Abstract
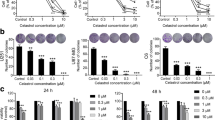
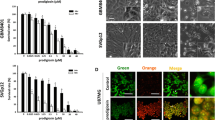
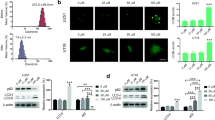
Docosahexaenoic acid (DHA), an important w-3 fatty acid exhibits differential behavior in cancer cells of neural origin when compared to that in normal healthy astrocytes. Treatment of C6 glioma and SH-SY5Y cell lines and primary astrocytes, representing the neoplastic cells and normal healthy cells respectively, with 100 µM DHA for 24 h showed significant loss of cell viability in the both the cancer cells as determined by MTT assay, whereas the primary astrocytes cultures were unaffected. Such loss of cell viability was due to apoptosis as confirmed by TUNEL staining and caspase-3 activation in cancer cells. Proteomic approach, employing 2-dimensional gel electrophoresis (2DE), difference gel electrophoresis (DIGE), and MALDI-TOF-TOF analysis identified six proteins which unlike in the astrocytes, were differently altered in the cancer cells upon exposure to DHA, suggesting their putative contribution in causing apoptosis in these cells. Of these, annexin A2, calumenin, pyruvate kinase M2 isoform, 14-3-3ζ were downregulated while aldo keto reductase-1B8 (AKR1B8) and glutathione–S-transferase P1 subunit (GSTP1) showed upregulation by DHA in the cancer cells. siRNA-mediated knockdown of AKR1B8 and GSTP1 inhibit DHA-induced apoptosis confirming their role in apoptotic process. Furthermore, western blot analysis identified upregulation of PPARα and the MAP kinases, JNK and p38 as well as increased ROS production selectively in the cell lines. Results suggest that DHA selectively induces apoptosis in the neural cell lines by regulating the expression of the above proteins to activate multiple apoptotic pathways which in association with excess ROS and activated MAPKs promote cell death.






Similar content being viewed by others
Abbreviations
- DHA:
-
Docosahexaenoic acid
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- 2DE:
-
2-Dimensional gel electrophoresis
- DIGE:
-
Difference gel electrophoresis
- AKR1B8:
-
Aldo keto reductase-1B8
- GSTP1:
-
Glutathione-S-transferase P1 subunit
- PPAR:
-
Peroxisome proliferator-activated receptors
- JNK:
-
c-Jun NH2-terminal kinase
- ROS:
-
Reactive oxygen species
- PKM2:
-
Pyruvate kinase-M2 isoform
- ER:
-
Endoplasmic reticulum
- 14-3-3ζ:
-
14-3-3 Zeta isoform
- ERK1/2:
-
Extracellular signal-regulated kinases 1 and 2
- pERK:
-
Phosphorylated ERK
- p-JNK:
-
Phosphorylated JNK
- p-p38:
-
Phosphorylated p38
- pCREB:
-
cAMP response element-binding protein
- ANXA2:
-
Annexin A2
- DMEM:
-
Dulbecco’s modified eagles medium
- FBS:
-
Fetal bovine serum
- DMSO:
-
Dimethyl sulfoxide
- SDS–PAGE:
-
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- DTT:
-
Dithiothreitol
References
Gharami K, Das M, Das S (2015) Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem Int 89:51–62
Joardar A, Sen AK, Das S (2006) Docosahexaenoic acid facilitates cell maturation and beta-adrenergic transmission in astrocytes. J Lipid Res 47:571–581
Kim HY (2007) Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem 282:18661–18665
Begum G, Kintner D, Liu Y, Cramer SW, Sun D (2012) DHA inhibits ER Ca2+ release and ER stress in astrocytes following in vitro ischemia. J Neurochem 20:622–630
Chang CY, Kuan YH, Li JR, Chen WY, Ou YC, Pan HC, Liao SL, Raung SL, Chang CJ, Chen CJ (2013) Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. J Nutr Biochem 24:2127–2137
Casañas-Sánchez V, Pérez JA, Fabelo N, Herrera-Herrera AV, Fernández C, Marín R, González-Montelongo MC, Díaz M (2014) Addition of docosahexaenoic acid, but not arachidonic acid, activates glutathione and thioredoxin antioxidant systems in murine hippocampal HT22 cells: potential implications in neuroprotection. J Neurochem 131:470–483
Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, Leung P, Hong-Gonzalez J, Freedland SJ, Said J, Gui D, Seeram NP, Popoviciu LM, Bagga D, Heber D, Glaspy JA, Aronson WJ (2006) Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res 12:4662–4670
Martin DD, Robbins ME, Spector AA, Wen BC, Hussey DH (1996) The fatty acid composition of human gliomas differs from that found in nonmalignant brain tissue. Lipids 31:1283–1288
Reynolds LM, Dalton CF, Reynolds GP (2001) Phospholipid fatty acids and neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci Lett 309:193–196
Kokoglu E, Tuter Y, Yazici Z, Sandikci KS, Sonmez H, Ulakoglu EZ, Ozyurt E (1998) Profiles of the fatty acids in the plasma membrane of human brain tumors. Cancer Biochem Biophys 16:301–312
Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT (2010) Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One 5:e10296, 1–13
Sun H, Hu Y, Gu Z, Owens RT, Chen YQ, Edwards IJ (2011) Omega-3 fatty acids induce apoptosis in human breast cancer cells and mouse mammary tissue through syndecan-1 inhibition of the MEK-Erk pathway. Carcinogenesis 32:1518–1524
Fasano E, Serini S, Piccioni E, Toesca A, Monego G, Cittadini AR, Ranelletti FO, Calviello G (2012) DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim Biophys Acta 1822:1762–1772
Das UN, Huang YS, Begin ME, Ells G, Horrobin DF (1987) Uptake and distribution of cis-unsaturated fatty acids and their effect on free radical generation in normal and tumor cells in vitro. Free Radic Biol Med 3:9–14
Jeong S, Jing K, Kim N, Shin S, Kim S, Song KS, Heo JY, Park JH, Seo KS, Han J, Wu T, Kweon GR, Park SK, Park JI, Lim K (2014) Docosahexaenoic acid-induced apoptosis is mediated by activation of mitogen-activated protein kinases in human cancer cells. BMC Cancer 14:481
Ding WQ, Vaught JL, Yamauchi H, Lind SE (2004) Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: the potential importance of down-regulation of superoxide dismutase 1 expression. Mol Cancer Ther 3:1109–1117
Maiti AK (2012) Reactive oxygen species reduction is a key underlying mechanism of drug resistance in cancer chemotherapy. Chemotherapy 1:104
Merendino N, Costantini L, Manzi L, Molinari R, D’Eliseo D, Velotti F (2013) Dietary ω–3 polyunsaturated fatty acid DHA: a potential adjuvant in the treatment of cancer. Biomed Res Int 2013:310186
Harvey KA, Xu Z, Saaddatzadeh MR, Wang H, Pollok K, Cohen-Gadol AA, Siddiqui RA (2015) Enhanced anticancer properties of lomustine in conjunction with docosahexaenoic acid in glioblastoma cell lines. J Neurosurg 122:547–556
D’Alessandro A, D’Amici GM, Timperio AM, Merendino N, Zolla L (2011) Docosohaexanoic acid-supplemented PACA44 cell lines and over-activation of Krebs cycle: an integrated proteomic, metabolomic and interactomic overview. J Proteomics 74:2138–2158
Gleissman H, Yang R, Martinod K, Lindskog M, Serhan CN, Johnsen JI, Kogner P (2010) Docosahexaenoic acid metabolome in neural tumors: identification of cytotoxic intermediates. FASEB J 24:906–915
Serhan CN, Chiang N (2008) Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol 153:S200–S215
Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG (2004) Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA 101:8491–8496
Mouradian M, Kikawa KD, Dranka BP, Komas SM, Kalyanaraman B, Pardini RS (2015) Docosahexaenoic acid attenuates breast cancer cell metabolism and the Warburg phenotype by targeting bioenergetic function. Mol Carcinog 54:810–820
Pal A, Das S (2015) Morphine causes persistent induction of nitrated neurofilaments in cortex and subcortex even during abstinence. Neuroscience 291:177–188
Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, Lupton JR, Chapkin RS (2005) The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis 26:1914–1921
