Abstract
Marine biogeochemistry deals with the budgets and transformations of biogeochemically reactive elements such as carbon, nitrogen and phosphorus . Inorganic nitrogen and phosphorus compounds are the major nutrients and control organic matter (biomass) production in the surface water. Due to various anthropogenic activities, the input of these nutrients into the Baltic Sea has increased drastically during the last century and has enhanced the net organic matter production by a factor of 2–4 (eutrophication). This has led to detrimental oxygen depletion and hydrogen sulphide production in the deep basins of the Baltic Sea. Model simulations based on the Baltic Sea Action Plan (BSAP) indicate that current eutrophication and thus extension of oxygen-depleted areas cannot be reversed within the next hundred years by the proposed nutrient reduction measures. Another environmental problem is related to decreasing pH (acidification) that is caused by dissolution of the rising atmospheric CO2. Estimates indicate a decrease in pH by about 0.15 during the last 1–2 centuries, and continuation of this trend may have serious ecological consequences. However, the concurrent increase in the alkalinity of the Baltic Sea may have significantly counteracted acidification.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Marine biogeochemistry deals with the internal transformation of sea water constituents and their transport into and out of the sea area in question. Internal processes take place against the background of the hydrographic setting and are mainly controlled by biological production and the decomposition of organic matter. The major elements involved in these processes and addressed in this chapter are carbon (C), nitrogen (N), phosphorus (P) and oxygen (O2). Their distributions and concentrations strongly influence the Baltic Sea ecosystem . On an annual timescale, the formation of organic matter is widely limited by the availability of N and P, which are the major nutrients . This in turn affects the O2 conditions in deeper water layers, where organic matter is mineralised and thus consumes O2 and may eventually lead to the formation of highly toxic hydrogen sulphide (H2S). Hence, eutrophication is a major issue when assessing past and possible future changes. Carbon not only plays a central role as backbone for any organic material, but its inorganic form, carbon dioxide (CO2), largely controls the marine acid/base balance, which due to the ongoing rise in atmospheric CO2 levels is gradually shifting towards more acidic conditions.
2 Major Biogeochemical Fluxes and Transformations
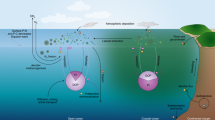
This section describes the main components of the biogeochemical cycles of nutrients, carbon and oxygen in the Baltic Sea (Fig. 18.1) and includes a brief introduction to available observations and biogeochemical models. The biogeochemical cycle starts with photosynthetic primary production by phytoplankton in the sunlit surface layer (euphotic zone). Production is controlled by the availability of nutrients supplied by internal recycling and input from surrounding land areas, the atmosphere and the North Sea. The productive season and the biogeochemical cycles in the Baltic Sea are also regulated by the physical processes of transport, mixing and stratification (such as deep-water renewal and the development of a seasonal thermocline ). The essential nutrients are dissolved inorganic nitrogen (DIN: ammonia NH3 (at equilibrium with ammonium, NH4 +), nitrite NO2 and nitrate NO3) and dissolved inorganic phosphorus (DIP: phosphate PO4). However, some evidence also exists for the use of dissolved organic N and dissolved organic P as nutrients. In addition, nitrogen (N2) fixation by cyanobacteria may add new bioavailable N to the Baltic Sea.
Sinking organic particles export nutrients below the productive surface layer. When this organic matter is decomposed, O2 in the deeper parts of the Baltic Sea is gradually consumed and the water becomes anoxic if it is not renewed. In the absence of O2, sulphate reduction produces H2S that is toxic to higher life forms. The interplay between production and mineralisation of organic matter is also reflected in the cycling of CO2 since the formation of organic matter uses up CO2, while, conversely, mineralisation of organic matter produces CO2 that accumulates in the deep water. A fraction of the export production reaches the sediments before being completely decomposed and mineralised, and permanent burial of organic material is an important sink especially for P. Depending on the redox conditions, P may also be bound to hydrated metal oxides (e.g. iron, Fe) in the sediments. Currents and waves may cause resuspension of sediments in shallow areas of the Baltic Sea. This process leads to a transport of lighter material and organic matter towards deeper parts of the Baltic Sea.
The major N sink in the Baltic Sea is denitrification , an anaerobic mineralisation of organic matter that transforms NO3 to N2. While the removal of N is enhanced at lower O2 concentrations, the sediment P sink is weakened and may even become a source under anoxic conditions, when older mineral-bound P can be released to the overlying water. Finally, nutrients supplied from external sources that are not permanently removed or accumulated inside the Baltic Sea will be exported to the Kattegat and Skagerrak.
Box 18.1 Basis for current knowledge
For over a century, the Baltic Sea has been one of the most heavily investigated seas in the world and much of the information summarised, for example in the books by Wulff et al. (2001), Feistel et al. (2008) and Leppäranta and Myrberg (2009), is based on analyses of historical data. Information on the physical, chemical and biological variables of the Baltic Sea is regularly gathered by national monitoring programmes in Denmark, Estonia, Finland, Germany, Latvia, Lithuania, Poland, Russia and Sweden. International cooperation between these countries and the European community is governed by the Baltic Marine Environment Protection Commission (HELCOM, www.helcom.fi) that provides data services and conducts environmental assessments based on the data collected. Other international sources of data and information concerning the Baltic Sea include the International Council for the Exploration of the Sea (ICES, www.ices.dk) and the European Monitoring and Evaluation Programme (EMEP, www.emep.int) that provides information about atmospheric emission and deposition of nutrients . In 2009, the Baltic Nest Institute, Stockholm University (www.balticnest.org), and several other institutes started a partnership on a system of distributed databases where each partner (see http://nest.su.se/bed/hydro_chem.shtml) makes the data hosted in its databases publicly available via a data portal (http://apps.nest.su.se/dataPortal/). The Baltic Nest Institute also provides access to the data portal via Data Assimilation System (DAS), http://nest.su.se/das/) and a decision support system Nest (http://apps.nest.su.se/nest/). The portal also provides access to all data collected in the Baltic Environmental Database (BED), maintained by the Baltic Nest Institute. Near-real-time observations are also available and distributed within the countries bordering the Baltic Sea by the partners participating in the Baltic Operational Oceanographic System (BOOS, www.boos.org).
The national and international funding of climate- and eutrophication -related research has been important drivers for an increased understanding of Baltic Sea biogeochemistry. For example, Wulff et al. (2001) presented results from international research projects that were funded by the Baltic Sea System Study (BASYS) and the EU Marine Science and Technology programme (EU/MAST). The combined scientific and political efforts have resulted in strategies to combat eutrophication in the Baltic Sea, for example a recent agreement to reduce nutrient loads (BSAP), HELCOM 2007). A current major international Baltic Sea research collaboration is the Joint Baltic Sea Research and Development Programme (BONUS, www.bonusportal.org), funded by the EU’s Seventh Framework Programme for Research (FP7, http://ec.europa.eu/research/fp7/) and national research-funding institutions in the Baltic Sea countries. Key results of the 16 projects of the BONUS 2007 are summarised on the website www.bonusportal.org/about_bonus/bonus_and_era-net/bonus_2009-2011/bonus_projects. The overall biogeochemical cycling of nutrients and carbon in the Baltic Sea is especially addressed by the BONUS projects ECOSUPPORT and BALTIC-C.
Current understanding of how the interactions between the external forcing and the internal physical and biogeochemical processes regulate the ecological status of the Baltic Sea has been synthesised, quantified and evaluated by model experiments. Numerical models (e.g. Fennel and Neumann 2004) are also used to study the effects of climate change on biogeochemical cycles and to evaluate various management strategies (see Sect. 18.5). The development of more complex coupled physical–biogeochemical models started with horizontally integrated basin models (Stigebrandt and Wulff 1987), and coupled models dividing the Baltic Sea into a number of dynamically interconnected sub-basins are still used because of their computational efficiency (Gustafsson 2012; Gustafsson et al. 2012). With the increasing availability of computational power, three-dimensional coupled models for the Baltic Sea that produce high-resolution results on long timescales (centuries) are now state of the art at the larger institutes (e.g. Neumann et al. 2002; Eilola et al. 2009). Generally, the biogeochemical functions of the coupled models are similar in that they describe transformations of N and P including inorganic nutrients and particulate organic matter comprising phytoplankton, dead organic matter and zooplankton . Organic matter is typically produced from the inorganic nutrients by several functional groups of phytoplankton that may differ between models, but in the Baltic Sea usually include diatoms , flagellates and cyanobacteria . Sinking organic material enters the model sediment as benthic N and P. Differences between biogeochemical models may, for example, be due to parameterisations of nutrient fluxes between sediment and water and also in the rates and parameterisations of transformations of nutrients within the water column.
3 Changes in External Forcing
External forcing has a strong impact on biogeochemical processes and internal fluxes. This refers both to the climate-driven physical forcing and to the input of biogeochemically reactive substances via rivers , water exchange with the North Sea and the atmosphere. The physical forcing controls the water transport, stratification , ice coverage, temperature and salinity in the Baltic Sea, which in turn affect the distribution of nutrients and C and thus have an impact on biogeochemical processes. Changes in hydrographic conditions, such as rising surface water temperature and changes in ice cover , are discussed in Chaps. 7 and 8 and will be addressed here only if necessary for interpreting changes in biogeochemical cycles. Changes in the biogeochemical forcing mainly relate to the input of the main biogeochemical actors: nutrients, organic C and CO2 (see also Chap. 17).
3.1 Nutrient Inputs
Compilations of data concerning the waterborne input (riverine input and point sources) and the atmospheric input of N and P into the entire Baltic Sea including the Kattegat are available through various HELCOM publications. The input data refer to both the inorganic and organic forms of N and P. Whereas the inorganic species are readily available for biological production, it is still an open question how much of the organic fraction is bioavailable and thus contributes to eutrophication. A summary of an input estimate (HELCOM 2005) based on 2000 data was given in the first assessment of climate change in the Baltic Sea (BACC Author Team 2008). An updated estimate based on data for 2001–2006 was recently published (HELCOM 2011). According to this later report, the annual waterborne inputs are 641,000 t N year−1 and 28,700 t P year−1. Adding an atmospheric deposition of 198,000 t N year−1 (Bartnicki et al. 2011) yields a total N input to the Baltic Sea of 840,000 t N year−1 (Table 18.1). According to HELCOM (2011), the atmospheric P deposition contributes 5 % to the total P input (1500 t P year−1) resulting in a total input of 30,200 t P year−1 (Table 18.1). These estimates differ only slightly from the values given in the previous HELCOM (2005) report and presented in the first assessment of climate change in the Baltic Sea (BACC Author Team 2008).
Attempts have been made to estimate nutrient inputs at the beginning of the last century when the influence of human activities was low (Larsson et al. 1985; Savchuk et al. 2008). Gustafsson et al. (2012) used data from different sources to reconstruct the N and P inputs to the various basins of the Baltic Sea including the Kattegat since 1850 (Fig. 18.2). For the period 1970–2006, annual land-borne loads for total N and total P, which include the input from rivers and point sources, are based on data compiled in the BED (Savchuk et al. 2012). Riverine inputs for the years before 1970 were reconstructed from historical data and present-day nutrient concentrations in pristine rivers in northern Scandinavia. The atmospheric deposition of N compounds for 1970–2006 was obtained both from measurements and model calculations. Prior to 1970, historical data were used to roughly estimate the atmospheric N input. Since neither regular monitoring data nor model simulations exist for the atmospheric deposition of P to the Baltic Sea, the values presented by Gustafsson et al. (2012, Fig. 18.2), which are based on deposition estimates for northern Europe (Ruoho-Airola et al. 2012 and references therein), differ from the HELCOM (2011) estimates (Table 18.1). Owing to the sparse database, the reconstruction of historical nutrient inputs is necessarily associated with a considerable uncertainty which is difficult to quantify.
Historical development of river water discharge (a), waterborne and atmospheric inputs of total nitrogen (b) and total phosphorus inputs (c) to the Baltic Sea (Gustafsson et al. 2012)
Figure 18.2 indicates that N and P inputs have both increased dramatically during the last 150 years. This is due to human activities such as intensified agriculture and increasing industrialisation. The rate of increase became stronger after the Second World War and peaked around 1980 at about three (N) and seven (P) times higher than in 1850. However, inputs have decreased significantly since the 1980s due to emission reduction measures and are presently at a level comparable to those around 1960. When evaluating land-borne and atmospheric inputs of nutrients to the Baltic Sea, the exchange with the North Sea must also be taken into account. According to budget calculations for the last 20 years, about 130 kt N year−1 is exported to the North Sea via the Danish Straits. This corresponds to about 15 % of the current total N inputs, while the loss of P to the North Sea amounts to 12 kt P year−1 corresponding to 25 % of the total input.
3.2 Carbon Inputs
Huge amounts of inorganic and organic carbon enter the Baltic Sea via rivers, affecting both the CO2 system and the oxygen budget. The inputs were estimated by Kulinski and Pempkowiak (2011) using the mean concentrations of total organic carbon (particulate and dissolved) and inorganic carbon (total CO2) in more than 60 rivers in connection with flow data. The input of total organic carbon amounted to 4090 kt C year−1 and consists mainly of dissolved organic carbon (DOC). More than 50 % of the annual DOC input is mineralised in the Baltic Sea (Kulinski and Pempkowiak 2011) and thus converted to CO2. It is possible that the organic carbon input increased during the last century due to the intensification of agriculture and possibly also to rising temperatures. However, this cannot be substantiated by data.
