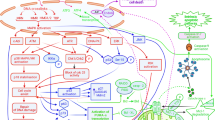
Abstract
Chemoresistance of cancer cells is a major problem in treating cancer. Knowledge of how cancer cells may die or resist cancer drugs is critical to providing certain strategies to overcome tumour resistance to treatment. Paclitaxel is known as a chemotherapy drug that can suppress the proliferation of cancer cells by inducing cell cycle arrest and induction of mitotic catastrophe. However, today, it is well known that paclitaxel can induce multiple kinds of cell death in cancers. Besides the induction of mitotic catastrophe that occurs during mitosis, paclitaxel has been shown to induce the expression of several pro-apoptosis mediators. It also can modulate the activity of anti-apoptosis mediators. However, certain cell-killing mechanisms such as senescence and autophagy can increase resistance to paclitaxel. This review focuses on the mechanisms of cell death, including apoptosis, mitotic catastrophe, senescence, autophagic cell death, pyroptosis, etc., following paclitaxel treatment. In addition, mechanisms of resistance to cell death due to exposure to paclitaxel and the use of combinations to overcome drug resistance will be discussed.





Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V (2017) Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med 6(12):2115–2125
Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M et al (2005) EGFR mutation and resistance of non–small-cell lung cancer to gefitinib. N Engl J Med 352(8):786–792
Shah MA, Schwartz GK (2001) Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res 7(8):2168–2181
Luqmani Y (2005) Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract 14(Suppl. 1):35–48
Wang H, Feng Z, Wang Y, Zhou R, Yang Z, Xu B (2016) Integrating enzymatic self-assembly and mitochondria targeting for selectively killing cancer cells without acquired drug resistance. J Am Chem Soc 138(49):16046–16055
Mortezaee K, Najafi M (2021) Immune system in cancer radiotherapy: resistance mechanisms and therapy perspectives. Crit Rev Oncol Hematol 157:103180
Fu X, Li M, Tang C, Huang Z, Najafi M (2021) Targeting of cancer cell death mechanisms by resveratrol: a review. Apoptosis 26(11):561–573. https://doi.org/10.1007/s10495-021-01689-7
Yu C, Yang B, Najafi M (2021) Targeting of cancer cell death mechanisms by curcumin: implications to cancer therapy. Basic Clin Pharmacol Toxicol 129(6):397–415. https://doi.org/10.1111/bcpt.13648
Hilska M, Collan YU, Laine VJO, Kössi J, Hirsimäki P, Laato M et al (2005) The significance of tumor markers for proliferation and apoptosis in predicting survival in colorectal cancer. Dis Colon Rectum 48(12):2197–2208
Aaltomaa S, Kärjä V, Lipponen P, Isotalo T, Kankkunen J, Talja M et al (2006) Expression of Ki-67, cyclin D1 and apoptosis markers correlated with survival in prostate cancer patients treated by radical prostatectomy. Anticancer Res 26(6C):4873–4878
Fu D, Lu C, Qu X, Li P, Chen K, Shan L et al (2019) LncRNA TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the miR-134-5p/MBTD1 axis. Aging (Albany NY) 11(19):8374
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV et al (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19(1):107–120. https://doi.org/10.1038/cdd.2011.96
Ramirez JAZ, Romagnoli GG, Kaneno R (2021) Inhibiting autophagy to prevent drug resistance and improve anti-tumor therapy. Life Sci 265:118745
Reyes-Castellanos G, Abdel Hadi N, Carrier A (2022) Autophagy contributes to metabolic reprogramming and therapeutic resistance in pancreatic tumors. Cells 11(3):426
Usman RM, Razzaq F, Akbar A, Farooqui AA, Iftikhar A, Latif A et al (2021) Role and mechanism of autophagy-regulating factors in tumorigenesis and drug resistance. Asia Pac J Clin Oncol 17(3):193–208
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G et al (2020) ROS in cancer therapy: the bright side of the moon. Exp Mol Med 52(2):192–203
Calaf GM, Ponce-Cusi R, Carrión F (2018) Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol Rep 40(4):2381–2388
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93(9):2325–2327
Li D, Fu D, Zhang Y, Ma X, Gao L, Wang X et al (2017) Isolation, purification, and identification of taxol and related taxanes from taxol-producing fungus Aspergillus niger subsp. taxi. J Microbiol Biotechnol 27(8):1379–1385
Menzin AW, King SA, Aikins JK, Mikuta JJ, Rubin SC (1994) Taxol (paclitaxel) was approved by FDA for the treatment of patients with recurrent ovarian cancer. Gynecol Oncol 54(1):103
Cortazar P, Justice R, Johnson J, Sridhara R, Keegan P, Pazdur R (2012) US Food and Drug Administration approval overview in metastatic breast cancer. J Clin Oncol 30(14):1705–1711. https://doi.org/10.1200/JCO.2011.39.2613
De Luca R, Profita G, Cicero G (2019) Nab-paclitaxel in pretreated metastatic breast cancer: evaluation of activity, safety, and quality of life. Onco Targets Ther 12:1621
Kelly WK, Curley T, Slovin S, Heller G, McCaffrey J, Bajorin D et al (2001) Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol 19(1):44–53
Ramalingam S, Belani CP (2004) Paclitaxel for non-small cell lung cancer. Expert Opin Pharmacother 5(8):1771–1780
Park DC, Kim JH, Lew YO, Kim DH, Namkoong SE (2004) Phase II trial of neoadjuvant paclitaxel and cisplatin in uterine cervical cancer. Gynecol Oncol 92(1):59–63
Elstad NL, Fowers KD (2009) OncoGel (ReGel/paclitaxel)—clinical applications for a novel paclitaxel delivery system. Adv Drug Deliv Rev 61(10):785–794
Foland TB, Dentler WL, Suprenant KA, Gupta ML Jr, Himes RH (2005) Paclitaxel-induced microtubule stabilization causes mitotic block and apoptotic-like cell death in a paclitaxel-sensitive strain of Saccharomyces cerevisiae. Yeast (Chichester, England) 22(12):971–978. https://doi.org/10.1002/yea.1284
Priyadarshini K, Keerthi AU (2012) Paclitaxel against cancer: a short review. Med Chem 2(7):139–141
Peltier S, Oger JM, Lagarce F, Couet W, Benoît JP (2006) Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res 23(6):1243–1250. https://doi.org/10.1007/s11095-006-0022-2
Lacoeuille F, Hindre F, Moal F, Roux J, Passirani C, Couturier O et al (2007) In vivo evaluation of lipid nanocapsules as a promising colloidal carrier for paclitaxel. Int J Pharm 344(1–2):143–149. https://doi.org/10.1016/j.ijpharm.2007.06.014
