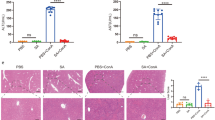
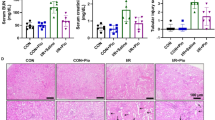
Abstract
Ischemia–reperfusion (IR) injury is one of the main causes of acute kidney disease (AKI). Several studies have shown that mitochondrial damage, which leads to increased production of reactive oxygen species (ROS), plays a vital role in the pathogenesis of IR-induced AKI. Increased ROS production can cause oxidative damage and activate the inflammasome in renal tubular cells, ultimately resulting in apoptosis or necrosis. Mitophagy is a type of selective autophagy that plays a protective role in AKI by regulating the quality of mitochondria and reducing the production of ROS. We previously reported that the augmenter of liver regeneration (ALR) exhibits antiapoptotic and antioxidant functions, although the precise mechanisms of action need to be studied further. In the current study, ALR was overexpressed and an in vitro model of IR injury was constructed. The overexpression of ALR reduced the production of mitochondria-derived ROS (mtROS), the activation of the NLRP3 inflammasome, and the rate of apoptosis. Moreover, this suppression of mtROS production and inflammasome activation was mediated through the PTEN-induced kinase 1 (PINK1)/Parkin pathway of mitophagy. These results suggest that ALR can alleviate IR-induced apoptosis via the PINK1/Parkin mitophagy pathway to reduce the production of mtROS and limit the activation of the NLRP3 inflammasome.






Similar content being viewed by others
Data availability
All datasets generated for this study are included in the article.
References
Mehta RL, Burdmann EA, Cerdá J, Feehally J, Finkelstein F, García-García G, Godin M, Jha V, Lameire NH, Levin NW, Lewington A, Lombardi R, Macedo E, Rocco M, Aronoff-Spencer E, Tonelli M, Zhang J, Remuzzi G (2016) Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 387(10032):2017–2025. https://doi.org/10.1016/S0140-6736(16)30240-9
Malek M, Nematbakhsh M (2015) Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev 4(2):20–27. https://doi.org/10.12861/jrip.2015.06
Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant E, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell R (2006) Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24(3):317–327. https://doi.org/10.1016/j.immuni.2006.02.004
Kim HJ, Lee DW, Ravichandran K, Keys DO, Akcay A, Nguyen Q, He ZB, Jani A, Ljubanovic D, Edelstein CL (2013) NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther 346(3):465–472. https://doi.org/10.1124/jpet.113.205732
Tang CY, Han HL, Yan MJ, Zhu SY, Liu J, Liu ZW, He LY, Tan JQ, Liu Y, Liu H, Sun L, Duan SB, Peng YM, Liu FY, Yin XM, Zhang ZH, Dong Z (2018) PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14(5):880–897. https://doi.org/10.1080/15548627.2017.1405880
Tatsuta T, Langer T (2008) Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 27(2):306–314. https://doi.org/10.1038/sj.emboj.7601972
Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y (2013) Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol 305(4):F495–F509. https://doi.org/10.1152/ajprenal.00642.2012
Hall AM, Schuh CD (2016) Mitochondria as therapeutic targets in acute kidney injury. Curr Opin Nephrol Hypertens 25(4):355–362. https://doi.org/10.1097/MNH.0000000000000228
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Turco DD, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi T, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburge G, Wood NW (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304(5674):1158–1160. https://doi.org/10.1126/science.1096284
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1):31–42. https://doi.org/10.1038/cdd.2012.81
Zhao C, Chen Z, Xu X, An X, Duan S, Huang Z, Zhang C, Wu L, Zhang B, Zhang A, Xing C, Yuan Y (2017) Pink1/Parkin-mediated mitophagy play a protective role in cisplatin induced renal tubular epithelial cells injury. Exp Cell Res 350(2):390–397. https://doi.org/10.1016/j.yexcr.2016.12.015
Wang Y, Tang C, Cai J, Chen G, Zhang D, Zhang Z, Dong Z (2018) PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis 9(11):1113. https://doi.org/10.1038/s41419-018-1152-2
Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin H, Zhang Z, Shen J, Zhou Y, Zhou W, Gu L, Lu R, Ni Z (2019) PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol 26:101254. https://doi.org/10.1016/j.redox.2019.101254
LaBrecque DR, Pesch LA (1975) Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol 248(2):273–284. https://doi.org/10.1113/jphysiol.1975.sp010973
Tury A, Mairet-Coello G, Lisowsky T, Griffond B, Fellmann D (2005) Expression of the sulfhydryl oxidase ALR (augmenter of liver regeneration) in adult rat brain. Brain Res 1048(1–2):87–97. https://doi.org/10.1016/j.brainres.2005.04.050
Liao XH, Zhang L, Liu Q, Sun H, Peng CM, Guo H (2010) Augmenter of liver regeneration protects kidneys from ischaemia/reperfusion injury in rats. Nephrol Dial Transplant 25(9):2921–2929. https://doi.org/10.1093/ndt/gfq151
Gandhi CR (2012) Augmenter of liver regeneration. Fibrogenes Tissue Repair 5(1):10. https://doi.org/10.1186/1755-1536-5-10
Weng J, Li W, Jia X, An W (2017) Alleviation of ischemia–reperfusion injury in liver steatosis by augmenter of liver regeneration is attributed to antioxidation and preservation of mitochondria. Transplantation 101(10):2340–2348. https://doi.org/10.1097/TP.0000000000001874
Gandhi CR, Chaillet JR, Nalesnik MA, Kumar S, Dangi A, Demetris AJ, Ferrell R, Wu T, Divanovic S, Stankeiwicz T, Shaffer B, Stolz DB, Harvey SA, Wang J, Starzl TE (2015) Liver-specific deletion of augmenter of liver regeneration accelerates development of steatohepatitis and hepatocellular carcinoma in mice. Gastroenterology 148(2):379–391. https://doi.org/10.1053/j.gastro.2014.10.008
Xia N, Yan RY, Liu Q, Liao XH, Sun H, Guo H, Zhang L (2015) Augmenter of liver regeneration plays a protective role against hydrogen peroxide-induced oxidative stress in renal proximal tubule cells. Apoptosis Int J Progr Cell Death 20(4):423–432. https://doi.org/10.1007/s10495-015-1096-2
Huang LL, Long RT, Jiang GP, Jiang X, Sun H, Guo H, Liao XH (2018) Augmenter of liver regeneration promotes mitochondrial biogenesis in renal ischemia-reperfusion injury. Apoptosis 23(11–12):695–706. https://doi.org/10.1007/s10495-018-1487-2
Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA (1992) Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci 103(Pt 3):857–862. https://doi.org/10.1242/jcs.103.3.857
Zhang L, Wang D, Yang K, Sheng D, Tan B, Wang Z, Ran H, Yi H, Zhong Y, Lin H, Chen Y (2018) Mitochondria-targeted artificial “nano-RBCs” for amplified synergistic cancer phototherapy by a single NIR irradiation. Adv Sci (Weinh) 5(8):1800049. https://doi.org/10.1002/advs.201800049
Lange H, Lisowsky T, Gerber J, Mühlenhoff U, Kispal G, Lill R (2001) An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep 2(8):715–720. https://doi.org/10.1093/embo-reports/kve161
Wu CK, Dailey TA, Dailey HA, Wang BC, Rose JP (2003) The crystal structure of augmenter of liver regeneration: a mammalian FAD-dependent sulfhydryl oxidase. Protein Sci 12(5):1109–1118. https://doi.org/10.1110/ps.0238103
Chi W, Hua X, Chen X, Bian F, Yuan X, Zhang L, Wang X, Chen D, Deng R, Li Z, Liu Y, Paiva CS, Pflugfelder SC, Li DQ (2017) Mitochondrial DNA oxidation induces imbalanced activity of NLRP3/NLRP6 inflammasomes by activation of caspase-8 and BRCC36 in dry eye. J Autoimmun 80:65–76. https://doi.org/10.1016/j.jaut.2017.02.006
Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, Wong J, Ding J, Seki E, Schnabl B, Hevener AL, Greenberg HB, Kisseleva T, Karin M (2018) New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560(7717):198–203. https://doi.org/10.1038/s41586-018-0372-z
Zinchuk V, Grossenbacher-Zinchuk O (2011) Quantitative colocalization analysis of confocal fluorescence microscopy images. Curr Protoc Cell Biol. https://doi.org/10.1002/0471143030.cb0419s52
Hirota Y, Yamashita SI, Kurihara Y, Jin X, Aihara M, Saigusa T, Kang D, Kanki T (2015) Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy 11(2):332–343. https://doi.org/10.1080/15548627.2015.1023047
Korkmaz A, Kolankaya D (2013) Inhibiting inducible nitric oxide synthase with rutin reduces renal ischemia/reperfusion injury. Can J Surg 56(1):6–14. https://doi.org/10.1503/cjs.004811