For the input of inorganic C, Kulinski and Pempkowiak (2011) reported a value of 6810 kt C year−1. Since the concentrations of total CO2 in river water are mainly controlled by alkalinity which is primarily a consequence of limestone dissolution, changes during the last century are very likely. Hjalmarsson et al. (2008) analysed historical alkalinity data from the northern Baltic Sea which go back to 1911. They concluded that the alkalinity input into the Gulf of Finland by the River Neva increased during the last century. This was attributed to acidic precipitation which in a limestone-rich catchment such as the River Neva may intensify weathering and thus increase alkalinity. An alkalinity increase also occurred in the eastern Gotland Sea. Surface alkalinities measured during the last decade under the framework of the Swedish Monitoring Programme (SMHI) were about 100 µmol kg−1 higher than that reported by Buch (1945) for 1928–1938. This constitutes a significant change and is equivalent to an increase in pH of 0.02–0.03 units.
The rising levels of atmospheric CO2 that cause enhanced CO2 dissolution and drive the pH in sea water to lower values must also be taken into account. Concentrations increased by about 40 % from a pre-industrial level of about 280 ppm to about 390 ppm in 2008 (Fig. 18.3). The rate of CO2 increase has grown steadily since 2000 and currently averages 2.0 ppm year−1. The historic development of the atmospheric CO2 level was reconstructed by analysing air occluded in ice cores (Fig. 18.3, Neftel et al. 1994). Direct measurement of CO2 started in the 1950s with the well-known time series of measurements at Mauna Loa on Hawaii (Keeling et al. 2008), and data from a northern hemisphere background station (Barrow, Alaska) are available since 1974 at a monthly resolution (Fig. 18.3). High-resolution measurements of atmospheric CO2 were also performed in the central Baltic Sea. A full annual cycle was obtained for 2005 and 2008 and indicated that the annual means over the Baltic Sea are almost identical to those at the Northern Hemisphere background station (Fig. 18.3, Schneider 2011).
Annual mean atmospheric carbon dioxide (CO2) concentration obtained from analysing air in ice cores (blue) (Neftel et al. 1994) and direct atmospheric measurements at the Northern Hemisphere background station Barrow in Alaska (full red circles) (Keeling et al. 2008) and over the Baltic Sea (open red circles) (Schneider 2011)
4 Changes in Internal Fluxes and Transformations
4.1 Organic Matter Production and Nutrient Availability
4.1.1 Current Process Understanding
The southern and central sub-basins of the Baltic Sea are eutrophic and mesotrophic systems (Wasmund et al. 2001) that mostly process C derived from phytoplankton primary production (Elmgren 1984; Shaffer 1987). In the northern boreal regions, where phytoplankton primary production is low, organic matter input from terrestrial sources becomes increasingly important for the marine C fluxes. Food web models (Sandberg et al. 2004; Sandberg 2007) and CO2 budgets have shown Bothnian Bay , the northernmost Baltic sub-basin, to be a net heterotrophic system (Algesten et al. 2004, 2006), whereas the Bothnian Sea oscillates between net autotrophy and heterotrophy (Algesten et al. 2004). In contrast, a CO2 air–sea flux balance (Löffler et al. 2012) indicated that on average the Bothnian Sea acts as a sink for atmospheric CO2 and must thus be considered as a net autotrophic system.
Baltic Sea phytoplankton communities undergo a distinct seasonal succession controlled by the temporal dynamics of stratification, light and nutrients and characterised by several blooms , that is periods of temporary mass occurrence of phytoplankton (Wasmund and Siegel 2008). Phytoplankton development starts when thermal surface layer stratification stabilises and light conditions improve, with a spring bloom dominated by diatoms and dinoflagellates . The spring bloom consumes nutrients within the euphotic zone that were accumulated during winter and ends when its limiting nutrient is exhausted. The amount of winter nutrients available to the phytoplankton spring bloom as well as the limiting nutrient differs between sub-basins. For present conditions (Table 18.2), winter nutrient concentrations are greatest in the Gulf of Riga and Gulf of Finland, which are sub-basins with high riverine nutrient loads relative to their surface area. The term nutrient limitation is often used quite loosely (Howarth 1988), but refers in aquatic ecology mostly to the nutrient that determines net primary production (Granéli et al. 1990). Assuming an N and P demand for primary production according to the Redfield ratio (N:P = 16), the winter DIN:DIP ratio is traditionally used to assess the limiting nutrient (Table 18.2). While the spring communities in the Kattegat (Granéli 1987; Granéli et al. 1990), the Baltic Proper (Granéli et al. 1990; Wasmund et al. 1998), the central Gulf of Finland and the Bothnian Sea are generally N limited (Tamminen and Andersen 2007), P limitation prevails in the Bothnian Bay (Tamminen and Andersen 2007). In the proximity of freshwater sources, limitation patterns can change spatially and interannually, depending on run-off (Pitkänen and Tamminen 1995; Seppälä et al. 1999; Tamminen and Seppälä 1999; Yurkovskis 2004).
The extent to which different nutrient sources sustain phytoplankton growth after the winter nutrients are depleted is under debate, but in the central Baltic Sea, first DIN (April) and then DIP (May to July) are depleted to the detection limit of the analytical methods (Nausch 1998; Nausch et al. 2004; Raateoja et al. 2011). Mineralisation of particulate organic P in the euphotic zone may in part cover the nutrient requirements of phytoplankton in early summer (Nausch and Nausch 2007). In addition, dissolved organic P (Nausch and Nausch 2007) and dissolved organic N (Eilola and Stigebrandt 1999; Jørgensen et al. 1999; Berg et al. 2001) deliver nutrients to pelagic communities . Preferential mineralisation of N and P compared to C might further contribute to sustain phytoplankton growth (Heiskanen et al. 1998; Osterroht and Thomas 2000; Lund-Hansen et al. 2004; Schneider et al. 2006; Riemann et al. 2008). Additional inorganic nutrients are to some extent also provided by vertical mixing (Stipa 2004; Reissmann et al. 2009). Nutrients below the thermocline can also be reached by motile phytoplankton, which migrate actively into the nutrient-rich waters below the thermocline (e.g. Olli 1999; Hajdu et al. 2007; Lips et al. 2011). Furthermore, it has been suggested that an early start to N fixation already in late spring may counteract N limitation during the decline phase of the spring bloom (Schneider et al. 2003, 2006).
During summer, when thermal stratification separates the euphotic zone entirely from the nutrient-rich waters below the thermocline, a diverse phytoplankton community is established and phytoplankton growth relies mainly on regenerated nutrients . However, in the Baltic Proper , the Gulf of Finland , and to some extent also in the Gulf of Riga and the Bothnian Sea (Wasmund et al. 2011), new production also takes place because N-fixing cyanobacteria blooms occur during summer. The magnitude of these blooms has been explained by excess inorganic dissolved phosphorus (eDIP), which due to the low N:P ratio in the winter nutrient pool still exists after the spring bloom NO3 depletion (Janssen et al. 2004; Kahru et al. 2007; Lips and Lips 2008; Lilover and Stips 2008; Eilola et al. 2009). However, eDIP is already exhausted before the summer cyanobacteria bloom starts. The mechanism by which eDIP is transferred to summer communities and utilised by cyanobacteria is complex and potentially involves P storage in particulate (Nausch et al. 2008) and dissolved organic matter (Nausch and Nausch 2007). In addition, cyanobacteria blooms also benefit from additional P in frontal and upwelling regions (Kononen et al. 1999; Nausch et al. 2009). Atmospheric deposition is a minor source of P to cyanobacteria blooms (Rolff et al. 2008).
Blooms of N-fixing cyanobacteria provide an important input of N to the Baltic Sea ecosystem, thus increasing organic matter production and sedimentation (Vahtera et al. 2007). Nitrogen fixation also circumvents N limitation in the central Baltic Sea, and P concentrations become the major control of the annual net production. Baltic cyanobacteria blooms are formed by the heterocystous species Nodularia spp., Aphanizomenon spp. and Anabaena spp. (Sohm et al. 2011), which are favoured by high water temperatures (Kanoshina et al. 2003; Laamanen and Kuosa 2005; Mazur-Marzec et al. 2006; Lips and Lips 2008; Jaanus et al. 2011) and calm conditions (Kanoshina et al. 2003; Mazur-Marzec et al. 2006). Estimates of the N input by cyanobacteria to the Baltic Sea cover a wide range. Based on field observations, values between 20 and 792 kt N year−1 were reported (median 173 kt N year−1, based on sources in Degerholm et al. 2008), whereas estimates derived from biogeochemical modelling resulted in 200–800 kt N year−1 (Savchuk and Wulff 2007; Neumann and Schernewski 2008). These numbers indicate that N input by N fixation is comparable to the annual riverine DIN load to the Baltic Proper (286 kt N year−1 in 2000, HELCOM 2004). This additional N input not only sustains the growth of cyanobacteria in summer communities, but is also transferred by exudation and organic matter mineralisation to non-N-fixing phytoplankton (Ohlendieck et al. 2007). In the central Baltic Sea, N fixation contributes a substantial part of N export from the euphotic zone (Larsson et al. 2001; Struck et al. 2004).
The amount of C converted into biomass by primary producers depends on the uptake ratio of C and nutrients during phytoplankton growth. However, under P limitation, the C:P and N:P ratios in Baltic plankton may exceed the Redfield ratio of C:N:P = 106:16:1 (e.g. Engel et al. 2002; Nausch et al. 2004; Kangro et al. 2007; Walve and Larsson 2007, 2010). However, lower C:P ratios are also observed in cases where a PO4 excess with regard to the Redfield N:P ratio exists in the nutrient pool (Wasmund et al. 1998). Therefore, the amount of C formed per unit N or P available to producers varies, which further influences C sedimentation and O2 consumption in sediments and bottom waters.
Measurements of phytoplankton primary production using the 14C method are associated with large methodological differences in the Baltic Sea (Andreasson et al. 2009; Larsson et al. 2010). Therefore, phytoplankton primary production is poorly known on a Baltic scale. Including river plumes and coastal areas, phytoplankton net primary production in the central Baltic Sea was estimated at about 200 g C m−2 year−1 by the end of the 1990s (Wasmund et al. 2001). A substantial part of this production is mineralised already in the euphotic zone. For the spring and early summer period in the eastern Gotland Sea, Wasmund et al. (2005) estimated that mineralisation reached 52 % of phytoplankton production. Consequently, net organic C production, which is the difference between primary production and respiration within the euphotic zone, is substantially less than phytoplankton primary production. Based on oxygen budgets, Stigebrandt (1991) estimated net production in the period before 1980 to range between 41 g C m−2 year−1 in the Kattegat, 38–49 g C m−2 year−1 in the Baltic Proper, and 32 g C m−2 year−1 in the Bothnian Sea . Direct measurements of the amount of C sinking out of the euphotic zone (export production) can be obtained using sediment traps. However, interpreting the data is complicated due to resuspension effects and organic matter degradation in the traps. Heiskanen and Leppänen (1995) estimated that only 32 % of phytoplankton primary production sinks out of the euphotic zone in the northern Baltic Sea, with most of the C export occurring during the spring bloom . A C budget model for the Kattegat (Carstensen et al. 2003) indicated that 54 % of net phytoplankton production is exported from the euphotic zone. However, the relationship between phytoplankton primary production and C export from the euphotic zone depends not only on nutrient supplies and the magnitude of primary production itself, but also on the structure of the pelagic ecosystem (e.g. Boyd and Trull 2007 and references therein).
4.1.2 Past Changes
Since the beginning of systematic monitoring in the 1970s, surface winter DIN and—with the exception of the Bothnian Bay—DIP have increased in all sub-basins of the Baltic Sea (see Fig. 18.4 for the central Baltic Sea) and reached a peak between 1980 and 1990 (HELCOM 2009). In sub-basins with a short residence time like the Danish Straits, DIN and DIP closely follow nutrient inputs, with pronounced concentration declines since 1990 (Carstensen et al. 2006). Decreasing winter DIN concentrations after the mid- or late 1980s have also been observed in all other Baltic Sea sub-basins except the Bothnian Bay (HELCOM 2009). The decline in DIN concentrations may be due to the decreased N inputs but also may be due to higher denitrification rates caused by increased hypoxia in the Baltic Sea. Winter DIP concentrations in the Baltic Proper and the Gulf of Finland do not show a distinct trend. They are more closely related to P concentrations beneath the Baltic Proper halocline and are thus linked to inflow dynamics and P release from bottom sediments (Conley et al. 2002; HELCOM 2009, see also Sect. 18.4.2.4). Consequently, winter DIP concentrations in the central Baltic Sea increased again after the inflow in 2003 and in the Gulf of Finland after the inflow in 1996 (HELCOM 2009). In the Gulf of Riga , winter DIP concentrations remained high during the 1990s due to release from bottom sediments (Müller-Karulis and Aigars 2011). The decline in winter DIN levels, while DIP remained high in the Baltic Proper, the Gulf of Finland and the Gulf of Riga, also led to a marked decrease in DIN:DIP ratios, which favours the growth of N-fixing cyanobacteria .
Simulated (line) and observed winter nutrient concentrations (dots) in the surface layer of the central Baltic Sea (Gustafsson et al. 2012)
Estimates of winter nutrient concentrations prior to the 1970s are available from a few modelling studies. A time slice experiment (Schernewski and Neumann 2005) as well as a transient simulation (Gustafsson et al. 2012) suggested that Baltic Proper winter nutrient concentrations in 1900 were 2.0–3.7 mmol m−3 for DIN and 0.23–0.35 mmol m−3 for DIP. Current DIN concentrations (Table 18.2) are thus within the upper range of past DIN concentrations, whereas the past DIP concentration was about a factor of two lower than present values. Nutrient concentrations remained near pristine values until the 1950s and then rose quickly (Gustafsson et al. 2012, Fig. 18.4).
The past increase in winter nutrient concentrations is also reflected in changes in primary production . In the Kattegat and Belt Sea area, where consistent time series of phytoplankton primary production measurements by the 14C method are available, eutrophication led to a doubling of primary production between 1950 and 1980 (Rydberg et al. 2006, Fig. 18.5). Estimates for the increase in primary production for the Baltic Proper range from an increase of 30–70 % since the beginning of the twentieth century (Elmgren 1989) to a doubling in the 1970s and 1980s (Wasmund et al. 2001). Also, decreasing δ13C values in bottom sediments indicate an increase in primary production due to eutrophication (Voss et al. 2000). Results from modelling studies differ. An increase in primary production since 1900 by a factor of three and even six were reported by Savchuk et al. (2008) and Gustafsson et al. (2012), respectively.