Torne SJ, Ansari KA, Vavia PR, Trotta F, Cavalli R (2010) Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv 17(6):419–425. https://doi.org/10.3109/10717541003777233
Yang FH, Zhang Q, Liang QY, Wang SQ, Zhao BX, Wang YT et al (2015) Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: paclitaxel-loaded glycyrrhizic acid micelles. Molecules 20(3):4337–4356. https://doi.org/10.3390/molecules20034337
Yang T, Feng J, Zhang Q, Wu W, Mo H, Huang L et al (2020) l-Carnitine conjugated chitosan-stearic acid polymeric micelles for improving the oral bioavailability of paclitaxel. Drug Deliv 27(1):575–584. https://doi.org/10.1080/10717544.2020.1748762
Chowdhury N, Singh M (2020) Current development of oral taxane formulations: a review. Crit Rev Ther Drug Carrier Syst 37(3):205–227. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2020029699
Ma P, Mumper RJ (2013) Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol 4(2):1000164. https://doi.org/10.4172/2157-7439.1000164
Foote M (2007) Using nanotechnology to improve the characteristics of antineoplastic drugs: improved characteristics of nab-paclitaxel compared with solvent-based paclitaxel. Biotechnol Annu Rev 13:345–357. https://doi.org/10.1016/s1387-2656(07)13012-x
Lee S, Margolin K (2011) Cytokines in cancer immunotherapy. Cancers (Basel) 3(4):3856–3893
Borst J, Ahrends T, Bąbała N, Melief CJ, Kastenmüller W (2018) CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 18(10):635–647
Mirzaei S, Mohammadi AT, Gholami MH, Hashemi F, Zarrabi A, Zabolian A et al (2021) Nrf2 signaling pathway in cisplatin chemotherapy: potential involvement in organ protection and chemoresistance. Pharmacol Res 167:105575. https://doi.org/10.1016/j.phrs.2021.105575
Najafi M, Mortezaee K, Rahimifard M, Farhood B, Haghi-Aminjan H (2020) The role of curcumin/curcuminoids during gastric cancer chemotherapy: a systematic review of non-clinical study. Life Sci 257:118051. https://doi.org/10.1016/j.lfs.2020.118051
Mortezaee K, Narmani A, Salehi M, Bagheri H, Farhood B, Haghi-Aminjan H et al (2021) Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci 269:119020. https://doi.org/10.1016/j.lfs.2021.119020
Farhood B, Mortezaee K, Haghi-Aminjan H, Khanlarkhani N, Salehi E, Nashtaei MS et al (2019) A systematic review of radiation-induced testicular toxicities following radiotherapy for prostate cancer. J Cell Physiol 234(9):14828–14837. https://doi.org/10.1002/jcp.28283
Farhood B, Ashrafizadeh M, Khodamoradi E, Hoseini-Ghahfarokhi M, Afrashi S, Musa AE et al (2020) Targeting of cellular redox metabolism for mitigation of radiation injury. Life Sci 250:117570. https://doi.org/10.1016/j.lfs.2020.117570
Wu Q, Allouch A, Martins I, Brenner C, Modjtahedi N, Deutsch E et al (2017) Modulating both tumor cell death and innate immunity is essential for improving radiation therapy effectiveness. Front Immunol 8:613
Wang T-H, Wang H-S, Soong Y-K (2000) Paclitaxel-induced cell death. Cancer 88(11):2619–2628. https://doi.org/10.1002/1097-0142(20000601)88:11%3c2619::AID-CNCR26%3e3.0.CO;2-J
Choi KH, Jeon JY, Lee Y-E, Kim SW, Kim SY, Yun YJ et al (2019) Synergistic activity of paclitaxel, sorafenib, and radiation therapy in advanced renal cell carcinoma and breast cancer. Transl Oncol 12(2):381–388
Fan W (1999) Possible mechanisms of paclitaxel-induced apoptosis. Biochem Pharmacol 57(11):1215–1221
Nawara HM, Afify SM, Hassan G, Zahra MH, Seno A, Seno M (2021) Paclitaxel-based chemotherapy targeting cancer stem cells from mono-to combination therapy. Biomedicines 9(5):500
Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM (2009) Paclitaxel and immune system. Eur J Pharm Sci 38(4):283–290
Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P (2016) Harnessing the immune system to improve cancer therapy. Ann Transl Med 4(14):261
Mullins DW, Burger CJ, Elgert KD (1999) Paclitaxel enhances macrophage IL-12 production in tumor-bearing hosts through nitric oxide. J Immunol 162(11):6811–6818
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, Gabaldon TA et al (2020) Localized interleukin-12 for cancer immunotherapy. Front Immunol. https://doi.org/10.3389/fimmu.2020.575597
Vicari AP, Luu R, Zhang N, Patel S, Makinen SR, Hanson DC et al (2009) Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother 58(4):615–628. https://doi.org/10.1007/s00262-008-0586-2
Shen J, Chen C, Li Z, Hu S (2020) Paclitaxel promotes tumor-infiltrating macrophages in breast cancer. Technol Cancer Res Treat. https://doi.org/10.1177/1533033820945821
Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA et al (2018) Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res 78(20):5891–5900. https://doi.org/10.1158/0008-5472.can-17-3480
Feng R, Morine Y, Ikemoto T, Imura S, Iwahashi S, Saito Y et al (2018) Nab-paclitaxel interrupts cancer-stromal interaction through C-X-C motif chemokine 10-mediated interleukin-6 downregulation in vitro. Cancer Sci 109(8):2509–2519. https://doi.org/10.1111/cas.13694
Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L et al (2008) Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 15(1):3–12
Lei X, Lei Y, Li J-K, Du W-X, Li R-G, Yang J et al (2020) Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett 470:126–133
Jafarzadeh E, Montazeri V, Aliebrahimi S, Sezavar AH, Ghahremani MH, Ostad SN (2022) Combined regimens of cisplatin and metformin in cancer therapy: a systematic review and meta-analysis. Life Sci. https://doi.org/10.1016/j.lfs.2022.120680
Moslehi M, Moazamiyanfar R, Dakkali MS, Rezaei S, Rastegar-Pouyani N, Jafarzadeh E et al (2022) Modulation of the immune system by melatonin; implications for cancer therapy. Int Immunopharmacol 108:108890
Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L (2021) The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Front Oncol. https://doi.org/10.3389/fonc.2021.610303
Muraro E, Comaro E, Talamini R, Turchet E, Miolo G, Scalone S et al (2015) Improved Natural Killer cell activity and retained anti-tumor CD8+ T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J Transl Med 13(1):204. https://doi.org/10.1186/s12967-015-0567-0
