Abstract
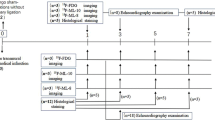
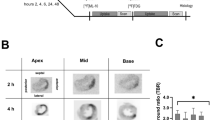
The purpose of this study was to employ novel tracers PET imaging approach to define the time course and intensity of myocardial repair after apoptosis and to correlate the imaging signal to immunohistochemical staining in myocardial infarction (MI). We designed novel αVβ3-targeted and radio-functionalized tracers for detection of apoptosis in H9C2 cells and myocardial tissue. MI rats were imaged with [18F]FDG, [18F]ANP-Cin or [18F]ANP-RGD2 using a small-animal PET/CT device. Rats were sacrificed, and tissue samples from viable and injured myocardial areas were sectioned for TUNEL assay and histology. The uncorrected radiochemical yield of [18F]ANP-Cin and [18F]ANP-RGD2 were 41.3 ± 5.4% and 21.17 ± 4.7%, respectively. Two tracers meet many criteria for cardiac imaging, including high stability, high binding, no toxicity, fast renal clearance and excellent biodistribution in rat models. The uptake of [18F]ANP-Cin was significantly higher on the 1st and 3rd day than the 7th or 28th day after MI induction, a timeframe associated with increased cardiomyocyte apoptosis. Higher uptake of [18F]ANP-Cin was observed in MI rats than in N-acetylcysteine (NAC)-treated rats on the 3rd days. In contrast with [18F]ANP-Cin, no hot-spots was observed with [18F]ANP-RGD2 on the 1st day and more hot-spots was observed from the 3rd day to the 7th day, then less on the 28th days in the high apoptotic site. There was no uptake of [18F]FDG in or around the apoptotic region. On the 7th day the uptake of [18F]ANP-RGD2 was higher in NAC-treated rats than MI rats. [18F]ANP-Cin and [18F]ANP-RGD2 are superior to [18F]FDG for PET/CT imaging for evaluation of cardiomyocyte apoptosis and tissue repair processes in the MI rats.








Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PET/CT:
-
Positron emission tomography/computed tomography
- MI:
-
Myocardial infarction
- PE:
-
Phosphatidylethanolamine
- RGD:
-
Arginyl-glycyl-aspartic acid
- SPF:
-
Specific pathogen free
- SD:
-
Sprague–Dawley
- NAC:
-
N-Acetylcysteine
- LAD:
-
Left anterior descending
- LVEDD:
-
Left ventricular end-diastolic dimension
- LVEF:
-
Left ventricular ejection fraction
- DMEM:
-
Dulbecco's modified Eagle's medium
- shRNA:
-
Short hairpin RNA
- 2D-OSEM:
-
Two-dimensional ordered-subsets expectation maximum
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- IHC:
-
Immunohistochemistry
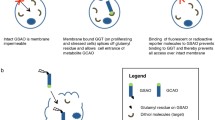
- [18F]ANP-Cin:
-
[18F]AlF-NOTA-PEG3-Cinnamycin
- [18F]ANP-RGD2 :
-
[18F]AlF-NOTA-PEG3-β-Glu-RGD2
References
Gabriel-Costa D (2018) The pathophysiology of myocardial infarction-induced heart failure. Pathophysiology 25:277–284
Wang W, Thompson DR, Ski CF, Liu M (2014) Health-related quality of life and its associated factors in Chinese myocardial infarction patients. Eur J Prev Cardiol 21:321–329
Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119:91–112
Tseliou E, de Couto G, Terrovitis J et al (2014) Angiogenesis, cardiomyocyte proliferation and anti-fibrotic effects underlie structural preservation post-infarction by intramyocardially-injected cardiospheres. PLoS ONE 9:e88590
Sahoo S, Losordo DW (2014) Exosomes and cardiac repair after myocardial infarction. Circ Res 114:333–344
Huang FY, Xia TL, Li JL et al (2019) The bifunctional SDF-1-AnxA5 fusion protein protects cardiac function after myocardial infarction. J Cell Mol Med 23:7673–7684