Changes in phytoplankton primary production in the Kattegat and the Belt Sea (Rydberg et al. 2006)
Net organic C production in the Gotland Basin was estimated by CO2 measurements and yielded an increase by a factor of about 2.5 since the 1920s (Schneider and Kuss 2004), which is in close agreement with the model estimate by Gustafsson et al. (2012). Higher primary production is also reflected in enhanced deposition of organic matter at the sediment surface. According to Jonsson and Carman (1994), the sedimentation rate increased by 70 % between the late 1920s and 1980s in the Baltic Proper, whereas Hansson and Gustafsson (2012) reported an increase of only 25 % after 1950. A larger increase was obtained from model simulations which yielded a rough doubling of sedimentation since the 1950s (Hansson and Gustafsson 2012) and an almost fourfold increase since 1900 (Gustafsson et al. 2012).
Cyanobacteria blooms have occurred in the Baltic Sea since the early brackish Littorina Sea stage (Chap. 2), in particular during hypoxic bottom water conditions (Bianchi et al. 2000). Observations suggest an increase in cyanobacteria after the 1960s (Finni et al. 2001). Furthermore, budgets for CO2 and total N in the surface water of the central Baltic Sea indicated an increase in N fixation by cyanobacteria of 30 % within the past two decades (Schneider et al. 2009). Model studies suggest a fourfold (Gustafsson et al. 2012) to 10-fold (Savchuk et al. 2008) increase in N fixation since 1900.
4.2 Organic Matter Decomposition and Nutrient Recycling
Decomposition of organic matter refers to both the decay of large organic compounds into smaller molecules and the oxidation of organic matter. The latter process is also referred to as mineralisation and plays a key role in the cycling of C and nutrients. Mineralisation is a biologically induced process that can be expected wherever organic matter exists. This text focuses on mineralisation in water layers and at the sediment surface below the permanent halocline that may be cut off from O2 supply for many years and thus generates an O2-depleted or anoxic environment. Under such conditions, several important biogeochemical reactions occur (Box 18.2) that will be discussed in the following subsections.
Box 18.2
4.2.1 Hydrographic Forcing of the Deep-Water Biogeochemistry
The O2 conditions in the Baltic Sea are the result of physical transport of O2 and consumption of O2 by biogeochemical processes. Owing to the positive freshwater budget and the bathymetry of the Baltic Sea, a strong salinity gradient generates a permanent density stratification (pycnocline) that inhibits the vertical exchange of O2 between the surface and deep waters. Lateral intrusions and inflows below the pycnocline, which is located at about 70 m in the central Gotland Basin , are the only effective means of O2 transport. The inflowing water requires a density high enough to sink down to the near-bottom layers of the central Baltic Sea deep basins. Those inflows occur irregularly, sometimes at intervals of many years. Matthäus and Franck (1992) used an intensity index to classify the inflow events during the last century (Fig. 18.6). During longer periods without a sufficient supply of O2 to the deep water, the continuous consumption of O2 by decomposition of organic matter results in O2 depletion and eventually in the development of anoxic conditions. Thus, the Baltic Sea is vulnerable to hypoxia and anoxia due to its hydrography (Conley et al. 2009a).
Intensity index for major Baltic Sea inflows during the last century (Schinke and Matthäus 1998) and the seasonal frequency distribution of different inflow categories (inset)
The most northern parts of the Baltic Sea, the Bothnian Sea and Bothnian Bay , are characterised by weaker density stratification, and vertical ventilation by deep convection may occur in particular years. Owing to the vertical ventilation and a lower primary production, this part of the Baltic Sea is not affected by O2 deficiency. A detailed description of Baltic Sea hydrography is given in Chap. 7.
4.2.2 Oxygen Depletion and H2S Formation
Current process understanding: The main reason for O2 demand in sea water is the mineralisation of organic matter. This is an oxidation process performed by heterotrophic organisms that use the chemical energy stored in organic matter and thus reverse chemically the primary production process (see Box 18.2). A large fraction of the organic matter produced in the euphotic zone is sinking into deeper water layers, and by mineralisation, inorganic nutrients and CO2 are again released. In addition to vertical transport, a considerable lateral transport of organic matter, produced in shallower areas, contributes to the C flux into the deep basins of the Baltic Sea and intensifies the mineralisation process (Emeis et al. 2000; Hille et al. 2006; Schneider et al. 2010).
Mineralisation of organic matter requires an electron acceptor (oxidant), which is provided first by O2. Once O2 and other oxidants such as NO3 and manganese oxides are depleted, sulphate will be used instead. Sulphate reduction produces H2S that at the deep-water pH mainly occurs as HS− (see Box 18.2) and is a toxin for many forms of life. These processes may occur in the water column, but mainly take place at the immediate sediment surface (fluffy layer) and in the upper layers of the surface sediment where particulate organic matter is accumulated. The primary mineralisation products such as ammonia , PO4, CO2 and H2S diffuse into the water column and may undergo further transformations depending on the redox conditions (see Sects. 18.4.2.3 and 18.4.2.4). A special mineralisation process, methanogenesis, takes place only in deeper organic-rich sediment layers where sulphate as an oxidant is entirely consumed. In this case, an internal oxidation/reduction (disproportionation) of organic matter is mediated by microorganisms and results in the formation of CO2 and methane (CH4). However, only a minor fraction of CH4 reaches the surface water and can escape into the atmosphere because of oxidation processes both in the sediment and in the water column (Schmale et al. 2010).
Past changes: Eutrophication is considered the main cause of low O2 concentrations in the deep water of the present-day Baltic Sea (Larsson et al. 1985; Conley et al. 2009b). The observed deep-water O2 conditions during the last 100 years (Fig. 18.7) show that the occurrence of H2S became much more frequent during recent decades. The increased availability of organic C due to eutrophication required more electron acceptors, and therefore, the depletion of O2 and formation of H2S were accelerated.
Time series of oxygen and hydrogen sulphide (shown as negative oxygen equivalents) for the Gotland Deep and Landsort Deep. The dashed lines mark the long-lasting stagnation period from 1976 to 1993 (adopted from Fonselius 1969 and updated to 2013 by monitoring data from the Swedish Meteorological Hydrological Institute and the Leibniz Institute for Baltic Sea Research, Germany)
Strong eutrophication started in the 1960s and peaked in the 1980s (Elmgren 2001; Gustafsson et al. 2012). In combination with a long-lasting stagnation period from 1976 to 1993 (Fig. 18.6) without any significant dense inflows from the North Sea, eutrophication caused high concentrations of H2S in the deep water of the Gotland Basin. Other locations such as the Landsort Deep, however, showed an increase in O2 concentration. In this case, the missing inflow events weakened the stratification due to decreasing salinity and thus enhanced the vertical O2 flux. Similarly, in the Gotland Basin , the decreasing stratification caused a deepening of the permanent halocline (Diekmann and Möllmann 2010), thus exposing larger bottom areas to ventilated surface waters, while H2S concentrations in bottom waters below the ventilation depth increased (Fig. 18.7). The shrinking of the hypoxic area during long-lasting stagnation periods is illustrated in Fig. 18.8. It shows that the hypoxic area was much smaller at the end of the 1976–1993 stagnation period than in 2006 after some recent inflows (Conley et al. 2002, 2009a; Savchuk 2010).
Sediment area (red) covered by hypoxic waters containing less than 2 mL L−1 dissolved oxygen: a at the end of a long-lasting stagnation period in 1993 (11,050 km2) and b in 2006 subsequent to some inflow events (67,700 km2) (Conley et al. 2009a)
Oxygen consumption by organic matter mineralisation is accompanied by the release of CO2. Hence, the accumulation of total CO2 in stagnant deep water can be used to estimate O2 conditions. The oldest deep-water data on total inorganic carbon (CT) originate from 1928–1938 (Buch 1945) and indicate a lower CT accumulation and thus a lower O2 deficiency than at present. This is certainly a consequence of the lower input of particulate organic matter into deeper water layers due to the lower biomass production at the beginning of the last century.
Sedimentary records suggest that periods of O2 deficiency and anoxic conditions in the Baltic Sea also occurred in the historic past (Conley et al. 2009a; Virtasalo et al. 2011) during warmer climate conditions such as the medieval climate anomaly (MCA, ca. 900–1350, also known as Medieval Warm Period , see Chap. 3). During colder periods, for example the Little Ice Age (ca. 1550–1850, see Chap. 3), the O2 supply of the deep water improved due to more intense ventilation. The mechanism behind the correlation between large-scale meteorological conditions in the different climate periods and O2 conditions in the Baltic Sea is not well understood and subject to ongoing research. It has also been hypothesised that human activities already had an impact on the O2 conditions of the Baltic Sea during the MCA. At that time, a doubling of the population in the catchment area occurred that led to more intense land use and possibly increased the release of soil nutrients (Zillén et al. 2008; Zillén and Conley 2010; see also Chap. 25).
4.2.3 Nitrogen Transformations
Current process understanding: Transformations between N species depend on the O2 conditions (redox potential) and are mediated by microbial activity. In sea water, the most important N compounds are ammonia , NO2 and NO3. This text considers those reactions that take place during the mineralisation of organic matter below the well-oxygenated surface layer. Ammonia is the primary product of the mineralisation process. In oxic conditions, nitrification occurs by which ammonia is rapidly oxidised first to NO2 and finally to NO3 (Box 18.2).
By vertical mixing, NO3 may become available for new production in the surface water. An N transformation with more far-reaching consequences for the N balance is denitrification, by which NO3 is reduced to elemental N2 and thus removed from the DIN pool. This occurs at low O2 concentrations (<5 µmol L−1, Devol 2008) by the use of NO3 for the oxidation of organic matter (Box 18.2). This heterotrophic denitrification is most effective in the organic-rich upper sediment layers that are in contact with an oxic water column where NO3 is produced by nitrification. But denitrification may also take place in the water column, in particular if a pelagic redoxcline exists. In this case, denitrification occurs mainly by oxidation of H2S through NO3 (Box 18.2) during mixing of oxic and anoxic waters at the redoxcline (chemolithotrophic denitrification).
Most of the studies on denitrification focused on the pelagic redoxcline. Incubation experiments have been used to determine denitrification rates (Rönner and Sörensen 1985; Brettar and Rheinheimer 1991, 1992; Hannig et al. 2007), which, however, did not allow an estimate of the large-scale effect of denitrification on the Baltic Sea DIN budget. Other studies used measurements of the N2 production in order to quantify the effect of denitrification (Rönner and Sörensen 1985; Löffler et al. 2011). Mass balances were used by Gustafsson and Stigebrandt (2007) and Schneider et al. (2010) to quantify the denitrification that takes place during the transition from oxic to anoxic conditions after renewal of the Gotland Sea deep water.
In addition to the process-orientated studies, attempts have been made to quantify the large-scale denitrification . Based on a mass balance model, Shaffer and Rönner (1984) reported a total denitrification for the Baltic Proper of 470 kt N year−1 which corresponds to a removal of about 50 % of the total annual N input to the Baltic Sea. Furthermore, they estimated that 80–90 % of the denitrification occurred in surface sediments and that denitrification at the pelagic redoxcline is thus of minor importance. Voss et al. (2005) concluded from isotopic measurements (δ15N) that sediments of the coastal rims play an important role in the removal of DIN. They found that the annual denitrification in coastal areas of the Baltic Proper may range between 580 and 855 kt N. This implies that the river N load is largely removed close to the coast and has only a minor effect on eutrophication of the central basins. However, this conflicts with the strong increase in DIN in the central Baltic Sea (Fig. 18.4) that roughly coincides with the increasing N loads during 1950–1980 (Fig. 18.2). Deutsch et al. (2010) investigated denitrification in sediments with different physical and chemical properties. The results were extrapolated to the entire Baltic Sea by the use of maps describing sediment characteristics for the entire Baltic Sea. For the Baltic Proper , sediment denitrification amounted to 191 kt N year−1. Together with denitrification at the pelagic redoxcline calculated from the rate measurements of Brettar and Rheinheimer (1991), this implies a total denitrification of 397 kt N year−1 for the Baltic Proper. For the remaining regions where no pelagic redoxcline exists, Deutsch et al. (2010) reported a sediment denitrification of 235 kt N year−1. Hence, the total denitrification in the Baltic Sea (823 kt N year−1) agrees with the upper limit given by Voss et al. (2005).
Past changes: Although reliable and consistent estimates of the present-day denitrification are not yet available, the annual rates under discussion indicate the paramount importance of this process for the Baltic Sea N budget and ultimately for many related biogeochemical processes. Hence, long-term changes in denitrification may have changed the cycling of nutrients and thus had an effect on the productivity of the Baltic Sea. Direct evidence for past changes does not exist, but it is reasonable to hypothesise that denitrification has increased concurrently with increasing production because organic matter provides the substrate and generates the necessary redox gradient for denitrification. This hypothesis is supported by observations by Vahtera et al. (2007) who found a negative correlation between the Baltic Sea DIN pool and the volume of hypoxic water. Since eutrophication has increased the extent of hypoxia during the last century, denitrification may constitute a negative feedback on eutrophication by partly removing the increasing input of DIN. On the other hand, higher denitrification rates and a concurrent increased PO4 release at hypoxic conditions (see Sect. 18.4.2.4) are decreasing the N:P ratio in the nutrient pool (Savchuk 2010). This will favour N fixation, which may then at least partly compensate DIN losses by denitrification.
