nominated this journal for some of its publications. Both have served as dear colleagues who contributed innumerably to my career over the years. Finally, I acknowledge the many professors, advisers, colleagues and students often cited who had a seminal and lasting impact on the development of my profession. In particular, those contributing essential material for this paper include Ed Boyle, Fred Mackenzie, Peter Santschi and Ed Sholkovitz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Church, T.M. Marine Chemistry in the Coastal Environment: Principles, Perspective and Prospectus. Aquat Geochem 22, 375–389 (2016). https://doi.org/10.1007/s10498-016-9296-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-016-9296-0