Dong Y, Undyala VVR, Przyklenk K (2016) Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment. Basic Res Cardiol 111:59
Robson PM, Dweck MR, Trivieri MG et al (2017) Coronary Artery PET/MR Imaging: Feasibility, Limitations, and Solutions. JACC Cardiovasc Imaging 10:1103–1112
Vance JE (2008) Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res 49:1377–1387
Hullin-Matsuda F, Makino A, Murate M, Kobayashi T (2016) Probing phosphoethanolamine-containing lipids in membranes with duramycin/cinnamycin and aegerolysin proteins. Biochimie 130:81–90
Ding L, Dong L, Chen X et al (2009) Increased expression of integrin-linked kinase attenuates left ventricular remodeling and improves cardiac function after myocardial infarction. Circulation 120:764–773
Su Y, Tian H, Wei L, Fu G, Sun T (2018) Integrin beta3 inhibits hypoxia-induced apoptosis in cardiomyocytes. Acta Biochim Biophys Sin (Shanghai) 50:658–665
Wei L, Zhou Q, Tian H, Su Y, Fu GH, Sun T (2020) Integrin beta3 promotes cardiomyocyte proliferation and attenuates hypoxia-induced apoptosis via regulating the PTEN/Akt/mTOR and ERK1/2 pathways. Int J Biol Sci 16:644–654
Liu S, Jiang Z, Qiao L et al (2018) Integrin beta-3 is required for the attachment, retention and therapeutic benefits of human cardiospheres in myocardial infarction. J Cell Mol Med 22:382–389
Sun M, Opavsky MA, Stewart DJ et al (2003) Temporal response and localization of integrins beta1 and beta3 in the heart after myocardial infarction: regulation by cytokines. Circulation 107:1046–1052
Xiong JP, Stehle T, Zhang R et al (2002) Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 296:151–155
Dissoki S, Abourbeh G, Salnikov O, Mishani E, Jacobson O (2015) PET molecular imaging of angiogenesis with a multiple tyrosine kinase receptor-targeted agent in a rat model of myocardial infarction. Mol Imaging Biol 17:222–230
Sherif HM, Saraste A, Nekolla SG et al (2012) Molecular imaging of early alphavbeta3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J Nucl Med 53:318–323
Vasudevan P, Gaebel R, Doering P et al (2019) 18F-FDG PET-based imaging of myocardial inflammation predicts a functional outcome following transplantation of mESC-derived cardiac induced cells in a mouse model of myocardial infarction. Cells 8:24–58
Liu Z, Larsen BT, Lerman LO et al (2016) Detection of atherosclerotic plaques in ApoE-deficient mice using (99m)Tc-duramycin. Nucl Med Biol 43:496–505
Murakami Y, Takamatsu H, Taki J et al (2004) 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur J Nucl Med Mol Imaging 31:469–474
Sun T, Tang G, Tian H et al (2015) Positron emission tomography imaging of cardiomyocyte apoptosis with a novel molecule probe [18F]FP-DPAZn2. Oncotarget 6:30579–30591
Yao S, Hu K, Tang G et al (2014) Positron emission tomography imaging of cell death with [(18)F]FPDuramycin. Apoptosis 19:841–850
Rasmussen T, Follin B, Kastrup J et al (2018) Angiogenesis PET tracer uptake ((68)Ga-NODAGA-E[(cRGDyK)](2)) in induced myocardial infarction and stromal cell treatment in minipigs. Diagnostics (Basel) 8:33
Lee MS, Park HS, Lee BC, Jung JH, Yoo JS, Kim SE (2016) Identification of angiogenesis rich-viable myocardium using RGD dimer based SPECT after myocardial infarction. Sci Rep 6:27520
Laitinen I, Notni J, Pohle K et al (2013) Comparison of cyclic RGD peptides for alphavbeta3 integrin detection in a rat model of myocardial infarction. EJNMMI Res 3:38