4.2.4 Phosphorus Transformations
Current process understanding: Organic P compounds in the water column are subject to mineralisation and other biological and abiotic transformation processes during transportation and settling. Decomposition of organic P results in small organic molecules and PO4 which is used by algae and bacteria . Although phosphate exists mainly as HPO4 2− at sea water pH around 8, it is commonly depicted by ‘PO4’. PO4 can also be adsorbed onto particulate material and settle to the sea floor (Froelich 1988). Mineralisation and transformation processes continue at the sediment–water interface and in surface sediment layers (e.g. Sundby et al. 1992).
In oxic conditions and Fe-rich systems, PO4 is adsorbed onto amorphic oxyhydroxides of ferric iron (Fe3+) (Mortimer 1941; Hingston et al. 1967). In the sediment pore water, PO4 diffuses upwards into the oxic sediment layer and can be bound to Fe-oxyhydroxides at the surface (Mortimer 1971; Krom and Berner 1981). Fe-bound P is abundant in surface sediments overlain by oxic bottom waters in the Baltic Sea (Balzer 1986; Jensen and Thamdrup 1993; Lukkari et al. 2009; Mort et al. 2010). Furthermore, in oxic conditions, activity of benthic fauna oxygenates the sediment and enhances binding of PO4. Benthic fauna can also mix PO4 from pore water to the water column and accelerate the release of P from the organic phase by enhancing microbial activity (Aller 1988; Kristensen 1988).
In sediments overlain by anoxic water, Fe in the oxyhydroxides is reduced resulting in the release of associated PO4 (Einsele 1936; Mortimer 1941). In organic-rich sediments common in the Baltic Sea, O2 consumption by mineralisation of organic matter can create reduced conditions, if the O2 is not replenished. When Fe-oxyhydroxides are not able to form, or they form only a thin layer on the sediment surface, PO4 is not efficiently retained (Mortimer 1971). Furthermore, in brackish and marine environments, sulphide can restrict participation of Fe in PO4 binding by precipitating ferrosulphides (Berner 1970; Caraco et al. 1989).
According to the ‘vicious circle’ hypothesis, eutrophication nourishes itself by increasing production and sedimentation of organic matter which enhances the formation of anoxia (e.g. Vahtera et al. 2007). When anoxia extends to previously oxic bottom areas rich in Fe-bound PO4, it releases more PO4 from the sediment. If vertical mixing in the water column supplies released PO4 to the productive layer, it further enhances eutrophication, leading to yet another progression of anoxia. However, if the sea area has been anoxic for a long period, the amount of Fe-bound PO4 sensitive to reduction-induced release may have already been diminished (Lukkari et al. 2009; Jilbert et al. 2011; Malmaeus and Karlsson 2012). Furthermore, under anoxia, regeneration of organic P may be enhanced (Ingall et al. 1993; Ingall and Jahnke 1994), for example via release of organic P compounds from Fe-oxyhydroxides (Suzumura and Kamatani 1995) and release of PO4 from microbial sources (Gächter et al. 1988; Ingall and Jahnke 1994; Hupfer et al. 2004).
Past changes: Important long-term changes affecting P transformations and cycling within the Baltic Sea are linked to increasing P inputs during the last century. This has a direct effect on the total PO4 inventory in the Baltic Sea and is reflected in the increase in surface water PO4. Hence, organic matter production was enhanced and resulted in an accelerated O2 depletion and finally the intensified formation of anoxia in the deeper below-halocline water layer since the 1970s (Conley et al. 2009a). This has restricted the binding of PO4 to Fe-oxyhydroxides in the sediments and PO4 recycles longer in the system maintaining higher PO4 levels in the water (Ingall et al. 1993; Hille et al. 2005; Jilbert et al. 2011). Figure 18.9 presents observations of PO4 concentration in the bottom water of the central Gotland Sea since about 1960. A trend cannot be detected because large changes occurred on short timescales. These can be explained by concomitant variations in O2 concentrations and by the abundance of H2S, which are controlled by irregular saltwater inflows from the North Sea.
4.3 The Marine CO2 (Acid/Base) System
The marine CO2 system is of biogeochemical importance as it regulates the pH of sea water and at the oceanic scale is a major control for atmospheric CO2. This has stimulated intensive research on the marine CO2 system during the last 20–30 years when the role of the oceans for the uptake of anthropogenic CO2 and the consequences for marine biogeochemistry became increasingly evident. Investigations of the marine CO2 system can also be used as a tool to study biogeochemical processes since biological production and decomposition of organic matter are intimately connected with the consumption or release of CO2.
4.3.1 Current Process Understanding
The marine CO2 system constitutes the major component of the acid/base balance in sea water. It is characterised by thermodynamic equilibria between hydrogen ions (pH) and the different CO2 species (Box 18.2 and Schneider 2011). The latter are dissolved CO2, carbonic acid (H2CO3), hydrogen carbonate (HCO3 −) and carbonate ions (CO3 2−). The sum of the different CO2 species is called total CO2, CT or dissolved inorganic carbon (DIC). Another important variable of the CO2 system is the CO2 partial pressure (pCO2), which is proportional to the CO2 concentration. Finally, another variable to be considered is the alkalinity , AT, which is defined as the excess of proton acceptors such as HCO3 − and CO3 2− ions over proton donors (H+). The AT controls the change in pH upon the addition of CO2 or other acidic substances and is thus regarded as the buffer capacity of sea water (Omstedt et al. 2010).
Box 18.3
The large-scale distribution of total CO2 in the Baltic Sea surface water is widely controlled by alkalinity , which originates mainly from the weathering of limestone in the catchment. The soils in the southern and eastern catchment are rich in limestone, and thus, their rivers carry large amounts of AT into the Baltic Sea. In contrast, the igneous rocks prevailing in the Scandinavian catchment result in low river water AT.
Data for total CO2 in surface water along a transect through the entire Baltic Sea (Beldowski et al. 2010) show highest values (>2000 µmol kg−1) in the Kattegat and minimum values of about 600 µmol kg−1 in the Bothnian Bay . However, due to uptake and release of CO2 during biological production and mineralisation of organic matter, a distinct seasonal cycle is superimposed on the AT-controlled background CT and is reflected in the surface water pCO2. The pCO2 seasonality of the surface water in the central Baltic Sea obtained from automated measurements on a cargo ship (Schneider 2011) reveals two typical minima (Fig. 18.10). These are caused by CO2 consumption during the two major productive periods which are the spring bloom and the N-fixing cyanobacteria bloom in midsummer.
Typical seasonality in the CO2 partial pressure (pCO2) of surface water (blue line) and in the atmosphere (green line) in the central Baltic Sea (adopted from Schneider 2011)
Later in the year, the pCO2 recovers and exceeds the atmospheric level. This is mainly due to the deepening of the mixed layer which leads to the transport of CO2-enriched deeper water layers to the surface. The pCO2 is directly linked to the pH which accordingly shows pronounced seasonality. High pH (of about 8.5) is observed during the spring/summer productive period, and low pH (around 7.9) can occur during the deepening of the mixed layer in autumn/winter (Wesslander et al. 2010).
The mainly biologically driven seasonal cycle in the surface water pCO2 also controls the annual CO2 gas exchange balance since the gas exchange is driven by the difference between the surface water pCO2 and the relatively stable atmospheric pCO2. It is not known whether the Baltic Sea is a net sink or a net source of atmospheric CO2. Whereas Thomas and Schneider (1999) reported a CO2 uptake of 0.9 mol m−2 year−1 by the central Baltic Sea, Wesslander et al. (2010) concluded that the Gotland Sea and Bornholm Sea are sources of atmospheric CO2 and release 1.6 and 2.4 mol m−2 year−1 CO2, respectively, to the atmosphere. These discrepancies are due to the different data sources, all showing insufficient temporal and spatial coverage in view of the huge daily, seasonal, interannual and regional variability in surface water pCO2.
The deep water of the major basins is subject to continual mineralisation of organic matter that leads to the accumulation of total CO2 during periods of stagnation. To estimate the effect of increasing CT on pH, concurrent alkalinity changes must be taken into account (Edman and Omstedt 2013). During oxic conditions, nitrification of ammonia takes place and results in a decrease in alkalinity. This slightly amplifies the strong decrease in pH caused by the gain in CT. As a consequence, the pH at O2 concentrations close to zero may be about one unit lower than the mean surface pH. When the water is turning to anoxic conditions, both denitrification and especially sulphate reduction, together with the associated release of ammonia from organic matter, strongly increase alkalinity . This damps a further decrease in pH during mineralisation at anoxic conditions and stabilises pH at about 7.0 (Ulfsbo et al. 2011) after a longer period of stagnation .
4.3.2 Past Changes
A large set of historical CO2 data for the northern Baltic Sea including the Gulf of Bothnia and Gulf of Finland is available for 1927–1938 when the first pioneering studies on the marine CO2 system were performed by Kurt Buch and co-workers at the Finnish Institute of Marine Research (FIMR) in Helsinki. They determined equilibrium constants for the CO2 system that agreed reasonably well with those determined by more sophisticated analytical techniques in recent years. This indicates that their data can be compared against the present-day status of the marine CO2 system. The current text restricts discussion on possible change in the CO2 system to the surface water because interpreting the deep-water CO2 data requires detailed information about the chronology of water mass stagnation and renewal events. This is not available for the Buch (1945) data, which give only seasonal mean values.
Surface water pCO2 reflects the interplay between biological production, mineralisation and mixing , and has undergone large changes during the past 80 years. In Buch’s (1945) data, the pCO2 minimum after the spring bloom in the eastern Gotland Sea was about 270 µatm. Taking into account the lower atmospheric pCO2 during the 1930s (about 320 µatm, Callendar 1940), a CO2 partial pressure difference, ∆pCO2, of about −50 µatm is obtained which indicates moderate undersaturation of the surface water with regard to atmospheric CO2. Recent pCO2 measurements show a spring bloom minimum of about 150 µatm which corresponds to a ∆pCO2 of −250 µatm (Schneider 2011). This undersaturation is about fivefold larger than at the beginning of the last century and can be attributed to increased organic matter production as a consequence of eutrophication. Although this is consistent with other historic production estimates, it must be taken into account that Buch’s (1945) spring value refers only to a single year and so its representation for the spring bloom can be questioned.
The changes in surface water ∆pCO2 during the past 80 years are even more pronounced in midsummer. Due to the intense N2 fixation in July, the ∆pCO2 in the central Baltic Sea currently amounts to almost −300 µatm (Schneider et al. 2009), whereas the mean ∆pCO2 at this time of year for 1927–1935 was less than −40 µatm (Buch 1945). This indicates low or even absent N fixation activity before the onset of the eutrophication .
Changes in the marine CO2 system are also due to the uptake of anthropogenic CO2. The increase in atmospheric CO2 from 280 ppm during the pre-industrial era to almost 400 ppm in 2010 has led to a corresponding increase in the mean surface water pCO2. According to the thermodynamics of the marine CO2 system, a decrease in pH of about 0.15 units can be expected if no other changes in the biogeochemical cycles had occurred. This phenomenon is called ‘ocean acidification’ and has stimulated many research projects on the ecological consequences of the decreasing pH. Monitoring programmes have included pH measurements for several decades, and large amounts of data are currently stored at national and international data centres. But due to methodological shortcomings, many of the older data are highly uncertain and are not suitable for trend analysis. Data with a higher accuracy and a monthly resolution are available for the early 1990s onwards at the SMHI and FIMR data centres. However, the expected trend of 0.02 pH units per decade, which corresponds to the current increase in atmospheric CO2 of 2.0 ppm year−1, could not be definitively identified. This may be due to the large seasonal amplitude of the pH signal and/or to the considerable interannual variability (Wesslander et al. 2010). It is also possible that other changes in the biogeochemical conditions may have counteracted the pH decrease. With reference to the data of Buch (1945), alkalinity in the eastern Gotland Sea increased by about 100 µmol kg−1 during the last century. The effect on pH is roughly an increase of 0.03 units by which the effect of the increasing atmospheric CO2 levels since the pre-industrial era (0.15 units) was reduced.
The increasing uptake of anthropogenic CO2 will also decrease the concentrations of carbonate ions and thus decrease the state of the calcium carbonate saturation (Ω) which is defined by
where k sp is the solubility product. This may affect the growth and possibly the survival of organisms that form shells consisting of calcite or aragonite which are the two biogenic CaCO3 crystal forms. Historic calcite and aragonite saturation values can be calculated from Buch’s (1945) measurements during 1928–1938. For surface water in the eastern Gotland Sea, a maximum oversaturation is obtained for calcite during midsummer (1.65), whereas a slight undersaturation (0.92) was found for aragonite. Despite an increase in atmospheric CO2 by about 80 ppm since Buch’s measurements, the surface water saturation for calcite and aragonite in midsummer did not decrease, but instead increased by a factor of 2.6 during the last 80 years according to pCO2 measurements performed in 2005 (Schneider et al. 2009). This is mainly a consequence of the low pCO2 that is caused by the eutrophication-related high production and that has completely overridden the effect of the rising atmospheric pCO2. However, enhanced production implies increased mineralisation and high CO2 concentrations in the deeper layers. Increased vertical mixing during winter therefore adds CO2 to the elevated atmospheric CO2 (Fig. 18.10). According to measurements in 2005 (Schneider et al. 2009), this results in a distinct calcite and aragonite undersaturation during winter of 0.56 and 0.31, respectively.
4.4 Burial of Carbon, Nitrogen and Phosphorus
4.4.1 Current Process Understanding
The biogeochemical cycling of C, N and P is linked via biological productivity (Mackenzie et al. 1993; Anderson et al. 2001), and their removal from the system through burial in the sediment is important for eutrophication and the CO2 balance. When C, N and P in the sediment are no longer significantly interacting with the water column, they can be considered as buried. Buried C and N in the sediments of the Baltic Sea are mainly in the form of organic compounds, whereas a substantial part of P occurs as inorganic forms such as associates with Fe-oxyhydroxides and various calcium compounds (Conley et al. 1997; Carman 1998; Aigars and Carman 2001; Mort et al. 2010). Burial of P formed in the marine system removes P from the nutrient cycle, while refractory P transported from land is buried passively (Ruttenberg and Berner 1993; Delaney 1998). In the Baltic, reactive P (i.e. potentially bioavailable that can promote primary productivity, Anderson et al. 2001) is mainly buried in organic form (Mort et al. 2010).