Bhola N, Arteaga C (2011) PD08-04: inhibition of the TGFb/TGFbR2 pathway prevents enrichment of drug-resistant breast cancer stem cells by anti-cancer chemotherapy. Cancer Res 71(24_Supplement):PD08-4-PD-4. https://doi.org/10.1158/0008-5472.SABCS11-PD08-04
Park SY, Kim MJ, Park SA, Kim JS, Min KN, Kim DK et al (2015) Combinatorial TGF-β attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget 6(35):37526–37543. https://doi.org/10.18632/oncotarget.6063
Zhang R, Wei Y-H, Zhao C-Y, Song H-Y, Shen N, Cui X et al (2018) EDIL3 depletion suppress epithelial-mesenchymal transition of lens epithelial cells via transforming growth factor β pathway. Int J Ophthalmol 11(1):18
Jeong D, Ban S, Oh S, Jin Lee S, Yong Park S, Koh YW (2017) Prognostic significance of EDIL3 expression and correlation with mesenchymal phenotype and microvessel density in lung adenocarcinoma. Sci Rep 7(1):8649. https://doi.org/10.1038/s41598-017-08851-9
Jiang SH, Wang Y, Yang JY, Li J, Feng MX, Wang YH et al (2016) Overexpressed EDIL3 predicts poor prognosis and promotes anchorage-independent tumor growth in human pancreatic cancer. Oncotarget 7(4):4226–4240. https://doi.org/10.18632/oncotarget.6772
Xia H, Chen J, Shi M, Gao H, Sekar K, Seshachalam VP et al (2015) EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol 63(4):863–873. https://doi.org/10.1016/j.jhep.2015.05.005
Gasca J, Flores ML, Jiménez-Guerrero R, Sáez ME, Barragán I, Ruíz-Borrego M et al (2020) EDIL3 promotes epithelial–mesenchymal transition and paclitaxel resistance through its interaction with integrin αVβ3 in cancer cells. Cell Death Discov 6(1):86. https://doi.org/10.1038/s41420-020-00322-x
Xu J-H, Hu S-L, Shen G-D, Shen G (2016) Tumor suppressor genes and their underlying interactions in paclitaxel resistance in cancer therapy. Cancer Cell Int 16(1):1–10
Chabalier C, Lamare C, Racca C, Privat M, Valette A, Larminat F (2006) BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle 5(9):1001–1007
Sung M, Giannakakou P (2014) BRCA1 regulates microtubule dynamics and taxane-induced apoptotic cell signaling. Oncogene 33(11):1418–1428
Gilmore PM, McCabe N, Quinn JE, Kennedy RD, Gorski JJ, Andrews HN et al (2004) BRCA1 interacts with and is required for paclitaxel-induced activation of mitogen-activated protein kinase kinase kinase 3. Cancer Res 64(12):4148–4154
Li J, Zhang Y, Zhao J, Kong F, Chen Y (2011) Overexpression of miR-22 reverses paclitaxel-induced chemoresistance through activation of PTEN signaling in p53-mutated colon cancer cells. Mol Cell Biochem 357(1):31–38. https://doi.org/10.1007/s11010-011-0872-8
Wang S-Q, Wang C, Chang L-M, Zhou K-R, Wang J-W, Ke Y et al (2016) Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget 7(45):72990–73002. https://doi.org/10.18632/oncotarget.12166
Liu Q, Sui R, Li R, Miao J, Liu J (2015) Biological characteristics of Taxol-resistant ovarian cancer cells and reversal of Taxol resistance by adenovirus expressing p53. Mol Med Rep 11(2):1292–1297
Guntur VP, Waldrep JC, Guo JJ, Selting K, Dhand R (2010) Increasing p53 protein sensitizes non-small cell lung cancer to paclitaxel and cisplatin in vitro. Anticancer Res 30(9):3557–3564
Vikhanskaya F, Vignati S, Beccaglia P, Ottoboni C, Russo P, D’Incalci M et al (1998) Inactivation of p53 in a human ovarian cancer cell line increases the sensitivity to paclitaxel by inducing G2/M arrest and apoptosis. Exp Cell Res 241(1):96–101
Debernardis D, Siré EG, De Feudis P, Vikhanskaya F, Valenti M, Russo P et al (1997) p53 status does not affect sensitivity of human ovarian cancer cell lines to paclitaxel. Cancer Res 57(5):870–874
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z et al (2020) PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis 11(9):1–12
Hutti JE, Pfefferle AD, Russell SC, Sircar M, Perou CM, Baldwin AS (2012) Oncogenic PI3K mutations lead to NF-κB-dependent cytokine expression following growth factor deprivation. Cancer Res 72(13):3260–3269. https://doi.org/10.1158/0008-5472.CAN-11-4141
Spangle JM, Roberts TM, Zhao JJ (2017) The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer 1868(1):123–131. https://doi.org/10.1016/j.bbcan.2017.03.002
Butler DE, Marlein C, Walker HF, Frame FM, Mann VM, Simms MS et al (2017) Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget 8(34):56698–56713. https://doi.org/10.18632/oncotarget.18082
Gu L, Zhu N, Findley HW, Zhou M (2004) Loss of PTEN expression induces NF-kB via PI3K/Akt pathway involving resistance to chemotherapy in acute lymphoblastic leukemia cell lines. Blood 104(11):4438
Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR (2019) Mechanisms of PTEN loss in cancer: it’s all about diversity. Semin Cancer Biol 59:66–79. https://doi.org/10.1016/j.semcancer.2019.02.001
Du F, Wu X, Liu Y, Wang T, Qi X, Mao Y et al (2013) Acquisition of paclitaxel resistance via PI3K-dependent epithelial-mesenchymal transition in A2780 human ovarian cancer cells. Oncol Rep 30(3):1113–1118. https://doi.org/10.3892/or.2013.2567
Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li Z et al (2018) Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis 9(2):1–11
Papadopoulos EI, Scorilas A (2015) Cisplatin and paclitaxel alter the expression pattern of miR-143/145 and miR-183/96/182 clusters in T24 bladder cancer cells. Clin Transl Sci 8(6):668–675. https://doi.org/10.1111/cts.12323
Xin Z, Tong Z, Tan J, Liu C (2021) MicroRNA-145-5p aggravates cell apoptosis and oxidative stress in tongue squamous cell carcinoma. Exp Ther Med 21(4):373. https://doi.org/10.3892/etm.2021.9804
Wang J, Sun Z, Yan S, Gao F (2019) Effect of miR-145 on gastric cancer cells. Mol Med Rep 19(5):3403–3410. https://doi.org/10.3892/mmr.2019.10015
Das TP, Suman S, Alatassi H, Ankem MK, Damodaran C (2016) Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis 7(2):e2111. https://doi.org/10.1038/cddis.2015.403
Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ et al (2006) Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res 66(1):212–220
Kim S-H, Juhnn Y-S, Song Y-S (2007) Akt involvement in paclitaxel chemoresistance of human ovarian cancer cells. Ann N Y Acad Sci 1095(1):82–89. https://doi.org/10.1196/annals.1397.012
Li Y, Chen K, Li L, Li R, Zhang J, Ren W (2015) Overexpression of SOX2 is involved in paclitaxel resistance of ovarian cancer via the PI3K/Akt pathway. Tumor Biol 36(12):9823–9828. https://doi.org/10.1007/s13277-015-3561-5