Cho DH, Nakamura T, Lipton SA (2010) Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci 67(20):3435–3447. https://doi.org/10.1007/s00018-010-0435-2
Xiao X, Hu Y, Quiros PM, Wei Q, Lopez-Otin C, Dong Z (2014) OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am J Physiol Renal Physiol 306(11):F1318–F1326. https://doi.org/10.1152/ajprenal.00036.2014
He L, Livingston MJ, Dong Z (2014) Autophagy in acute kidney injury and repair. Nephron Clin Pract 127(1–4):56–60. https://doi.org/10.1159/000363677
Sztolsztener ME, Brewinska A, Guiard B, Chacinska A (2013) Disulfide bond formation: sulfhydryl oxidase ALR controls mitochondrial biogenesis of human MIA40. Traffic 14(3):309–320. https://doi.org/10.1111/tra.12030
Boussabbeh M, Ben Salem I, Prola A, Guilbert A, Bacha H, Abid-Essefi S, Lemaire C (2015) Patulin induces apoptosis through ROS-mediated endoplasmic reticulum stress pathway. Toxicol Sci 144(2):328–337. https://doi.org/10.1093/toxsci/kfu319
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. https://doi.org/10.1038/nature09663
Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, He F, Boassa D, Perkins G, Ali SR, McGeough MD, Ellisman MH, Seki E, Gustafsson AB, Hoffman HM, Diaz-Meco MT, Moscat J, Karin M (2016) NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164(5):896–910. https://doi.org/10.1016/j.cell.2015.12.057
Kang R, Zeng L, Xie Y, Yan Z, Zhou B, Cao L, Klionsky DJ, Tracey KJ, Li J, Wang H, Billiar TR, Jiang J, Tang D (2016) A novel PINK1- and PARK2-dependent protective neuroimmune pathway in lethal sepsis. Autophagy 12(12):2374–2385. https://doi.org/10.1080/15548627.2016.1239678
Kim MJ, Bae SH, Ryu JC, Kwon Y, Oh JH, Kwon J, Moon JS, Kim K, Miyawaki A, Lee MG, Shin J, Kim YS, Kim CH, Ryter SW, Choi AMK, Rhee SG, Ryu JH, Yoon JH (2016) SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 12(8):1272–1291. https://doi.org/10.1080/15548627.2016.1183081
Li H, Miao W, Ma J, Xv Z, Bo H, Li J, Zhang JLL (2016) Acute exercise-induced mitochondrial stress triggers an inflammatory response in the myocardium via NLRP3 inflammasome activation with mitophagy. Oxid Med Cell Longev 2016:1987149. https://doi.org/10.1155/2016/1987149
Chen K, Feng L, Hu W, Chen J, Wang X, Wang L, He Y (2019) Optineurin inhibits NLRP3 inflammasome activation by enhancing mitophagy of renal tubular cells in diabetic nephropathy. FASEB J 33(3):4571–4585. https://doi.org/10.1096/fj.201801749RRR
Xu Y, Wang J, Xu W, Ding F, Ding W (2019) Prohibitin 2-mediated mitophagy attenuates renal tubular epithelial cells injury by regulating mitochondrial dysfunction and NLRP3 inflammasome activation. Am J Physiol Renal Physiol 316(2):F396–F407. https://doi.org/10.1152/ajprenal.00420.2018
Chen L, Guo Y, Qu S, Li K, Yang T, Yang Y, Zheng Z, Liu H, Wang X, Deng S, Zhang Y, Zhu X, Li Y (2021) The protective effects of Shengmai formula against myocardial injury induced by ultrafine particulate matter exposure and myocardial ischemia are mediated by the PI3K/AKT/p38 MAPK/Nrf2 pathway. Front Pharmacol 12:619311. https://doi.org/10.3389/fphar.2021.619311
Dagda RK, Zhu J, Kulich SM, Chu CT (2008) Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson’s disease. Autophagy 4(6):770–782. https://doi.org/10.4161/auto.6458
Acknowledgements
The authors would like to thank all the reviewers who participated in the review, as well as Medjaden (http://www.medjaden.com) for providing English editing services during the preparation of this manuscript.
Funding
This study has no funding support.
Author information
Authors and Affiliations
Contributions
DZ performed the experiments and wrote the manuscript. JZ analyzed the confocal laser-scanning microscopy experiments. XG revised and corrected the manuscript. XW designed the study and helped to analyze and interpret the data. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, D., Zhong, J., Gong, X. et al. Augmenter of liver regeneration reduces mitochondria-derived ROS and NLRP3 inflammasome activation through PINK1/Parkin-mediated mitophagy in ischemia-reperfusion-induced renal tubular injury. Apoptosis 28, 335–347 (2023). https://doi.org/10.1007/s10495-022-01794-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01794-1