It has been hypothesised that N and P are preferentially released during the mineralisation of organic matter and that the remainder that is deposited on the sea floor and buried is enriched in C (e.g. Froelich et al. 1982; Andersen and Jensen 1992; Ingall et al. 1993; Anderson et al. 2001). Hence, atomic ratios of organic C, N and P in sediment, compared to those in plankton (Redfield et al. 1963), have been used to draw conclusions regarding the decomposition or origin of the buried material (e.g. Froelich et al. 1982; Ruttenberg and Goñi 1997). However, atomic ratios are affected by many biogeochemical processes and properties of the sedimentation environment (e.g. Ingall and Van Cappellen 1990; Ingall and Jahnke 1994; Anderson et al. 2001; Jilbert et al. 2011). C:N:P ratios presented in the literature for the Baltic Sea sediments vary depending on sea area, bottom type and sediment depth (e.g. Carman and Cederwall 2001), the variation being clearly higher in the C:P ratio than that in the C:N ratio.
In the Baltic Sea, characteristic conditions that affect sedimentation and burial of C, N and P are, for example, high input of terrestrial material, shallow water column, intense production of organic matter and variable topography of the sea floor (Winterhalter et al. 1981; Carman and Cederwall 2001). Burial is most effective in the deep basins, which accumulate resuspended material from erosion and transportation areas. Carman and Cederwall (2001) estimated the contributions of erosion (19–33 %), transportation (38–46 %) and accumulation (28–41 %) areas to the sea floor of the Baltic Proper , the Gulf of Bothnia, the Gulf of Finland and the Gulf of Riga .
Figure 18.11 shows the spatial variation in particulate organic C content in the sediments (Leipe et al. 2011). Sedimentation rates of organic matter vary spatially, especially in the archipelagos because of the fractured topography (Winterhalter et al. 1981) and temporally because of the seasonality of plankton growth and senescence (Bianchi et al. 2002). Anoxia which is common in the Baltic Sea can favour burial of organic matter but diminish the burial of P because the formation of Fe-oxyhydroxides that bind PO4 is inhibited (Van Cappellen and Ingall 1994; Ingall et al. 1993; Mort et al. 2010; Jilbert et al. 2011). In the Baltic Sea, Edlund and Carman (2001) reported higher organic C:P ratios for anoxic than for oxic sites and Jilbert et al. (2011) suggested increasing rates of P mineralisation in more reduced conditions. However, it is also conceivable that the shift to higher C:P ratios is caused by a slower organic C mineralisation. In oxic areas, benthic fauna affect burial of C, N and P (Aller 1988; Kristensen 1988; Andersen and Jensen 1991; Andersen and Kristensen 1992; Norkko et al. 2011).
Distribution of the percentage of particulate organic carbon (POC) in surface sediments in the Baltic Sea (Leipe et al. 2011)
4.4.2 Past Changes
It has been estimated that the sedimentation of organic matter and thus the burial of C, N and P increased in the Baltic Sea during the latter half of the twentieth century as a result of eutrophication (Jonsson and Carman 1994; Bonsdorff et al. 1997; Emeis et al. 2000; Vaalgamaa and Conley 2008). Jonsson and Carman (1994) suggested an increase in organic matter deposition by a factor larger than 1.7 between the 1920s and 1980s in the northern Baltic Proper and Emeis et al. (2000) estimated a fourfold increase in sediment accumulation in the Bornholm, Gdansk and Gotland basins since 1900. Shaffer (1987) estimated that in 1900, the burial rate of C may have been about 0.2 mol m−2 year−1 in the Baltic Proper, compared to about 0.8 mol m−2 year−1 in 1980. More recent literature presents variable C burial rates of up to about 5 mol m−2 year−1 for the Baltic Proper. Burial rates reported for N and P in the Baltic Sea vary from 77 to 1286 mmol m−2 year−1 and 2 to 51 mmol m−2 year−1, respectively. According to Mort et al. (2010), burial of P has not clearly increased in the Baltic Proper, and Jilbert et al. (2011) suggested that burial of organic P has increased in hypoxic basins in the Baltic Proper because of enhanced burial of organic matter.
Several authors have reported that increasing nutrient inputs and anoxia have altered the C:N:P ratios in the Baltic Sea sediments (e.g. Emeis et al. 2000; Hille 2006; Mort et al. 2010). For example, Emeis et al. (2000) reported a twofold increase in the C:P ratios in the Gdansk basin since 1950. Similar findings were made by Hille (2006) who analysed the elemental ratios of C, N and P in sediment cores from the Gotland Sea. The C:P ratios (Fig. 18.12a) increased by a factor of more than two during the last century. In contrast, the C:N ratios (Fig. 18.12b) remained relatively stable with values around 10 which is consistent with measurements performed at the beginning of the last century in different regions of the northern Baltic Sea (Gripenberg 1934).
Mean molar C:P (a) and C:N (b) ratios and standard deviation in sediments from the Gotland Basin. Sediment above 10–16 cm depth (grey bar) represents material accumulated during approximately the last 100 years (data from Hille 2006)
5 Response to Potential Future Changes
5.1 Nutrient Inputs
The HELCOM has adopted several recommendations since the 1970s to reduce nutrient loading of the Baltic Sea. A first attempt was to implement a 50 % reduction for N and P loads until 1995 (HELCOM 1988). This reduction target was not achieved (HELCOM 2009). With eight coastal countries being members of the European Union (EU), EU policies have become important for the Baltic Sea. Following the Water Framework Directive (WFD), nutrient reduction measures are to be installed that lead to a ‘good ecological status’ (European Parliament 2000). While the WFD is restricted to coastal waters, the EU Marine Strategy Framework Directive (MSFD, European Parliament 2008) refers to good environmental status in open sea areas as well as coastal waters.
Based on definitions of good environmental status, maximum allowable nutrient loads have been estimated. These loads are formulated as targets in the Baltic Sea Action Plan . The BSAP was adopted by the HELCOM member states in 2007. Compared to the period 1997–2003, a 19 % reduction for N and a 42 % reduction for P loads should be reached by 2021. However, the BSAP is currently (as of 2013) under revision and new reduction targets may be defined. While the P loads to the Baltic Sea can probably be reduced in the future, the perspective is less optimistic for the N loads. Even if the BSAP aims for a reduction in N loads, an increase in livestock may counteract this effort. With further economic development especially in some eastern European countries, animal protein consumption by humans is expected to increase. Since it is unlikely that increasing demand for animal proteins will be covered entirely by imports, a more likely scenario is an intensive development of the agricultural sector in the transitional countries. There is potential for large animal farms to be established in regions with low or inefficient current use of agricultural land. Such development could have a strong impact on the N flux from the catchment (see also Chap. 17). Eriksson Hägg et al. (2010) calculated that an increased protein consumption scenario would lead to a 16–39 % increase in N fluxes from the catchment. Taking into account climate-induced changes in river discharge , the increase in the N flux could be 3–72 % depending on the climate scenario. If N fluxes increase and P fluxes decrease, this would probably increase primary production in spring, but decrease the midsummer cyanobacteria blooms (Humborg et al. 2007).
Atmospheric deposition of N may increase in the future due to increased precipitation and increased shipping and agriculture . The recent share of atmospheric deposition in N loads is about 25 %, while the atmospheric load of P accounts for only a few percentage of the total load (HELCOM 2009).
5.2 Biogeochemical Cycles
Understanding of the impact of future climate change and other anthropogenic drivers on biogeochemical cycles is still limited. Several factors may influence the biogeochemical cycles in different ways. Hydrographic conditions affect the mixing depth, vertical exchange of nutrients and O2, and inflow dynamics. Higher temperatures decrease the solubility of O2 in sea water and accelerate many biogeochemical processes. Changing run-off and precipitation patterns influence the nutrient loads to the Baltic Sea. Taking into account that the Baltic Sea ecosystem is a complex, nonlinear system, the effect of changes in external forcing is best quantified by numerical models . The BONUS (www.bonusportal.org) programme supports several projects that aim to quantify the response of the Baltic Sea ecosystem to climate change.
One focus of climate change effects on the Baltic Sea is the future development of O2 conditions because it is a key parameter for biogeochemical cycles. Future warming will increase hypoxia through several interacting processes. Temperature controls stratification, mineralisation of organic matter and the solubility of O2. Conley et al. (2009b) postulated that higher temperatures are likely to exacerbate hypoxia. Meier et al. (2011) showed with the aid of a model ensemble that future climate change could expand O2-depleted regions. Reasons for increasing hypoxic and anoxic areas (Fig. 18.13) are increasing nutrient loads due to enhanced river run-off, reduced O2 solubility and accelerated recycling of organic matter. The same model ensemble projected that hypoxic and anoxic periods in the main southern and central basins of the Baltic Sea could become more frequent and last longer (Neumann et al. 2012). This may lead to intensified P mobility and reduced denitrification efficiency. As a consequence, the pelagic DIN and DIP concentrations as well as primary production would increase (Meier et al. 2012). Such changes in biogeochemical fluxes imply that even if nutrient loads are reduced according to the BSAP, the Baltic Sea ecosystem would not recover but would stabilise close to its present state.
Furthermore, warming will preferentially favour cyanobacteria blooms . Blooms are expected to start earlier in summer, and N fixation might increase (Neumann 2010; Neumann et al. 2012; Meier et al. 2012). It was also shown that increasing nutrient loads due to climate-induced change in river run-off have a smaller impact on the modelled phytoplankton development than changes caused by socio-economic development. The pessimistic ‘business-as-usual’ scenario for nutrient loads could lead to increasing adverse effects on the marine environment in a future climate (Meier et al. 2011).
Omstedt et al. (2012) used a complex model system that included biogeochemical processes in the catchment area (see Chap. 17) to simulate the future development of the hypoxic areas. For both the business-as-usual and the BSAP scenarios, they found almost the same long-term trend pattern as Meier et al. (2011) (Fig. 18.13), however, with an overall offset of about 20,000 km2.
5.3 Acidification
In addition to eutrophication , ocean acidification (decreasing pH) caused by increasing atmospheric CO2 is considered a major potential threat for the Baltic Sea. Taking into account only the effect of the CO2 increase and ignoring other potential changes in the marine CO2 system, the pH decrease may be calculated on the basis of the acid/base equilibria in sea water. Assuming the worst-case scenario, which is an increase from about 400 ppm CO2 at present to 950 ppm at the end of the century (van Vuuren et al. 2011), yields a decrease in the mean pH in the Baltic Sea surface water of about 0.4 pH units. Since pH is a logarithmic quantity, this is equivalent to an increase in hydrogen ion (H+) concentration by a factor of 2.5. This corresponds to a decrease in calcium carbonate saturation by a factor of approximately 2 and may affect the survival of organisms such as mussels that form calcite or aragonite shells (Brander and Havenhand 2011).
To estimate the possible future development of pH in the Baltic Sea until 2100, Omstedt et al. (2012) developed a biogeochemical model that included the cycling of C and thus the marine CO2 system which mainly controls the acid/base system in sea water. The model was coupled with a catchment model in order to account for climate-induced change in the riverine inputs of total CO2 and alkalinity and of dissolved organic C. Both these classes of C compound affect sea water pH, either directly or in case of organic C by mineralisation . Their release by weathering and soil organic matter transformation and transport in a changing climate were simulated by sub-models for the catchment area. Simulations with the coupled models were performed for a variety of different climate, nutrient load and land cover scenarios. The results were used to identify the contributions of the different forcing factors to a possible change in sea water pH.
Figure 18.14 shows the simulated pH development until 2100 for a best-case and a worst-case forcing scenario (green and red line, respectively). The best case refers to a climate change scenario based on CO2 emission reductions resulting in atmospheric CO2 concentrations of about 550 ppm in 2100 (ECHAM, B1). It is combined with reduced nutrient loads to the Baltic Sea according to the BSAP. For the worst case, a climate change scenario (ECHAM, A2) is used that yields final CO2 concentration of about 850 ppm and the nutrient loads are assumed to increase according to a business-as-usual scenario . A decrease in pH of about 0.40 and 0.26 in surface waters of the central Baltic Sea is obtained for the worst-case and best-case scenarios, respectively (Fig. 18.14). Analysis of the model simulations showed that changes in surface water pH are mainly due to rising atmospheric CO2 concentrations. Increasing nutrient loads that cause enhanced biological production only increased the seasonal pH amplitude; they had no effect on mean pH.
Simulation of surface water and deep-water pH in the eastern Gotland Sea. The temporal development was calculated for (1) a worst-case scenario : atmospheric CO2 increase to 850 ppm and nutrient inputs according to business-as-usual (red line) and (2) a best-case scenario: moderate increase in atmospheric CO2 to 550 ppm and nutrient inputs according to the Baltic Sea Action Plan (green line) (Omstedt et al. 2012)
Temporal change in the pH of the Gotland Sea deep water is less regular because of the interplay between stagnation periods and water renewal events. Nevertheless, the model simulations show a pH decrease for both scenarios. However, in the best-case scenario, pH declines only until 2000, then shows no further change. In contrast, for the worst-case scenario, the simulations show a continuous decrease in deep-water pH (Fig. 18.14). Furthermore, model experiments indicate that pH in the deep water results not only from increasing atmospheric CO2 but is also partly controlled by rising temperatures and changing inputs from the catchment area.