Li D, Zhao L-N, Zheng X-L, Lin P, Lin F, Li Y et al (2014) Sox2 is involved in paclitaxel resistance of the prostate cancer cell line PC-3 via the PI3K/Akt pathway. Mol Med Rep 10(6):3169–3176. https://doi.org/10.3892/mmr.2014.2630
Ozen M, Karatas OF, Gulluoglu S, Bayrak OF, Sevli S, Guzel E et al (2015) Overexpression of miR-145–5p inhibits proliferation of prostate cancer cells and reduces SOX2 expression. Cancer Investig 33(6):251–258
Ying L, Zhu Z, Xu Z, He T, Li E, Guo Z et al (2015) Cancer associated fibroblast-derived hepatocyte growth factor inhibits the paclitaxel-induced apoptosis of lung cancer A549 cells by up-regulating the PI3K/Akt and GRP78 signaling on a microfluidic platform. PLoS ONE 10(6):e0129593. https://doi.org/10.1371/journal.pone.0129593
Kim K-J, Kim J-W, Sung JH, Suh KJ, Lee JY, Kim SH et al (2020) PI3K-targeting strategy using alpelisib to enhance the antitumor effect of paclitaxel in human gastric cancer. Sci Rep 10(1):12308. https://doi.org/10.1038/s41598-020-68998-w
Lin Y-H, Chen BY-H, Lai W-T, Wu S-F, Guh J-H, Cheng A-L et al (2015) The Akt inhibitor MK-2206 enhances the cytotoxicity of paclitaxel (Taxol) and cisplatin in ovarian cancer cells. Naunyn-Schmiedeberg’s Arch Pharmacol 388(1):19–31. https://doi.org/10.1007/s00210-014-1032-y
Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G et al (2020) Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol 38(5):423–433. https://doi.org/10.1200/jco.19.00368
Choudhary GS, Al-harbi S, Mazumder S, Hill BT, Smith MR, Bodo J et al (2015) MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis 6(1):e1593. https://doi.org/10.1038/cddis.2014.525
Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD (2010) PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol 184(6):3213–3222. https://doi.org/10.4049/jimmunol.0903025
Song T, Chai G, Liu Y, Xie M, Chen Q, Yu X et al (2015) Mechanism of synergy of BH3 mimetics and paclitaxel in chronic myeloid leukemia cells: Mcl-1 inhibition. Eur J Pharm Sci 70:64–71. https://doi.org/10.1016/j.ejps.2015.01.003
Song L, Coppola D, Livingston S, Cress WD, Haura EB (2005) Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther 4(3):267–276
Yamanaka K, Miyake H, Zangemeister-wittke U, Jansen B, Gleave M (2004) Novel bispecific antisense oligonucleotides inhibiting both Bcl-2 and Bcl-xL expression induce apoptosis and enhance chemosensitivity in human androgen-independent prostate cancer cells. Cancer Res 64(7_Supplement):677
Kasai S, Sasaki T, Watanabe A, Nishiya M, Yasuhira S, Shibazaki M et al (2017) Bcl-2/Bcl-xL inhibitor ABT-737 sensitizes pancreatic ductal adenocarcinoma to paclitaxel-induced cell death. Oncol Lett 14(1):903–908. https://doi.org/10.3892/ol.2017.6211
Parrondo R, de Las PA, Reiner T, Perez-Stable C (2013) ABT-737, a small molecule Bcl-2/Bcl-xL antagonist, increases antimitotic-mediated apoptosis in human prostate cancer cells. PeerJ 1:e144
Basu A, Haldar S (2003) Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxol- or 2-methoxyestradiol-induced apoptosis. FEBS Lett 538(1):41–47. https://doi.org/10.1016/S0014-5793(03)00131-5
Flores ML, Castilla C, Ávila R, Ruiz-Borrego M, Sáez C, Japón MA (2012) Paclitaxel sensitivity of breast cancer cells requires efficient mitotic arrest and disruption of Bcl-xL/Bak interaction. Breast Cancer Res Treat 133(3):917–928. https://doi.org/10.1007/s10549-011-1864-9
Or C-HR, Huang C-W, Chang C-C, Lai Y-C, Chen Y-J, Chang C-C (2020) Obatoclax, a Pan-BCL-2 inhibitor, downregulates survivin to induce apoptosis in human colorectal carcinoma cells via suppressing WNT/β-catenin signaling. Int J Mol Sci 21(5):1773
Jiménez-Guerrero R, Gasca J, Flores ML, Pérez-Valderrama B, Tejera-Parrado C, Medina R et al (2018) Obatoclax and paclitaxel synergistically induce apoptosis and overcome paclitaxel resistance in urothelial cancer cells. Cancers (Basel) 10(12):490
Stamelos VA, Fisher N, Bamrah H, Voisey C, Price JC, Farrell WE et al (2016) The BH3 mimetic obatoclax accumulates in lysosomes and causes their alkalinization. PLoS ONE 11(3):e0150696. https://doi.org/10.1371/journal.pone.0150696
Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP et al (2014) Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta Rev Cancer 1845(2):136–154
Wang S, Yao Y, Yao M, Fu P, Wang W (2018) Interleukin-22 promotes triple negative breast cancer cells migration and paclitaxel resistance through JAK-STAT3/MAPKs/AKT signaling pathways. Biochem Biophys Res Commun 503(3):1605–1609. https://doi.org/10.1016/j.bbrc.2018.07.088
Yang C, He L, He P, Liu Y, Wang W, He Y et al (2015) Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol 32(2):14
Wang L, Zhang F, Cui JY, Chen L, Chen YT, Liu BW (2018) CAFs enhance paclitaxel resistance by inducing EMT through the IL-6/JAK2/STAT3 pathway. Oncol Rep 39(5):2081–2090. https://doi.org/10.3892/or.2018.6311
Zhang X, Wu X, Zhang F, Mo S, Lu Y, Wei W et al (2017) Paclitaxel induces apoptosis of esophageal squamous cell carcinoma cells by downregulating STAT3 phosphorylation at Ser727. Oncol Rep 37(4):2237–2244. https://doi.org/10.3892/or.2017.5503
Liu H, Tekle C, Chen Y-W, Kristian A, Zhao Y, Zhou M et al (2011) B7–H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther 10(6):960–971
Sun C-C, Li S-J, Zhang F, Zhang Y-D, Zuo Z-Y, Xi Y-Y et al (2016) The novel miR-9600 suppresses tumor progression and promotes paclitaxel sensitivity in non–small-cell lung cancer through altering STAT3 expression. Mol Ther Nucleic Acids 5:e387. https://doi.org/10.1038/mtna.2016.96
Gao J, Shao Z, Yan M, Fu T, Zhang L, Yan Y (2018) Targeted regulationof STAT3 by miR-29a in mediating Taxol resistance of nasopharyngeal carcinoma cell line CNE-1. Cancer Biomark 22:641–648. https://doi.org/10.3233/CBM-170964
Su W-P, Cheng F-Y, Shieh D-B, Yeh C-S, Su W-C (2012) PLGA nanoparticles codeliver paclitaxel and Stat3 siRNA to overcome cellular resistance in lung cancer cells. Int J Nanomed 7:4269–4283. https://doi.org/10.2147/IJN.S33666
Fan Z, Cui H, Yu H, Ji Q, Kang L, Han B et al (2016) MiR-125a promotes paclitaxel sensitivity in cervical cancer through altering STAT3 expression. Oncogenesis 5(2):e197. https://doi.org/10.1038/oncsis.2016.1