Hui Ma SL, Zhang Z, Tang G, Yuan G, Zhao J, Shu Su (2019) Preliminary biological evaluation of 68Ga-labeled cyclic RGD dimer as an integrin αvβ3-targeting radiotracer for tumor PET imaging. J Radioanal Nucl Chem 24:857–865
Andrade JN, Tang J, Hensley MT et al (2015) Rapid and efficient production of coronary artery ligation and myocardial infarction in mice using surgical clips. PLoS ONE 10:e0143221
Pasupathy S, Tavella R, Grover S et al (2017) Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM trial [N-acetylcysteine in acute myocardial infarction]). Circulation 136:894–903
Yuan G, Liu S, Ma H et al (2020) Targeting phosphatidylethanolamine with fluorine-18 labeled small molecule probe for apoptosis imaging. Mol Imaging Biol 22:914–923
Hu K, Tang X, Tang G et al (2015) 18F-FP-PEG2-beta-Glu-RGD2: a symmetric integrin alphavbeta3-targeting radiotracer for tumor PET imaging. PLoS ONE 10:e0138675
Emoto K, Umeda M (2001) Membrane lipid control of cytokinesis. Cell Struct Funct 26:659–665
Abdelwahid E, Kalvelyte A, Stulpinas A, de Carvalho KA, Guarita-Souza LC, Foldes G (2016) Stem cell death and survival in heart regeneration and repair. Apoptosis 21:252–268
Dobrucki LW, Meoli DF, Hu J, Sadeghi MM, Sinusas AJ (2009) Regional hypoxia correlates with the uptake of a radiolabeled targeted marker of angiogenesis in rat model of myocardial hypertrophy and ischemic injury. J Physiol Pharmacol 60(Suppl 4):117–123
Golestani R, Mirfeizi L, Zeebregts CJ et al (2015) Feasibility of [18F]-RGD for ex vivo imaging of atherosclerosis in detection of alphavbeta3 integrin expression. J Nucl Cardiol 22:1179–1186
Chen H, Niu G, Wu H, Chen X (2016) Clinical application of radiolabeled RGD peptides for PET imaging of integrin alphavbeta3. Theranostics 6:78–92
Pfaff M, Tangemann K, Muller B et al (1994) Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem 269:20233–20238
Deas O, Dumont C, Mollereau B et al (1997) Thiol-mediated inhibition of FAS and CD2 apoptotic signaling in activated human peripheral T cells. Int Immunol 9:117–125
Peng YW, Buller CL, Charpie JR (2011) Impact of N-acetylcysteine on neonatal cardiomyocyte ischemia-reperfusion injury. Pediatr Res 70:61–66
Funding
This work was supported by the National Natural Science Foundation (Nos. 81770505, 91949121, 81671719), Nanfang Hospital of Southern Medical University (No. 123456), Research Project of Shanghai Municipal Health and Family Planning Commission (No. 201740060).
Author information
Authors and Affiliations
Contributions
TS designed the study, supervised the project, wrote the original manuscript and revised the paper. TS and LJW conducted the cell and animal experiments, PET/CT imaging. HT conducted the cell and animal experiments and discussed the results. HM radio-synthesized the tracers. WLZ and XC discussed the results and analyzed the data. DH.N and S.L.W conducted PET/CT imaging. GHT supervised the project, discussed the results, analyzed the data and revised the paper. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval
All animal care and experimental procedures were approved by the Animal Care and Use Committee of Sun Yat-sen University (Approval Number: IACUC-DB-16-1106).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, T., Wei, L., Tian, H. et al. Novel PET/CT tracers for targeted imaging of membrane receptors to evaluate cardiomyocyte apoptosis and tissue repair process in a rat model of myocardial infarction. Apoptosis 26, 460–473 (2021). https://doi.org/10.1007/s10495-021-01681-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01681-1