6 Conclusion
The first assessment of climate change in the Baltic Sea (BACC Author Team 2008) did not include a chapter explicitly dedicated to Baltic Sea biogeochemistry. However, some aspects such as nutrient inputs, the relationship between nutrient availability and biomass production, and the consequences of eutrophication for deep-water O2 conditions were addressed in Chap. 5 Climate-related Marine Ecosystem Change. Since then, much progress has been achieved, particularly in the reconstruction of past changes and model simulations of future eutrophication scenarios. This is mainly due to BONUS-funded research and is presented here. In general, this chapter has focussed on the description of the basic processes controlling the complex relationship between nutrient transformations and organic matter production and mineralisation. This implies a consideration of both the O2/H2S budget and the marine CO2 system, and so is related to both eutrophication and acidification.
Data on the concentrations of nutrients, O2 and H2S in the Baltic Sea are available for about the last 40 years and clearly indicate that major biogeochemical changes have occurred. Nitrate and PO4 concentrations in the winter surface water of the Baltic Proper increased by a factor of approximately 3. This is consistent with the enhanced nutrient inputs by river water and atmospheric deposition during the last century. However, inputs have decreased since about 1980 and are currently at a level that is roughly comparable to that in 1960. This is reflected in a decrease in NO3 concentrations in the winter surface water of the Baltic Proper since 1980. A similar trend does not exist for PO4 concentrations. This is partly explained by the long residence time of P in the Baltic Sea and its temporary storage as PO4–Fe-oxyhydroxide. But considerable uncertainties in the input estimates must also be taken into account. As a consequence of the increasing nutrient concentrations that mainly control the net organic matter production and the export into deeper water layers, the frequency of H2S occurrence has increased. This may have enhanced the recycling of PO4 and favoured blooms of N-fixing cyanobacteria. Data concerning the marine CO2 system are available for the 1920s and 1930s. They indicate that net biomass production has increased since then by a factor of about 2.5. The data also show that alkalinity in the Baltic Proper has increased and has considerably damped the decline in pH caused by rising levels of atmospheric CO2.
Model simulations of scenarios concerning the future biogeochemistry of the Baltic Sea indicate that climate change is likely to exacerbate eutrophication effects in the Baltic Sea. However, they also indicate that the implementation of the BSAP could help to decrease slightly the area covered with hypoxic and anoxic waters despite climate change effects. In contrast, the business-as-usual nutrient input scenario increased the hypoxic area by about 30 % and more than doubled the anoxic area. Simulations of the future development of pH in the Baltic Sea were performed with a model system that included the cycling of organic C and CO2 in the Baltic Sea as well as the catchment area. It was shown that the rising atmospheric CO2 mainly controls future pH changes in Baltic Sea surface water and that eutrophication and enhanced biological production are not affecting the mean pH. The worst-case CO2 emission scenario (resulting in an atmospheric concentration of 850 ppm) projected a fall in pH in Baltic Sea surface water of about 0.40 by 2100. A more optimistic future CO2 emission scenario (550 ppm) yielded a decrease in pH of approximately 0.26.
References
Aigars J, Carman R (2001) Seasonal and spatial variations of carbon and nitrogen distribution in the surface sediments of the Gulf of Riga, Baltic Sea. Chemosphere 43:313-320
Algesten G, Wikner J, Sobek S, Tranvik LJ, Jansson M (2004) Seasonal variation of CO2 saturation in the Gulf of Bothnia: Indications of marine net heterotrophy. Global Biogeochem Cy 18: GB4021. doi:10.1029/2004GB002232
Algesten G, Brydsten L, Jonsson P, Kortelainen P, Löfgren S, Rahm L, Räike A, Sobeck S, Tranvik L, Wikner J, Jansson M (2006) Organic carbon budget for the Gulf of Bothnia. J Marine Syst 63:155-161
Aller RC (1988) Benthic fauna and biogeochemical processes in marine sediments: The role of burrow structures. In: Blackburn TH, Sørensen J (eds), Nitrogen Cycling in Coastal Marine Environments. SCOPE, John Wiley & Sons, Chichester, p 301-338
Andersen FØ, Jensen HS (1991) The influence of chironomids on decomposition of organic matter and nutrient exchange in a lake sediment. Verh Internat Verein Limnol 24:3051-3055
Andersen FØ, Jensen HS (1992) Regeneration of inorganic phosphorus and nitrogen from decomposition of seston in a freshwater sediment. Hydrobiologia 228:71-81
Andersen FØ, Kristensen E (1992) The importance of benthic macrofauna in decomposition of microalgae in a coastal marine sediment. Limnol Oceanogr 37:1392-1403
Anderson LD, Delaney ML, Faul KL (2001) Carbon to phosphorus ratios in sediments: Implications for nutrient cycling. Global Biogeochem Cy 15:65-79
Andreasson KIM, Wikner J, Abrahamsson B, Melrose C, Nyberg S (2009) Primary production measurements - an intercalibration during a cruise in the Kattegat and the Baltic Sea SMHI Report Oceanografi 100/2009
Balzer W (1986) Forms of phosphorus and its accumulation in coastal sediments of Kieler Bucht. Ophelia 26:19-35
Bartnicki J, Semeena V, Fagerli H (2011) Atmospheric deposition of nitrogen to the Baltic Sea in the period 1995-2006. Atmos Chem Phys 11:10057-10069
Beldowski J, Löffler A, Schneider B, Joensuu L (2010) Distribution and biogeochemical control of total CO2 and total alkalinity in the Baltic Sea. J Marine Syst 81:252-259
Berg GM, Glibert PM, Jørgensen NOG, Balode M, Purina I (2001) Variability in inorganic and organic nitrogen uptake associated with riverine nutrient input in the Gulf of Riga, Baltic Sea. Estuaries 24:204-214
Berner RA (1970) Sedimentary pyrite formation. Am J Sci 268:1-23
Bianchi TS, Egelhaupt E, Westmann P, Andren T, Rolff C (2000) Cyanobacteria blooms in the Baltic Sea: Natural or human-induced? Limnol Oceanogr 45:716-726
Bianchi TS, Rolff C, Widbom B, Elmgren R (2002) Phytoplankton pigments in Baltic Sea seston and sediments: Seasonal variability, fluxes, and transformations. Estuar Coast Shelf Sci 55:369-383
Bonsdorff E, Blomquist EM, Mattila J, Norkko A (1997) Coastal eutrophication: causes, consequences and perspectives in the Archipelago areas of the Northern Baltic Sea. Estuar Coast Shelf Sci 44:63-72
Boyd PW, Trull TW (2007) Understanding the export of biogenic particles in oceanic waters: Is there consensus? Progr Oceanogr 72:276-316
Brander K, Havenhand J (2011) Impacts of climate change, including acidification, on marine ecosystems and fisheries. In: Reckermann M, Brander K, MacKenzie BR, Omstedt A (eds), Climate Impacts on the Baltic Sea: From Science to Policy. Springer Earth System Sciences, p 129-160
Brettar I, Rheinheimer G (1991) Denitrification in the central Baltic: evidence for H2S-oxidation as motor of denitrification at the oxic-anoxic interface. Mar Ecol Progr Ser 77:157-169
Brettar I, Rheinheimer G (1992) Influence of carbon availability on denitrification in the central Baltic Sea. Limnol Oceanogr 37:1146-1163
Buch K (1945) Kolsyrejämvikten i Baltiska Havet. Fennia 68:5
Callendar GS (1940) Variations of the amount of carbon dioxide in different air currents. Q J Roy Meteorol Soc LXVI 287:395-400
Caraco N, Cole JJ, Likens GE (1989) Evidence for sulphate-controlled phosphorus release from sediments of aquatic systems. Nature 341:316-318
Carman R (1998) Burial pattern of carbon, nitrogen and phosphorus in the soft bottom sediments of the Baltic Sea. Vie Milieu 48:229-241
Carman R, Cederwall H (2001) Sediments and macrofauna in the Baltic Sea – characteristics, nutrient contents and distribution. In: Wulff FV, Rahm LA, Larsson P (eds), A Systems Analysis of the Baltic Sea, Ecological Studies 148, p 289-327
Carstensen J, Conley D, Müller-Karulis B (2003) Spatial and temporal resolution of carbon fluxes in a shallow coastal ecosystem, the Kattegat. Mar Ecol Progr Ser 252:35-50
Carstensen J, Conley DJ, Andersen JH, Ærtebjerg G (2006) Coastal eutrophication and trend reversal: A Danish case study. Limnol Oceanogr 51:398-408
Conley DJ, Stockenberg A, Carman R, Johnstone RW, Rahm L, Wulff F (1997) Sediment-water nutrient fluxes in the Gulf of Finland, Baltic Sea. Estuar Coast Shelf Sci 45:591-598
Conley DJ, Humborg C, Rahm L, Savchuk OP, Wulff F (2002) Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ Sci Technol 36:5315-5320
Conley DJ, Björck S, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hietanen S, Kortekaas M, Kuosa H, Meier HEM, Müller-Karulis B, Nordberg K, Norkko A, Nürnberg G, Pitkänen H, Rabalais NN, Rosenberg R, Savchuk OP, Slomp CP, Voss M, Wulff F, Zillen L (2009a) Hypoxia related processes in the Baltic Sea. Environ Sci Technol 43:3412-3420
Conley DJ, Carstensen J, Vaquer-Sunyer R, Duarte CM (2009b) Ecosystem thresholds with hypoxia. Hydrobiologia 629:21-29
Degerholm J, Gundersen K, Bergman B, Söderbäck E (2008) Seasonal significance of N2 fixation in coastal and offshore waters of the northwestern Baltic Sea. Mar Ecol Progr Ser 360:73-84
Delaney ML (1998) Phosphorus accumulation in marine sediments and the oceanic phosphorus cycle. Global Biogeochem Cy 12:563-572
Deutsch B, Forster S, Wilhelm M, Dippner JW, Voss M (2010), Denitrification in sediments as a major nitrogen sink in the Baltic Sea: an extrapolation using sediment characteristics. Biogeosciences 7:3259-3271
Devol AH (2008) Denitrification including anammox. In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ (eds), Nitrogen in the Marine Environment 2nd Ed, Elsevier, p 263-301
Diekmann R, Möllmann C (eds) (2010) Integrated ecosystem assessments of seven Baltic Sea areas covering the last three decades. ICES Cooperative Research Report 302
Edlund G, Carman R (2001) Distribution and diagenesis of organic and inorganic phosphorus in sediments of the Baltic proper. Chemosphere 45:1053-1061
Edman M, Omstedt A (2013) Modeling the dissolved CO2 system in the redox environment of the Baltic Sea. Limnol Oceanogr 58:74-92
Eilola K, Stigebrandt A (1999) On the seasonal nitrogen dynamics of the Baltic proper biogeochemical reactor. J Mar Res 57:693-713
Eilola K, Meier HEM, Almroth E (2009) On the dynamics of oxygen, phosphorus and cyanobacteria in the Baltic Sea; A model study. J Marine Syst 75:163-184
Einsele W (1936) Über die Beziehungen des Eisenkreislaufs zum Phosphatkreislauf im eutrophen See. Arch Hydrobiol 29:664-686
Elmgren R (1984) Trophic dynamics in the enclosed, brackish Baltic Sea. Rap Proces 183:152-169
Elmgren R (1989) Man’s impact on the ecosystem of the Baltic Sea: Energy flows today and at the turn of the century. Ambio 18:326-332
Elmgren R (2001) Understanding human impact on the Baltic ecosystem: changing views in recent decades. Ambio 30:222-231
Emeis K-C, Struck U, Leipe T, Pollehne F, Kunzendorf H, Christiansen C (2000) Changes in the C, N, P burial rates in some Baltic Sea sediments over the last 150 years – relevance to P regeneration rates and the phosphorus cycle. Mar Geol 167:43-59
Engel A, Meyerhöfer M, Bröckel von K (2002) Chemical and biological composition of suspended particles and aggregates in the Baltic Sea in summer (1999). Estuar Coast Shelf Sci 55:729-741
Eriksson Hägg H, Humborg C, Mörth CM, Medina MR, Wulff F (2010) Scenario analysis on protein consumption and climate change effects on riverine input to the Baltic Sea. Environ Sci Technol 44:2379-2385
European Parliament (2000) Directive 2000/60/EC Of The European Parliament And Of The Council of 23 October 2000 establishing a framework for Community action in the field of water policy
European Parliament (2008) Directive 2008/56/EC Of The European Parliament And Of The Council of 17 June 2008 establishing a framework for Community action in the field of marine environmental policy (Marine Strategy Framework Directive)
Feistel R, Nausch G, Wasmund N (eds) (2008) State and Evaluation of the Baltic Sea, 1952–2005. A detailed 50-year survey of Meteorology and Climate, Physics, Chemistry, biology, and Marine Environment. John Wiley & Sons
Fennel W, Neumann T (2004) Introduction to Modelling the Marine Ecosystems. Elsevier Oceanography Series 72
Finni T, Kononen K, Olsonen R, Wallström K (2001) The history of cyanobacteria blooms. Ambio 30:172-178
Fonselius SH (1969) Hydrography of the Baltic deep basins. III. Fishery board of Sweden, Series Hydrography, 13, 20, 23