Höll M, Koziel R, Schäfer G, Pircher H, Pauck A, Hermann M et al (2016) ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol Carcinog 55(1):27–39
Fukai T, Ushio-Fukai M (2020) Cross-talk between NADPH oxidase and mitochondria: role in ROS signaling and angiogenesis. Cells 9(8):1849
Ushio-Fukai M (2007) VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9(6):731–739
Kim J, Kim J, Bae J-S (2016) ROS homeostasis and metabolism: a critical liaison for cancer therapy. Exp Mol Med 48(11):e269
Lin H-L, Liu T-Y, Chau G-Y, Lui W-Y, Chi C-W (2000) Comparison of 2-methoxyestradiol-induced, docetaxel-induced, and paclitaxel-induced apoptosis in hepatoma cells and its correlation with reactive oxygen species. Cancer 89(5):983–994. https://doi.org/10.1002/1097-0142(20000901)89:5%3c983::AID-CNCR7%3e3.0.CO;2-G
Sun H, Yu T, Li J (2011) Co-administration of perifosine with paclitaxel synergistically induces apoptosis in ovarian cancer cells: more than just AKT inhibition. Cancer Lett 310(1):118–128. https://doi.org/10.1016/j.canlet.2011.06.010
Liu W, Gu J, Qi J, Zeng XN, Ji J, Chen ZZ et al (2015) Lentinan exerts synergistic apoptotic effects with paclitaxel in A549 cells via activating ROS-TXNIP-NLRP 3 inflammasome. J Cell Mol Med 19(8):1949–1955
Young MM, Kester M, Wang H-G (2013) Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res 54(1):5–19. https://doi.org/10.1194/jlr.R031278
Huang W-C, Chen C-L, Lin Y-S, Lin C-F (2011) Apoptotic sphingolipid ceramide in cancer therapy. J Lipids 2011:565316. https://doi.org/10.1155/2011/565316
Adamovich Y, Adler J, Meltser V, Reuven N, Shaul Y (2014) AMPK couples p73 with p53 in cell fate decision. Cell Death Differ 21(9):1451–1459. https://doi.org/10.1038/cdd.2014.60
Zhang X, Huang J, Yu C, Xiang L, Li L, Shi D et al (2020) Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. Onco Targets Ther 13:513–523. https://doi.org/10.2147/OTT.S228453
Zhao Y, Zeng X, Tang H, Ye D, Liu J (2019) Combination of metformin and paclitaxel suppresses proliferation and induces apoptosis of human prostate cancer cells via oxidative stress and targeting the mitochondria-dependent pathway. Oncol Lett 17(5):4277–4284. https://doi.org/10.3892/ol.2019.10119
Subramaniam Y, Subban K, Chelliah J (2021) A novel synergistic anticancer effect of fungal cholestanol glucoside and paclitaxel: apoptosis induced by an intrinsic pathway through ROS generation in cervical cancer cell line (HeLa). Toxicol In Vitro 72:105079. https://doi.org/10.1016/j.tiv.2021.105079
Li Y, Guo M, Lin Z, Zhao M, Xiao M, Wang C et al (2016) Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. Int J Nanomed 11:6693–6702. https://doi.org/10.2147/IJN.S122666
Zou J, Zhu B, Li Y (2020) Functionalization of silver nanoparticles loaded with paclitaxel-induced A549 cells apoptosis through ROS-mediated signaling pathways. Curr Top Med Chem 20(2):89–98. https://doi.org/10.2174/1568026619666191019102219
Li X, Lu X, Xu H, Zhu Z, Yin H, Qian X et al (2012) Paclitaxel/tetrandrine coloaded nanoparticles effectively promote the apoptosis of gastric cancer cells based on “Oxidation Therapy.” Mol Pharm 9(2):222–229. https://doi.org/10.1021/mp2002736
Chandler NM, Canete JJ, Callery MP (2004) Increased expression of NF-κB subunits in human pancreatic cancer cells1, 2. J Surg Res 118(1):9–14
Naugler WE, Karin M (2008) NF-κB and cancer—identifying targets and mechanisms. Curr Opin Genet Dev 18(1):19–26
Magné N, Toillon R-A, Bottero V, Didelot C, Van Houtte P, Gérard J-P et al (2006) NF-κB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett 231(2):158–168
Collins TS, Lee L-F, Ting JPY (2000) Paclitaxel up-regulates interleukin-8 synthesis in human lung carcinoma through an NF-κB- and AP-1-dependent mechanism. Cancer Immunol Immunother 49(2):78–84. https://doi.org/10.1007/s002620050605
Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S et al (2000) Paclitaxel sensitivity of breast cancer cells with constitutively active NF-κB is enhanced by IκBα super-repressor and parthenolide. Oncogene 19(36):4159–4169
Li Q, Ma Z, Liu Y, Kan X, Wang C, Su B et al (2016) Low doses of paclitaxel enhance liver metastasis of breast cancer cells in the mouse model. FEBS J 283(15):2836–2852
Oyaizu H, Adachi Y, Okumura T, Okigaki M, Oyaizu N, Taketani S et al (2001) Proteasome inhibitor 1 enhances paclitaxel-induced apoptosis in human lung adenocarcinoma cell line. Oncol Rep 8(4):825–829. https://doi.org/10.3892/or.8.4.825
Liu GH, Wang SR, Wang B, Kong BH (2006) Inhibition of nuclear factor-kappaB by an antioxidant enhances paclitaxel sensitivity in ovarian carcinoma cell line. Int J Gynecol Cancer 16(5):1777–1782. https://doi.org/10.1111/j.1525-1438.2006.00652.x
Bellarosa D, Binaschi M, Maggi CA, Goso C (2005) Sabarubicin-(MEN 10755) and paclitaxel show different kinetics in nuclear factor-kappaB (NF-kB) activation: effect of parthenolide on their cytotoxicity. Anticancer Res 25(3B):2119
Yan H, Wang S, Yu H, Zhu J, Chen C (2013) Molecular pathways and functional analysis of miRNA expression associated with paclitaxel-induced apoptosis in hepatocellular carcinoma cells. Pharmacology 92(3–4):167–174
Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi Y et al (2010) MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem 285(28):21496–21507. https://doi.org/10.1074/jbc.M109.083337
Huang L, Hu C, Chao H, Wang R, Lu H, Li H et al (2019) miR-29c regulates resistance to paclitaxel in nasopharyngeal cancer by targeting ITGB1. Exp Cell Res 378(1):1–10. https://doi.org/10.1016/j.yexcr.2019.02.012
Portugal J, Mansilla S, Bataller M (2010) Mechanisms of drug-induced mitotic catastrophe in cancer cells. Curr Pharm Des 16(1):69–78. https://doi.org/10.2174/138161210789941801
Mc Gee MM (2015) Targeting the mitotic catastrophe signaling pathway in cancer. Mediat Inflamm 2015:146282. https://doi.org/10.1155/2015/146282
Denisenko TV, Sorokina IV, Gogvadze V, Zhivotovsky B (2016) Mitotic catastrophe and cancer drug resistance: a link that must to be broken. Drug Resist Updat 24:1–12. https://doi.org/10.1016/j.drup.2015.11.002
Sia J, Szmyd R, Hau E, Gee HE (2020) Molecular mechanisms of radiation-induced cancer cell death: a primer. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.00041