Boneva NB, Yamashima T (2012) New insights into ‘‘GPR40-CREB interaction in adult neurogenesis’’ specific for primates. Hippocampus 22:896–905
Calderon F, Kim HY (2004) Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 90:979–988
Cao D, Xue R, Xu J, Liu Z (2005) Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem 16:538–546
Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY (2009) Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem 111:510–521
He C, Qu X, Cui L, Wang J, Kang JX (2009) Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci USA 106:11370–11375
Robson LG, Dyall S, Sidloff D, Michael-Titus AT (2010) Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurons throughout development and in aged animals. Neurobiol Aging 31:678–687
Han S, Zhang KH, Lu PH, Xu XM (2004) Effects of annexins II and V on survival of neurons and astrocytes in vitro. Acta Pharmacol Sin 25:602–610
Roseman BJ, Bollen A, Hsu J, Lamborn K, Israel MA (1994) Annexin II marks astrocytic brain tumors of high histologic grade. Oncol Res 6:561–567
Zhai H, Acharya S, Gravanis I, Mehmood S, Seidman RJ, Shroyer KR, Hajjar KA, Tsirka SE (2011) Annexin A2 promotes glioma cell invasion and tumor progression. J Neurosci 31:14346–14360
Madureira PA, Hill R, Miller VA, Giacomantonio C, Lee PW, Waisman DM (2011) Annexin A2 is a novel cellular redox regulatory protein involved in tumorigenesis. Oncotarget 2:1075–1093
D’Eliseo D, Velotti F (2016) Omega-3 fatty acids and cancer cell cytotoxicity: implications for ulti-targeted cancer therapy. J Clin Med 5:15
Cao WD, Kawai N, Miyake K, Zhang X, Fei Z, Tamiya T (2014) Relationship of 14-3-3zeta (ζ), HIF-1α, and VEGF expression in human brain gliomas. Brain Tumor Pathol 31:1–10
Hekman M, Albert S, Galmiche A, Rennefahrt UE, Fueller J, Fischer A, Puehringer D, Wiese S, Rapp UR (2006) Reversible membrane interaction of BAD requires two C-terminal lipid binding domains in conjunction with 14-3-3 protein binding. J Biol Chem 281:17321–17336
Cao W, Yang X, Zhou J, Teng Z, Cao L, Zhang X, Fei Z (2010) Targeting 14-3-3 protein, difopein induces apoptosis of human glioma cells and suppresses tumor growth in mice. Apoptosis 15:230–241
Niemantsverdriet M, Wagner K, Visser M, Backendorf C (2008) Cellular functions of 14-3-3 zeta in apoptosis and cell adhesion emphasize its oncogenic character. Oncogene 27:1315–1319
Lee JH, Kwon EJ, Kim do H (2013) Calumenin has a role in the alleviation of ER stress in neonatal rat cardiomyocytes. Biochem Biophys Res Commun 439:327–332
Nagano K, Imai S, Zhao X, Yamashita T, Yoshioka Y, Abe Y, Mukai Y, Kamada H, Nakagawa S, Tsutsumi Y, Tsunoda S (2015) Identification and evaluation of metastasis-related proteins, oxysterol binding protein-like 5 and calumenin, in lung tumors. Int J Oncol 47:195–203
Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta 1833:3460–3470
Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z (2012) PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 150:685–696
Shi HS, Li D, Zhang J, Wang YS, Yang L, Zhang HL, Wang XH, Mu B, Wang W, Ma Y, Guo FC, Wei YQ (2010) Silencing of pkm2 increases the efficacy of docetaxel in humanlung cancer xenografts in mice. Cancer Sci 101:1447–1453
Guo W, Zhang Y, Chen T, Wang Y, Xue J, Zhang Y, Xiao W, Mo X, Lu Y (2011) Efficacy of RNAi targeting of pyruvate kinase M2 combined with cisplatin in a lung cancer model. J Cancer Res Clin Oncol 137:65–72
Goldberg MS, Sharp PA (2012) Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med 209:217–224
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334:1278–1283
Bobba A, Amadoro G, La Piana G, Petragallo VA, Calissano P, Atlante A (2015) Glucose-6-phosphate tips the balance in modulating apoptosis in cerebellar granule cells. FEBS Lett 589:651–658