Froelich PN (1988) Kinetic control of dissolved phosphate in natural rivers and estuaries: A primer on the phosphate buffer mechanism. Limnol Oceanogr 33:649-668
Froelich PN, Bender ML, Luedtke NA, Heath GR, DeVries T (1982) The marine phosphorus cycle. Am J Sci 282:474-511
Gächter R, Meyer JS, Mares A (1988) Contribution of bacteria to release and fixation of phosphorus in lake sediments. Limnol Oceanogr 33:1542-1558
Granéli E (1987) Nutrient limitation of phytoplankton biomass in a brackish water bay highly influenced by river discharge. Estuar Coast Shelf Sci 25:555-565
Granéli E, Wallström K, Larsson U, Graneli W, Elmgren R (1990) Nutrient limitation of primary production in the Baltic Sea area. Ambio 19:142-151
Gripenberg S (1934) A study of the sediments of the North Baltic and adjoining seas. Merentutkimuslaitoksen Julk 166, Valtioneuvoston kirjapaino, Helsinki
Gustafsson E (2012) Modelled long-term development of hypoxic area and nutrient pools in the Baltic Proper. J Marine Syst 94:120-134
Gustafsson BG, Stigebrandt A (2007) Dynamics of nutrients and oxygen/hydrogen sulphide in the Baltic Sea deep water. J Geophys Res 112: G02023. doi:10.1029/2006JG000304
Gustafsson B, Schenk F, Bleckner T, Meier HEM, Müller-Karulis B, Neumann T, Ruoho-Airola T, Savchuk OP, Zorita E (2012) Reconstructing the development of Baltic Sea eutrophication 1850–2006. Ambio 41:534-548
Hajdu S, Hoglander H, Larsson U (2007) Phytoplankton vertical distributions and composition in Baltic Sea cyanobacterial blooms. Harmful Algae 6:189-205
Hannig M, Lavik G, Kuypers MMM, Woebken D, Martens-Habbena W, Jürgens K (2007) Shift from denitrification to anammox after inflow events in the central Baltic Sea. Limnol Oceanogr 52:1336-1345
Heiskanen AS, Leppänen JM (1995) Estimation of export production in the coastal Baltic Sea: effect of resuspension and microbial decomposition on sedimentation measurements. Hydrobiologia 316:211-224
Heiskanen AS, Haapala J, Gundersen K (1998) Sedimentation and pelagic retention of particulate C, N and P in the coastal northern Baltic Sea. Estuar Coast Shelf Sci 46:703-712
HELCOM (1988) Declaration of the Protection of the Marine Environment of the Baltic Sea Area, www.helcom.fi/stc/files/MinisterialDeclarations/MinDecl1988.pdf
HELCOM (2004) The fourth Baltic Sea pollution load compilation (PLC-4). Baltic Sea Environment Proceedings 93
HELCOM (2005) Nutrient pollution to the Baltic Sea in 2000. Baltic Sea Environment Proceedings 100
HELCOM (2007) Toward a Baltic Sea unaffected by eutrophication. Background document to HELCOM Ministerial Meeting, Krakow, Poland, Tech rep, Helsinki Commission, Helsinki, Finland
HELCOM (2009) Eutrophication in the Baltic Sea. An integrated thematic assessment of the effects of nutrient enrichment in the Baltic Sea region. Baltic Sea Environment Proceedings 115b
HELCOM (2011) Fifth Baltic Sea Pollution Load Compilation (PLC-5). Baltic Sea Environment Proceedings 128
Hille S (2006) New aspects of sediment accumulation and reflux of nutrients in the Eastern Gotland Basin (Baltic Sea) and its impact on nutrient cycling. Dissertation, University of Rostock, Germany
Hille S, Nausch G, Leipe T (2005) Sedimentary deposition and reflux of phosphorus (P) in the Eastern Gotland Basin and their coupling with P concentrations in the water column. Oceanologia 47:663-679
Hille S, Leipe T, Seifert T (2006) Spatial variability of recent sedimentation rates in the Eastern Gotland Basin (Baltic Sea). Oceanologia 48:297-317
Hingston FJ, Atkinson RJ, Posner AM, Quirk JP (1967) Specific adsorption of anions. Nature 215:1459-1461
Hjalmarsson S, Wesslander K, Anderson LG, Omstedt A, Perttilä M, Mintrop L (2008) Distribution, long-term development and mass balance calculations of total alkalinity in the Baltic Sea. Cont Shelf Res 28:593-601
Howarth R (1988) Nutrient limitation of net primary production in marine ecosystems. Ann Rev Ecol System 19:89-110
Humborg C, Mörth CM, Sundom M, Wulff F (2007) Riverine transport of biogenic elements to the Baltic Sea – Past and possible future perspectives. Hydrol Earth Syst Sci 11:1593-1607
Hupfer M, Rübe B, Schmieder P (2004) Origin and diagenesis of polyphosphate in lake sediments: A 31P-NMR study. Limnol Oceanogr 49:1-10
Ingall ED, Jahnke R (1994) Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim Cosmochim Acta 58:2571-2575
Ingall ED, van Cappellen P (1990) Relation between sedimentation rate and burial of organic phosphorus and organic carbon in marine sediments. Geochim Cosmochim Acta 54:373-386
Ingall ED, Bustin RM, van Cappellen P (1993) Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim Cosmochim Acta 57:303-316
Jaanus A, Andersson A, Olenina I, Toming K, Kaljurand K (2011) Changes in phytoplankton communities along a north–south gradient in the Baltic sea between 1990 and 2008. Boreal Environ Res A 16:191-208
Janssen F, Neumann T, Schmidt M (2004) Inter-annual variability in cyanobacteria blooms in the Baltic Sea controlled by wintertime hydrographic conditions. Mar Ecol Progr Ser 275:59-68
Jensen HS, Thamdrup B (1993) Iron-bound phosphorus in marine sediments as measured by bicarbonate-dithionite extraction. Hydrobiologia 253:47-59
Jilbert T, Slomp CP, Gustafsson BG, Boer W (2011) Beyond the Fe-P-redox connection: preferential regeneration of phosphorus from organic matter as a key control on Baltic Sea nutrient cycles. Biogeosciences 8:1699-1720
Jonsson P, Carman R (1994) Changes in deposition of organic matter and nutrients in the Baltic Sea during the twentieth century. Mar Pollut Bull 28:417-426
Jørgensen NOG, Tranvik LJ, Berg GM (1999) Occurrence and bacterial cycling of dissolved nitrogen in the Gulf of Riga, the Baltic Sea. Mar Ecol Progr Ser 191:1-18
Kahru M, Savchuk O, Elmgren R (2007) Satellite measurements of cyanobacterial bloom frequency in the Baltic Sea: interannual and spatial variability. Mar Ecol Progr Ser 343:15-23
Kangro K, Olli K, Tamminen T, Lignell R (2007) Species-specific responses of a cyanobacteria-dominated phytoplankton community to artificial nutrient limitation in the Baltic Sea. Mar Ecol Progr Ser 336:15-27
Kanoshina I, Lips U, Leppänen J-M (2003) The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2:29-41
Keeling RF, Piper SC, Bollenbacher AF, Walker JS (2008) Atmospheric CO2 records from sites in the SIO air sampling network. In Trends: A Compendium of Data on Global Change. US Department of Energy, Oak Ridge, Tennessee
Kononen K, Huttunen M, Kanoshina I, Laanemets J, Moisander P, Pavelson J (1999) Spatial and temporal variability of a dinoflagellate-cyanobacterium community under a complex hydrodynamical influence: a case study at the entrance to the Gulf of Finland. Mar Ecol Progr Ser 186:43-57
Kristensen E (1988) Benthic fauna and biogeochemical processes in marine sediments: Microbial activities and fluxes. In: Blackburn TH, Sørensen J (eds), Nitrogen cycling in coastal marine environments. John Wiley & Sons, p 275-299
Krom MD, Berner RA (1981) The diagenesis of phosphorus in a nearshore marine sediment. Geochim Cosmochim Acta 45:207-216
Kulinski K, Pempkowiak J (2011) The carbon budget of the Baltic Sea. Biogeosciences 8:3219-3230
Laamanen M, Kuosa H (2005) Annual variability of biomass and heterocysts of the N2-fixing cyanobacterium Aphanizomenon flos-aquae in the Baltic Sea with reference to Anabaena spp. and Nodularia spumigena. Boreal Environ Res 10:19-30
Larsson U, Elmgren R, Wulff F (1985) Eutrophication and the Baltic Sea: causes and consequences. Ambio 14:9-14
Larsson U, Hajdu S, Walve J, Elmgren R (2001) Baltic Sea nitrogen fixation estimated from the summer increase in upper mixed layer total nitrogen. Limnol Oceanogr 46:811-820
Larsson U, Nyberg S, Andreasson K, Lindahl O, Wikner J (2010) Växtplanktonproduktion- mätninger med problem. Havet 2010
Leipe T, Tauber F, Vallius H, Virtasalo J, Uścinowicz S, Kowalski N, Hille S, Lindgren S, Myllyvirta T (2011) Particulate organic carbon (POC) in surface sediments of the Baltic Sea. Geo Mar Lett 31:175-188
Leppäranta M, Myrberg K (2009) Physical Oceanography of the Baltic Sea. Praxis publishing, Chester, UK
Lilover MJ, Stips A (2008) The variability of parameters controlling the cyanobacteria bloom biomass in the Baltic Sea. J Marine Syst 74:S108-S115
Lips I, Lips U (2008) Abiotic factors influencing cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Hydrobiologia 614:133-140
Lips U, Lips I, Liblik T, Kikas V, Altoja K, Buhhalko N, Ruenk N (2011) Vertical dynamics of summer phytoplankton in a stratified estuary (Gulf of Finland, Baltic Sea). Ocean Dynam 61:903-915
Löffler A, Schneider B, Schmidt M, Nausch G (2011) Estimation of denitrification in Baltic Sea deep water from gas tension measurements. Mar Chem 125:91-100
Löffler A, Schneider B, Perttilä M, Rehder G (2012) Air–sea CO2 exchange in the Gulf of Bothnia, Baltic Sea. Cont Shelf Res C 37:46-56
Lukkari K, Leivuori M, Kotilainen A (2009) The chemical character and behaviour of phosphorus in poorly oxygenated sediments from open sea to organic-rich inner bay in the Baltic Sea. Biogeochemistry 96:25-48
Lund-Hansen LC, Pejrup M, Floderus S (2004). Pelagic and seabed fluxes of particulate matter and carbon, and C:N ratios resolved by sediment traps during a spring bloom, southwest Kattegat J Sea Res 52:87-98
Mackenzie FT, Ver LM, Sabine C, Lane M, Lerman A (1993) C, N, P, S global biogeochemical cycles and modeling of global change. In: Wollast R, Mackenzie FT, Chou L (eds), Interactions of C, N, P and S Biogeochemical Cycles and Global Change. NATO ASI Series 14, Springer, Berlin, p 1-61
Malmaeus JM, Karlsson OM (2012) Estimating the pool of mobile phosphorus in offshore soft sediments of the Baltic Proper. Air Soil Water Res 5:1-13
Matthäus W, Franck H (1992) Characteristics of major Baltic Inflows – a statistical analysis. Cont Shelf Res 12:1375-1400
Mazur-Marzec H, Krezel A, Kobos J, Plinski M (2006) Toxic Nodularia spumigena blooms in the coastal waters of the Gulf of Gdansk: a ten-year survey. Oceanologia 48:255-273
Meier HEM, Andersson HC, Eilola K, Gustafsson B, Kuznetsov I, Müller-Karulis B, Neumann T, Savchuk OP (2011) Hypoxia in future climates - a model ensemble study for the Baltic Sea. Geophys Res Lett 38:L24608. doi:10.1029/2011GL049929
Meier HEM, Müller-Karulis B, Anderson HC, Dietrich C, Eilola H, Gustafsson BG, Höglund A, Hordoir R, Kuznetsov I, Neumann T, Ranjbar Z, Savchuk OP, Schimanke S (2012) Impact of climate change on ecological quality indicators and biogeochemical fluxes in the Baltic Sea – a multi-model ensemble study. Ambio 41:558-573
Mort HP, Slomp CP, Gustafsson BG, Andersen TJ (2010) Phosphorus recycling and burial in Baltic Sea sediments with contrasting redox conditions. Geochim Cosmochim Acta 74:1350-1362
Mortimer CH (1941) The exchange of dissolved substances between mud and water in lakes. J Ecol 29:280-329
Mortimer CH (1971) Chemical exchanges between sediments and water in the Great Lakes – speculations on probable regulatory mechanisms. Limnol Oceanogr 16:387-404
Müller-Karulis B, Aigars J (2011) Modeling the long-term dynamics of nutrients and phytoplankton in the Gulf of Riga. J Marine Syst 87:161-176
Nausch M (1998) Alkaline phosphatase activities and the relationship to inorganic phosphate in the Pomeranian Bight (southern Baltic Sea). Aquat Microb Ecol 16:87-94
Nausch M, Nausch G (2007). Bioavailable dissolved organic phosphorus and phosphorus use by heterotrophic bacteria. Aquat Biol 1:151-160
Nausch M, Nausch G, Wasmund N (2004) Phosphorus dynamics during the transition from nitrogen to phosphate limitation in the central Baltic Sea. Mar Ecol Progr Ser 266:15-25
Nausch M, Nausch G, Wasmund N, Nagel K (2008) Phosphorus pool variations and their relation to cyanobacteria development in the Baltic Sea: A three-year study. J Marine Syst 71:99-111
Nausch M, Nausch G, Lass HU, Mohrholz V, Nagel K, Siegel H, Wasmund N (2009) Phosphorus input by upwelling in the eastern Gotland Basin (Baltic Sea) in summer and its effects on filamentous cyanobacteria. Estuar Coast Shelf Sci 83:434-442
Neftel A, Friedli H, Moor E, Lötscher H, Oeschger H, Siegenthaler U, Stauffer B (1994) Historical CO2 record from the Siple Station ice core. In Trends: A Compendium of Data on Global Change. US Department of Energy, Oak Ridge, Tennessee
Neumann T (2010) Climate-change effects on the Baltic Sea ecosystem: A model study. J Marine Syst 81:213-224
Neumann T, Schernewski G (2008) Eutrophication in the Baltic Sea and shifts in nitrogen fixation analyzed with a 3D ecosystem model. J Marine Syst 74:592-602