Fragkos M, Beard P (2011) Mitotic catastrophe occurs in the absence of apoptosis in p53-null cells with a defective G1 checkpoint. PLoS ONE 6(8):e22946. https://doi.org/10.1371/journal.pone.0022946
Khing TM, Choi WS, Kim DM, Po WW, Thein W, Shin CY et al (2021) The effect of paclitaxel on apoptosis, autophagy and mitotic catastrophe in AGS cells. Sci Rep 11(1):23490. https://doi.org/10.1038/s41598-021-02503-9
Khodamoradi E, Hoseini-Ghahfarokhi M, Amini P, Motevaseli E, Shabeeb D, Musa AE et al (2020) Targets for protection and mitigation of radiation injury. Cell Mol Life Sci 77(16):3129–3159. https://doi.org/10.1007/s00018-020-03479-x
Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS (2003) Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol 23(16):5556–5571. https://doi.org/10.1128/MCB.23.16.5556-5571.2003
Lin Y-W, Raj EN, Liao W-S, Lin J, Liu K-K, Chen T-H et al (2017) Co-delivery of paclitaxel and cetuximab by nanodiamond enhances mitotic catastrophe and tumor inhibition. Sci Rep 7(1):9814. https://doi.org/10.1038/s41598-017-09983-8
Isham CR, Bossou AR, Negron V, Fisher KE, Kumar R, Marlow L et al (2013) Pazopanib enhances paclitaxel-induced mitotic catastrophe in anaplastic thyroid cancer. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3004358
Bai Z, Gao M, Zhang H, Guan Q, Xu J, Li Y et al (2017) BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe. Cancer Lett 402:81–92. https://doi.org/10.1016/j.canlet.2017.05.016
Soares AS, Costa VM, Diniz C, Fresco P (2014) Combination of Cl-IB-MECA with paclitaxel is a highly effective cytotoxic therapy causing mTOR-dependent autophagy and mitotic catastrophe on human melanoma cells. J Cancer Res Clin Oncol 140(6):921–935. https://doi.org/10.1007/s00432-014-1645-z
Wang X, Wu E, Wu J, Wang T-L, Hsieh H-P, Liu X (2013) An antimitotic and antivascular agent BPR0L075 overcomes multidrug resistance and induces mitotic catastrophe in paclitaxel-resistant ovarian cancer cells. PLoS ONE 8(6):e65686. https://doi.org/10.1371/journal.pone.0065686
Chen N-C, Chyau C-C, Lee Y-J, Tseng H-C, Chou F-P (2016) Promotion of mitotic catastrophe via activation of PTEN by paclitaxel with supplement of mulberry water extract in bladder cancer cells. Sci Rep 6(1):20417. https://doi.org/10.1038/srep20417
Klimaszewska-Wisniewska A, Halas-Wisniewska M, Tadrowski T, Gagat M, Grzanka D, Grzanka A (2016) Paclitaxel and the dietary flavonoid fisetin: a synergistic combination that induces mitotic catastrophe and autophagic cell death in A549 non-small cell lung cancer cells. Cancer Cell Int 16(1):10. https://doi.org/10.1186/s12935-016-0288-3
Michalakis J, Georgatos SD, Romanos J, Koutala H, Georgoulias V, Tsiftsis D et al (2005) Micromolar taxol, with or without hyperthermia, induces mitotic catastrophe and cell necrosis in HeLa cells. Cancer Chemother Pharmacol 56(6):615–622. https://doi.org/10.1007/s00280-005-1002-7
Jiang L, Siu MK, Wong OG, Tam K-F, Lu X, Lam EW et al (2011) iASPP and chemoresistance in ovarian cancers: effects on paclitaxel-mediated mitotic catastrophe. Clin Cancer Res 17(21):6924–6933
Zhao F, Siu MKY, Jiang L, Tam KF, Ngan HYS, Le XF et al (2014) Overexpression of forkhead box protein M1 (FOXM1) in ovarian cancer correlates with poor patient survival and contributes to paclitaxel resistance. PLoS ONE 9(11):e113478. https://doi.org/10.1371/journal.pone.0113478
Khongkow P, Gomes AR, Gong C, Man EPS, Tsang JWH, Zhao F et al (2016) Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene 35(8):990–1002. https://doi.org/10.1038/onc.2015.152
Burgess A, Rasouli M, Rogers S (2014) Stressing mitosis to death. Front Oncol 4:140. https://doi.org/10.3389/fonc.2014.00140
Chan KS, Koh CG, Li HY (2012) Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis 3(10):e411. https://doi.org/10.1038/cddis.2012.148
Henriques AC, Ribeiro D, Pedrosa J, Sarmento B, Silva PMA, Bousbaa H (2019) Mitosis inhibitors in anticancer therapy: when blocking the exit becomes a solution. Cancer Lett 440–441:64–81. https://doi.org/10.1016/j.canlet.2018.10.005
Cheng B, Crasta K (2017) Consequences of mitotic slippage for antimicrotubule drug therapy. Endocr Relat Cancer 24(9):T97-t106. https://doi.org/10.1530/erc-17-0147
Sloss O, Topham C, Diez M, Taylor S (2016) Mcl-1 dynamics influence mitotic slippage and death in mitosis. Oncotarget 7(5):5176
Bennett A, Sloss O, Topham C, Nelson L, Tighe A, Taylor SS (2016) Inhibition of Bcl-xL sensitizes cells to mitotic blockers, but not mitotic drivers. Open Biol 6(8):160134
Lin Y, Jiang M, Chen W, Zhao T, Wei Y (2019) Cancer and ER stress: mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother 118:109249
Linder B, Kögel D (2019) Autophagy in cancer cell death. Biology 8(4):82. https://doi.org/10.3390/biology8040082
Lin L, Baehrecke EH (2015) Autophagy, cell death, and cancer. Mol Cell Oncol 2(3):e985913. https://doi.org/10.4161/23723556.2014.985913
Zhong Z, Sanchez-Lopez E, Karin M (2016) Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166(2):288–298
Gerada C, Ryan KM (2020) Autophagy, the innate immune response and cancer. Mol Oncol 14(9):1913–1929
Kong Y, Feng Z, Chen A, Qi Q, Han M, Wang S et al (2019) The natural flavonoid galangin elicits apoptosis, pyroptosis, and autophagy in glioblastoma. Front Oncol 9:942
Yu Y-F, Hu P-C, Wang Y, Xu X-L, Rushworth GM, Zhang Z et al (2017) Paclitaxel induces autophagy in gastric cancer BGC823 cells. Ultrastruct Pathol 41(4):284–290. https://doi.org/10.1080/01913123.2017.1334019
Lee Y, Na J, Lee MS, Cha EY, Sul JY, Park JB et al (2018) Combination of pristimerin and paclitaxel additively induces autophagy in human breast cancer cells via ERK1/2 regulation. Mol Med Rep 18(5):4281–4288. https://doi.org/10.3892/mmr.2018.9488
Eum K-H, Lee M (2011) Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol Cell Biochem 348(1):61–68. https://doi.org/10.1007/s11010-010-0638-8
Ajabnoor GMA, Crook T, Coley HM (2012) Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis 3(1):e260. https://doi.org/10.1038/cddis.2011.139
Ghaforui-Fard S, Vafaee R, Taheri M (2019) Taurine-upregulated gene 1: a functional long noncoding RNA in tumorigenesis. J Cell Physiol 234(10):17100–17112. https://doi.org/10.1002/jcp.28464