Chung YT, Matkowskyj KA, Li H, Bai H, Zhang W, Tsao MS, Liao J, Yang GY (2012) Overexpression and oncogenic function of aldo–keto reductase family 1B10 (AKR1B10) in pancreatic carcinoma. Mod Pathol 25:758–766
Yan LJ (2014) Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res 2014:137919
Merendino N, Loppi B, D’Aquino M, Molinari R, Pessina G, Romano C, Velotti F (2005) Docosahexaenoic acid induces apoptosis in the human PaCa-44 pancreatic cancer cell line by active reduced glutathione extrusion and lipid peroxidation. Nutr Cancer 52:225–233
Brockmann A, Bluwstein A, Kögel A, May S, Marx A, Tschan MP, Brunner T (2015) Thiazolides promote apoptosis in colorectal tumor cells via MAP kinase-induced Bim and Puma activation. Cell Death Dis 6:e1778
Li X, Liang Q, Liu W, Zhang N, Xu L, Zhang X, Zhang J, Sung JJ, Yu J (2015) Ras association domain family member 10 suppresses gastric cancer growth by cooperating with GSTP1 to regulate JNK/c-Jun/AP-1 pathway. Oncogene. doi:10.1038/onc.2015.300 (Epub ahead of print)
van Beelen VA, Aarts JM, Reus A, Mooibroek H, Sijtsma L, Bosch D, Rietjens IM, Alink GM (2006) Differential induction of electrophile-responsive element-regulated genes by n-3 and n-6 polyunsaturated fatty acids. FEBS Lett 580:4587–4590
Lo HW, Ali-Osman F (2002) Cyclic AMP mediated GSTP1 gene activation in tumor cells involves the interaction of activated CREB-1 with the GSTP1 CRE: a novel mechanism of cellular GSTP1 gene regulation. J Cell Biochem 87:103–116
Saeki K, Yuo A, Suzuki E, Yazaki Y, Takaku F (1999) Aberrant expression of cAMP-response-element-binding protein (‘CREB’) induces apoptosis. Biochem J 343:249–255
Narayanan BA, Narayanan NK, Reddy BS (2001) Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int J Oncol 19:1255–1262
Martinasso G, Oraldi M, Trombetta A, Maggiora M, Bertetto O, Canuto RA, Muzio G (2007) Involvement of PPARs in cell proliferation and apoptosis in human colon cancer specimens and in normal and cancer cell lines. PPAR Res 2007:93416
Diep QN, Touyz RM, Schiffrin EL (2000) Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension 36:851–855
Kato T, Kolenic N, Pardini RS (2007) Docosahexaenoic acid (DHA), a primary tumor suppressive omega-3 fatty acid, inhibits growth of colorectal cancer independent of p53 mutational status. Nutr Cancer 58:178–187
Shaul YD, Seger R (2007) The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 1773:1213–1226
Das M, Ghosh M, Das S (2016) Thyroid Hormone-induced differentiation of astrocytes is associated with transcriptional upregulation of β-arrestin-1 and β-adrenergic receptor-mediated endosomal signaling. Mol Neurobiol 53(8):5178–5190
Zamarbide M, Etayo-Labiano I, Ricobaraza A, Martínez-Pinilla E, Aymerich MS, Luis Lanciego J, Pérez-Mediavilla A, Franco R (2014) GPR40 activation leads to CREB and ERK phosphorylation in primary cultures of neurons from the mouse CNS and in human neuroblastoma cells. Hippocampus 24:733–739
Cherkaoui-Malki M, Meyer K, Cao WQ, Latruffe N, Yeldandi AV, Rao MS, Bradfield CA, Reddy JK (2001) Identification of novel peroxisome proliferator-activated receptor alpha (PPARalpha) target genes in mouse liver using cDNA microarray analysis. Gene Expr 9:291–304
Acknowledgments
The study was funded by the Council of Scientific and Industrial Research (CSIR), New Delhi, India. MD was a recipient of fellowship also from the CSIR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Das, M., Das, S. Identification of cytotoxic mediators and their putative role in the signaling pathways during docosahexaenoic acid (DHA)-induced apoptosis of cancer cells. Apoptosis 21, 1408–1421 (2016). https://doi.org/10.1007/s10495-016-1298-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1298-2