Neumann T, Fennel W, Kremp C (2002) Experimental simulations with an ecosystem model of the Baltic Sea: A nutrient load reduction experiment. Global Biogeochem Cy 16:7-1–7-19
Neumann T, Eilola K, Gustafsson B, Müller-Karulis B, Kuznetsov I, Meier HEM, Savchuk OP (2012) Extreme values of temperature, oxygen and blooms in the Baltic Sea in a changing climate. Ambio 41:576-585
Norkko J, Reed DC, Timmermann K, Norkko A, Gustafsson B, Bonsdorf E, Slomp CP, Carstensen J, Conley DJ (2011) A welcome can of worms? Hypoxia mitigation by an invasive species. Global Change Biol 18:422-434
Ohlendieck U, Gundersen K, Meyerhöfer M, Fritsche P, Nachtigall K, Bergmann B (2007) The significance of nitrogen fixation to new production during early summer in the Baltic Sea. Biogeosciences 4:63-73
Olli K (1999) Diel vertical migration of phytoplankton and heterotrophic flagellates in the Gulf of Riga. J Marine Syst 23:145-163
Omstedt, A, Edman M, Claremar B, Frodin P, Gustafsson E, Humborg C, Mörth M, Rutgersson A, Schurgers G, Smith B, Wällstedt T, Yurova A (2012) Future changes of the Baltic Sea acid-base (pH) and oxygen balances. Tellus B 64:19586. doi:10.3402/tellusb.v64i0.19586
Omstedt A, Edman M, Anderson LG, Laudon H (2010) Factors influencing the acid-base (pH) balance in the Baltic Sea: a sensitivity analysis. Tellus B 62:280-295
Osterroht C, Thomas H (2000) New production enhanced by nutrient supply from non-Redfield remineralisation of freshly produced organic material. J Marine Syst 25:33-46
Pitkänen H, Tamminen T (1995) Nitrogen and phosphorus as production limiting factors in the estuarine waters of the eastern Gulf of Finland. Mar Ecol Progr Ser 129:283-294
Raateoja M, Kuosa H, Hällfors S (2011) Fate of excess phosphorus in the Baltic Sea: A real driving force for cyanobacterial blooms? J Sea Res 65:315-321
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea-water. In: Hill MN (ed) The Sea: Ideas and Observations on Progress in the Study of the Seas. Vol 2Interscience Publishers, New York, p 26-77
Reissmann JH, Burchard H, Feistel R, Hagen E, Lass HU, Mohrholz V, Nausch G, Umlauf L, Wieczorek G (2009) Vertical mixing in the Baltic Sea and consequences for eutrophication – A review. Progr Oceanogr 82:47-80
Riemann L, Holmfeldt K, Titelman J (2008) Importance of viral lysis and dissolved DNA for bacterioplankton activity in a P-limited estuary, northern Baltic Sea. Microb Ecol 57:286-294
Rolff C, Elmgren R, Voss M (2008) Deposition of nitrogen and phosphorus on the Baltic Sea: seasonal patterns and nitrogen isotope composition. Biogeosciences 5:1657-1667
Rönner U, Sörensen F (1985) Denitrification rates in the low-oxygen waters of the stratified Baltic Proper. Appl Environ Microbiol 50:801-806
Ruoho-Airola T, Eilola K, Savchuk OP, Parviainen M, Tarvainen V (2012) Atmospheric nutrient input to the Baltic Sea from 1850 to 2006: A reconstruction from modeling results and historical data. Ambio 41:549-557
Ruttenberg KC, Berner RA (1993) Authigenic apatite formation and burial in sediments from non-upwelling, continental margin environments. Geochim Cosmochim Acta 57:991-1007
Ruttenberg KC, Goñi MA (1997) Depth trends in phosphorus distribution and C:N:P ratios of organic matter in Amazon Fan sediments: Indices of organic matter source and burial history. In: Flood RD, Piper DJW, Klaus A, Peterson LC (eds) Proceedings of the Ocean Drilling Program, Scientific Results, Vol 155
Rydberg L, Ærtebjerg G, Edler L (2006) Fifty years of primary production measurements in the Baltic entrance region, trends and variability in relation to land-based input of nutrients. J Sea Res 56:1-16
Sandberg J (2007) Cross-ecosystem analyses of pelagic food web structure and processes in the Baltic Sea. Ecol Model 201:243-261
Sandberg J, Andersson A, Johansson S, Wikner J (2004). Pelagic food web structure and carbon budget in the northern Baltic Sea: potential importance of terrigenous carbon. Mar Ecol Progr Ser 268:13-29
Savchuk OP (2010) Large-scale dynamics of hypoxia in the Baltic Sea. In: Yakushev E (ed) Chemical Structure of Pelagic Redox Interfaces: Observation and Modelling. Springer-Verlag, Berlin, Heidelberg
Savchuk OP, Wulff F (2007) Modeling the Baltic Sea eutrophication in a decision support system. Ambio 36:141-148
Savchuk OP, Wulff F, Hille S, Humborg C, Pollehne F (2008) The Baltic Sea a century ago - a reconstruction from model simulations, verified by observations. J Marine Syst 74:485-494
Savchuk OP, Rodriguez Medina M, Gustafsson BG (2012) Nutrient loads to the Baltic Sea, 1970–2006, Baltic Nest Institute Tech Rep 5, Stockholm, Sweden
Schernewski G, Neumann T (2005) The trophic state of the Baltic Sea a century ago: a model simulation study. J Marine Syst 53:109-124
Schinke H, Matthäus W (1998) On the causes of major Baltic inflows – an analysis of long time series. Cont Shelf Res 18:67-97
Schmale O, Beaubien SE, Rehder G, Greiner J, Lombardi S (2010) Gas seepage in the Dnepr paleo-delta area (NW-Black Sea) and its regional impact on the water column methane cycle. J Marine Syst 80:90-100
Schneider B (2011) The CO2 system of the Baltic Sea: Biogeochemical control and impact of anthropogenic CO2. In: Schernewski G, Hofstede J, Neumann T (eds) Global Change and Baltic Coastal Zones. Coastal Research Library 1, Springer Science + Business
Schneider B, Kuss J (2004) Past and present productivity of the Baltic Sea as inferred from pCO2 data. Cont Shelf Res 24:1611-1622
Schneider B, Nausch G, Nagel K, Wasmund N (2003) The surface water CO2 budget for the Baltic Proper: a new way to determine nitrogen fixation. J Marine Syst 42:53-64
Schneider B, Kaitala S, Maunula P (2006) Identification and quantification of plankton bloom events in the Baltic Sea by continuous pCO2 and chlorophyll a measurements on a cargo ship. J Marine Syst 59:238-248
Schneider B, Kaitala S, Raateoja M, Sadkowiak B (2009) A nitrogen fixation estimate for the Baltic Sea based on continuous pCO2 measurements on a cargo ship and total nitrogen data. Cont Shelf Res 29:1535-1540
Schneider B, Nausch G, Pohl C (2010) Mineralization of organic matter and nitrogen transformation in the Gotland Sea deep water. Mar Chem 119:153-161
Seppälä J, Tamminen T, Kaitala S (1999) Experimental evaluation of nutrient limitation of phytoplankton communities in the Gulf of Riga. J Marine Syst 23:107-126
Shaffer G (1987) Redfield ratios, primary production, and organic-carbon burial in the Baltic Sea. Deep Sea Res 34:769-784
Shaffer G, Rönner U (1984) Denitrification in the Baltic proper deep water. Deep Sea Res 31:197-220
Sohm JA, Webb EA, Capone DG (2011) Emerging patterns of marine nitrogen fixation. Nat Rev Microbiol 9:499-508
Stigebrandt A (1991) Computations of oxygen fluxes through the sea-surface and the net production of organic matter with application to the Baltic and adjacent seas. Limnol Oceanogr 36:444-454
Stigebrandt A, Wulff F (1987) A model for the dynamics of nutrients and oxygen in the Baltic Sea. J Mar Res 45:729-759
Stipa T (2004) The vernal bloom in heterogeneous convection: a numerical study of Baltic restratification. J Marine Syst 44:19-30
Struck U, Pollehne F, Bauerfeind E, Bodungen von B (2004) Sources of nitrogen for the vertical particle flux in the Gotland Sea (Baltic Proper) – results from sediment trap studies. J Marine Syst 45:91-101
Sundby B, Gobeil C, Silverberg N, Mucci A (1992) The phosphorus cycle in coastal marine sediments. Limnol Oceanogr 37:1129-1145
Suzumura M, Kamatani A (1995) Origin and distribution of inositol hexaphosphate in estuarine and coastal sediments. Limnol Oceanogr 40:1254-1261
Tamminen T, Andersen T (2007) Seasonal phytoplankton nutrient limitation patterns as revealed by bioassays over Baltic Sea gradients of salinity and eutrophication. Mar Ecol Progr Ser 340:121-138
Tamminen T, Seppälä J (1999) Nutrient pools, transformations, ratios, and limitation in the Gulf of Riga, the Baltic Sea, during four successional stages. J Marine Syst 23:83-106
BACC Author Team (2008) Assessment of Climate Change for the Baltic Sea Basin. Regional Climate Series, Springer-Verlag, Berline, Heidelberg
Thomas H, Schneider B (1999) The seasonal cycle of carbon dioxide in Baltic Sea surface waters. J Marine Syst 22:53-67
Ulfsbo A, Hulth S, Anderson L (2011) pH and biogeochemical processes in the Gotland Basin of the Baltic Sea. J Mar Chem 127:20-30
Vaalgamaa S, Conley DJ (2008) Detecting environmental change in estuaries: Nutrient and heavy-metal distribution in long sediment cores from the Gulf of Finland, Baltic Sea. Estuar Coast Shelf Sci 76:45-56
Vahtera E, Conley DJ, Gustafsson BG, Kuosa H, Pitkänen H, Savchuk OP, Tamminen T, Viitasalo M, Voss M, Wasmund N, Wulff F (2007) Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the Baltic Sea. Ambio 36:186-194
Van Cappellen P, Ingall ED (1994) Benthic phosphorus regeneration, net primary production, and ocean anoxia: A model of the coupled marine biogeochemical cycles of carbon and phosphorus. Paleoceanography 9:677-692
van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J-F, Masui T, Meinshausen M, Nakicenovic N, Smith SJ, Rose S K (2011) The representative concentration pathways: an overview. Clim Change 109:5-31
Virtasalo JJ, Leipe T, Moros M, Kotilainen AT (2011) Physicochemical and biological influences on sedimentary-fabric formation in a salinity and oxygen-restricted semi-enclosed sea: Gotland Deep, Baltic Sea. Sedimentology 58:352-375
Voss, M, Larsen, B, Leivuori, M, Vallius, H. (2000). Stable isotope signals of eutrophication in Baltic Sea sediments. J Marine Syst 25:287-298
Voss M, Emeis K-C, Hille S, Neumann T, Dippner JW (2005) The nitrogen cycle of the Baltic Sea from an isotopic perspective. Global Biogeochem Cy 19:GB3001. doi:10.1029/2004GB002338
Walve J, Larsson U (2007) Blooms of Baltic Sea Aphanizomenon sp. (Cyanobacteria) collapse after internal phosphorus depletion. Aquat Microb Ecol 49:57-69
Walve J, Larsson U (2010) Seasonal changes in Baltic Sea seston stoichiometry: the influence of diazotrophic cyanobacteria. Mar Ecol Progr Ser 407:13-25
Wasmund N, Siegel HB (2008) Phytoplankton. In: Feistel R, Nausch G, Wasmund N (eds). State and evolution of the Baltic Sea, 1952-2005: a detailed 50-year survey of meteorology and climate, physics, chemistry, biology, and marine environment, p 441-481
Wasmund N, Nausch G, Matthäus W (1998) Phytoplankton spring blooms in the southern Baltic Sea – spatio-temporal development and long-term trends. J Plankton Res 20:1099-1117
Wasmund N, Andrushaitis A, Lysiak-Pastuszak E, Müller-Karulis B, Nausch G, Neumann T, Ojaveer H, Olenina I, Postel L, Witek Z (2001) Trophic status of the south-eastern Baltic Sea: a comparison of coastal and open areas. Estuar Coast Shelf Sci 53:849-864
Wasmund N, Nausch G, Schneider B (2005) Primary production rates calculated by different concepts – an opportunity to study the complex production system in the Baltic Proper. J Sea Res 54:244-255
Wasmund N, Busch S, Gromisz S, Hajdu S, Höglander H, Jaanus A, Johansen M, Jurgensone I, Karlsson C, Knownacka J, Krasniewski W, Olenina I (2011) Cyanobacteria biomass indicator. HELCOM Baltic Sea Environment Fact Sheets 2011 www.helcom.fi/BSAP_assessment/ifs/ifs2011/en_GB/CyanobacteriaBiomass/, viewed Apr 29, 2013
Wesslander K, Omstedt A, Schneider B (2010) Interannual and seasonal variations in the air-sea CO2 balancein the central Baltic Sea and the Kattegat. Cont Shelf Res 30:1511-1521
Winterhalter B, Flodén T, Ignatius H, Axberg S, Niemistö L (1981) Geology of the Baltic Sea. In: Voipio A (ed) The Baltic Sea. Elsevier Oceanograpthy Series, 30, p 1-121
Wulff F, Rahm L, Larsson P (eds) (2001) A Systems Analysis of the Baltic Sea. Ecological Studies 148, Springer, Berlin
Yurkovskis A (2004) Long-term land-based and internal forcing of the nutrient state of the Gulf of Riga (Baltic Sea). J Marine Syst 50:181-197
Zillén L, Conley DJ (2010) Hypoxia and cyanobacteria blooms - are they really natural features of the late Holocene history of the Baltic Sea? Biogeosciences 7:2567-2580
Zillén L, Conley DJ, Andrén T, Andrén E, Björck S (2008) Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth Sci Rev 91:77-92
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Schneider, B., Eilola, K., Lukkari, K., Muller-Karulis, B., Neumann, T. (2015). Environmental Impacts—Marine Biogeochemistry. In: The BACC II Author Team, . (eds) Second Assessment of Climate Change for the Baltic Sea Basin. Regional Climate Studies. Springer, Cham. https://doi.org/10.1007/978-3-319-16006-1_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-16006-1_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16005-4
Online ISBN: 978-3-319-16006-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)