Gu L, Li Q, Liu H, Lu X, Zhu M (2020) Long noncoding RNA TUG1 promotes autophagy-associated paclitaxel resistance by sponging miR-29b-3p in ovarian cancer cells. Onco Targets Ther 13:2007–2019. https://doi.org/10.2147/OTT.S240434
Chen K, Shi W (2016) Autophagy regulates resistance of non-small cell lung cancer cells to paclitaxel. Tumor Biol 37(8):10539–10544. https://doi.org/10.1007/s13277-016-4929-x
Zhang S-F, Wang X-Y, Fu Z-Q, Peng Q-H, Zhang J-Y, Ye F et al (2015) TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy 11(2):225–238
Peng X, Gong F, Chen Y, Jiang Y, Liu J, Yu M et al (2014) Autophagy promotes paclitaxel resistance of cervical cancer cells: involvement of Warburg effect activated hypoxia-induced factor 1-α-mediated signaling. Cell Death Dis 5(8):e1367. https://doi.org/10.1038/cddis.2014.297
Pastor F, Dumas K, Barthélémy M-A, Regazzetti C, Druelle N, Peraldi P et al (2017) Implication of REDD1 in the activation of inflammatory pathways. Sci Rep 7(1):7023. https://doi.org/10.1038/s41598-017-07182-z
Zeng Q, Liu J, Cao P, Li J, Liu X, Fan X et al (2018) Inhibition of REDD1 sensitizes bladder urothelial carcinoma to paclitaxel by inhibiting autophagy. Clin Cancer Res 24(2):445–459
Zou C-F, Jia L, Jin H, Yao M, Zhao N, Huan J et al (2011) Re-expression of ARHI (DIRAS3) induces autophagy in breast cancer cells and enhances the inhibitory effect of paclitaxel. BMC Cancer 11(1):22. https://doi.org/10.1186/1471-2407-11-22
Xu S, Wang P, Zhang J, Wu H, Sui S, Zhang J et al (2019) Ai-lncRNA EGOT enhancing autophagy sensitizes paclitaxel cytotoxicity via upregulation of ITPR1 expression by RNA-RNA and RNA-protein interactions in human cancer. Mol Cancer 18(1):89. https://doi.org/10.1186/s12943-019-1017-z
Wang H, Li D, Li X, Ou X, Liu S, Zhang Y et al (2016) Mammalian target of rapamycin inhibitor RAD001 sensitizes endometrial cancer cells to paclitaxel-induced apoptosis via the induction of autophagy. Oncol Lett 12(6):5029–5035. https://doi.org/10.3892/ol.2016.5338
Zamora A, Alves M, Chollet C, Therville N, Fougeray T, Tatin F et al (2019) Paclitaxel induces lymphatic endothelial cells autophagy to promote metastasis. Cell Death Dis 10(12):956. https://doi.org/10.1038/s41419-019-2181-1
Zhan Y, Wang K, Li Q, Zou Y, Chen B, Gong Q et al (2018) The novel autophagy inhibitor alpha-hederin promoted paclitaxel cytotoxicity by increasing reactive oxygen species accumulation in non-small cell lung cancer cells. Int J Mol Sci 19(10):3221
Wang K, Liu X, Liu Q, Ho Ih, Wei X, Yin T et al (2020) Hederagenin potentiated cisplatin- and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis 11(8):611. https://doi.org/10.1038/s41419-020-02880-5
Datta S, Choudhury D, Das A, Mukherjee DD, Dasgupta M, Bandopadhyay S et al (2019) Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of β-catenin pathway. Apoptosis 24(5):414–433. https://doi.org/10.1007/s10495-019-01526-y
Xi G, Hu X, Wu B, Jiang H, Young CYF, Pang Y et al (2011) Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett 307(2):141–148. https://doi.org/10.1016/j.canlet.2011.03.026
Xu L, Liu J-H, Zhang J, Zhang N, Wang Z-H (2015) Blockade of autophagy aggravates endoplasmic reticulum stress and improves paclitaxel cytotoxicity in human cervical cancer cells. Cancer Res Treat 47(2):313–321. https://doi.org/10.4143/crt.2013.222
Liu S, Li X (2015) Autophagy inhibition enhances sensitivity of endometrial carcinoma cells to paclitaxel. Int J Oncol 46(6):2399–2408. https://doi.org/10.3892/ijo.2015.2937
Zou S, Du X, Lin H, Wang P, Li M (2018) Paclitaxel inhibits the progression of cervical cancer by inhibiting autophagy via lncRNARP11-381N20. 2. Eur Rev Med Pharmacol Sci 22(10):3010–3017
Wang R-X, Xu X-E, Huang L, Chen S, Shao Z-M (2019) eEF2 kinase mediated autophagy as a potential therapeutic target for paclitaxel-resistant triple-negative breast cancer. Ann Transl Med 7(23):783. https://doi.org/10.21037/atm.2019.11.39
Wen J, Yeo S, Wang C, Chen S, Sun S, Haas MA et al (2015) Autophagy inhibition re-sensitizes pulse stimulation-selected paclitaxel-resistant triple negative breast cancer cells to chemotherapy-induced apoptosis. Breast Cancer Res Treat 149(3):619–629. https://doi.org/10.1007/s10549-015-3283-9
Zhang Q, Si S, Schoen S, Chen J, Jin X-B, Wu G (2013) Suppression of autophagy enhances preferential toxicity of paclitaxel to folliculin-deficient renal cancer cells. J Exp Clin Cancer Res 32(1):99. https://doi.org/10.1186/1756-9966-32-99
Zeh HJ, Bahary N, Boone BA, Singhi AD, Miller-Ocuin JL, Normolle DP et al (2020) A randomized phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and gemcitabine/nab-paclitaxel in pancreatic cancer patients. Clin Cancer Res 26(13):3126–3134
Wyld L, Bellantuono I, Tchkonia T, Morgan J, Turner O, Foss F et al (2020) Senescence and cancer: a review of clinical implications of senescence and senotherapies. Cancers 12(8):2134. https://doi.org/10.3390/cancers12082134
Wang B, Kohli J, Demaria M (2020) Senescent cells in cancer therapy: friends or foes? Trends Cancer 6(10):838–857. https://doi.org/10.1016/j.trecan.2020.05.004
Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
Franco MS, Roque MC, Oliveira MC (2019) Short and long-term effects of the exposure of breast cancer cell lines to different ratios of free or co-encapsulated liposomal paclitaxel and doxorubicin. Pharmaceutics 11(4):178
Chen JY-F, Hwang C-C, Chen W-Y, Lee J-C, Fu T-F, Fang K et al (2010) Additive effects of C2-ceramide on paclitaxel-induced premature senescence of human lung cancer cells. Life Sci 87(11):350–357. https://doi.org/10.1016/j.lfs.2010.06.017
Ling YH, Zou Y, Perez-Soler R (2000) Induction of senescence-like phenotype and loss of paclitaxel sensitivity after wild-type p53 gene transfection of p53-null human non-small cell lung cancer H358 cells. Anticancer Res 20(2a):693–702
Uruski P, Sepetowska A, Konieczna C, Pakuła M, Wyrwa M, Tussupkaliyev A et al (2021) Primary high-grade serous ovarian cancer cells are sensitive to senescence induced by carboplatin and paclitaxel in vitro. Cell Mol Biol Lett 26(1):44. https://doi.org/10.1186/s11658-021-00287-4
Zhou J, Jiang Y-Y, Wang H-P, Chen H, Wu Y-C, Wang L et al (2020) Natural compound Tan-I enhances the efficacy of paclitaxel chemotherapy in ovarian cancer. Ann Transl Med 8(12):752. https://doi.org/10.21037/atm-20-4072
Chou Y-S, Yen C-C, Chen W-M, Lin Y-C, Wen Y-S, Ke W-T et al (2016) Cytotoxic mechanism of PLK1 inhibitor GSK461364 against osteosarcoma: mitotic arrest, apoptosis, cellular senescence, and synergistic effect with paclitaxel. Int J Oncol 48(3):1187–1194. https://doi.org/10.3892/ijo.2016.3352
Schmidt S, Schneider L, Essmann F, Cirstea IC, Kuck F, Kletke A et al (2010) The centrosomal protein TACC3 controls paclitaxel sensitivity by modulating a premature senescence program. Oncogene 29(46):6184–6192. https://doi.org/10.1038/onc.2010.354
Mohiuddin M, Kasahara K (2021) The mechanisms of the growth inhibitory effects of paclitaxel on gefitinib-resistant non-small cell lung cancer cells. Cancer Genomics Proteomics 18(5):661–673
Prencipe M, Fitzpatrick P, Gorman S, Mosetto M, Klinger R, Furlong F et al (2009) Cellular senescence induced by aberrant MAD2 levels impacts on paclitaxel responsiveness in vitro. Br J Cancer 101(11):1900–1908. https://doi.org/10.1038/sj.bjc.6605419
Weiner-Gorzel K, Dempsey E, Milewska M, McGoldrick A, Toh V, Walsh A et al (2015) Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Med 4(5):745–758
Tan Y, Chen Q, Li X, Zeng Z, Xiong W, Li G et al (2021) Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res 40(1):153. https://doi.org/10.1186/s13046-021-01959-x
Wang Y-Y, Liu X-L, Zhao R (2019) Induction of pyroptosis and its implications in cancer management. Front Oncol 9:971
Shi Y, Ren J, Liang C, Wang F, Li W, Li X (2019) GSDME influences sensitivity of breast cancer MCF-7 cells to paclitaxel by regulating cell pyroptosis. Chin J Cancer Biother pp 146–151
Zhang C-c, Li C-g, Wang Y-f, Xu L-h, He X-h, Zeng Q-z et al (2019) Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis 24(3):312–325. https://doi.org/10.1007/s10495-019-01515-1
Wang X, Li H, Li W, Xie J, Wang F, Peng X et al (2020) The role of caspase-1/GSDMD-mediated pyroptosis in Taxol-induced cell death and a Taxol-resistant phenotype in nasopharyngeal carcinoma regulated by autophagy. Cell Biol Toxicol 36(5):437–457. https://doi.org/10.1007/s10565-020-09514-8
Cheng Z, Li Z, Gu L, Li L, Gao Q, Zhang X et al (2020) Ophiopogonin B-inducing pyroptosis through caspase-1/gsdmd pathway contributes to alleviation of paclitaxel resistance in lung cancer cells. J Cancer 13(2):715–727. https://doi.org/10.7150/jca.66432
Xiao Y, Zhang T, Ma X, Yang QC, Yang LL, Yang SC et al (2021) Microenvironment-responsive prodrug-induced pyroptosis boosts cancer immunotherapy. Adv Sci. 8(24):e2101840. https://doi.org/10.1002/advs.202101840
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Wu Y, Yu C, Luo M, Cen C, Qiu J, Zhang S et al (2020) Ferroptosis in cancer treatment: another way to Rome. Front Oncol. https://doi.org/10.3389/fonc.2020.571127
Jiang M, Qiao M, Zhao C, Deng J, Li X, Zhou C (2020) Targeting ferroptosis for cancer therapy: exploring novel strategies from its mechanisms and role in cancers. Transl Lung Cancer Res 9(4):1569–1584. https://doi.org/10.21037/tlcr-20-341
Ye J, Jiang X, Dong Z, Hu S, Xiao M (2019) Low-concentration PTX and RSL3 inhibits tumor cell growth synergistically by inducing ferroptosis in mutant p53 hypopharyngeal squamous carcinoma. Cancer Manag Res 11:9783–9792. https://doi.org/10.2147/CMAR.S217944
Sugiyama A, Ohta T, Obata M, Takahashi K, Seino M, Nagase S (2020) xCT inhibitor sulfasalazine depletes paclitaxel-resistant tumor cells through ferroptosis in uterine serous carcinoma. Oncol Lett 20(3):2689–2700. https://doi.org/10.3892/ol.2020.11813
You JH, Lee J, Roh J-L (2021) PGRMC1-dependent lipophagy promotes ferroptosis in paclitaxel-tolerant persister cancer cells. J Exp Clin Cancer Res 40(1):350. https://doi.org/10.1186/s13046-021-02168-2
Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D et al (2020) CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 19(1):43. https://doi.org/10.1186/s12943-020-01168-8
Wei D, Ke Y-Q, Duan P, Zhou L, Wang C-Y, Cao P (2021) MicroRNA-302a-3p induces ferroptosis of non-small cell lung cancer cells via targeting ferroportin. Free Radic Res 55(7):821–830. https://doi.org/10.1080/10715762.2021.1947503
Qiu Y, Yu Q, Ji M, Zhang Z, Kang L, Fu Y et al (2021) Activation ferroptosis enhanced the therapy sensitivity of TNBC to paclitaxel via NCOA4 mediated ferritinophagy. Res Sq. PPR: PPR310530. https://doi.org/10.21203/rs.3.rs-360631/v1
Su Z, Yang Z, Xu Y, Chen Y, Yu Q (2015) Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 14:48. https://doi.org/10.1186/s12943-015-0321-5
Su Z, Yang Z, Xie L, DeWitt JP, Chen Y (2016) Cancer therapy in the necroptosis era. Cell Death Differ 23(5):748–756. https://doi.org/10.1038/cdd.2016.8
Diao Y, Ma X, Min W, Lin S, Kang H, Dai Z et al (2016) Dasatinib promotes paclitaxel-induced necroptosis in lung adenocarcinoma with phosphorylated caspase-8 by c-Src. Cancer Lett 379(1):12–23. https://doi.org/10.1016/j.canlet.2016.05.003
Khing TM, Po WW, Sohn UD (2019) Fluoxetine enhances anti-tumor activity of paclitaxel in gastric adenocarcinoma cells by triggering apoptosis and necroptosis. Anticancer Res 39(11):6155. https://doi.org/10.21873/anticanres.13823
Jang MS, Lee SJ, Kang NS, Kim E (2011) Cooperative phosphorylation of FADD by Aur-A and Plk1 in response to taxol triggers both apoptotic and necrotic cell death. Cancer Res 71(23):7207–7215. https://doi.org/10.1158/0008-5472.can-11-0760
Ando Y, Ohuchida K, Otsubo Y, Kibe S, Takesue S, Abe T et al (2020) Necroptosis in pancreatic cancer promotes cancer cell migration and invasion by release of CXCL5. PLoS ONE 15(1):e0228015. https://doi.org/10.1371/journal.pone.0228015
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparing first draft. The scientific edition was performed by MN. All authors wrote and approved the article.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain human or animal studies performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, S., Tang, Y., Wang, R. et al. Mechanisms of cancer cell death induction by paclitaxel: an updated review. Apoptosis 27, 647–667 (2022). https://doi.org/10.1007/s10495-022-01750-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01750-z