Abstract
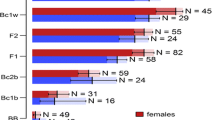
Host adaptation in herbivorous arthropods is one of the first steps to sympatric speciation, and spider mites (Acari: Tetranychidae) are useful model organisms for studying this phenomenon. Many researchers have studied the process of host adaptation via artificial selection experiments. Recent analyses suggest that hybridisation has diversified host ranges, although empirical evidence is scarce. We explored the host exploitation of two strains of Eotetranychus asiaticus established from Ternstroemia gymnanthera (Pentaphylacaceae) and Japanese cinnamon, Cinnamomum yabunikkei (Lauraceae), and evaluated the effect of hybridisation on offspring host use. Transplant experiments showed that females oviposited and immature mites developed only on their native hosts, suggesting specialisation to the secondary metabolites of each host plant. However, F1 hybrids from reciprocal crosses developed on both host plants (survival rate: 92–100%) with normal female-biased sex ratios. Furthermore, all backcrosses to the parental strains yielded B1 offspring that were also viable on both host plants with normal sex ratios (69–87% and 39–92% females on T. gymnanthera and C. yabunikkei, respectively). B1 survival rates in interstrain crosses were varied (11–63%) and lower than those in intrastrain crosses (88–93%). We could not detect any reproductive barriers in these experiments, and host preference may be the sole factor determining pre-mating isolation. The survival rates and sex ratios we observed suggest cytochromosome interactions. In conclusion, hybridisation, which results in heterozygotes and recombination, is an underexplored way to provide spider mites with a novel host plant.


Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Agrawal AA (2000) Host-range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81:500–508
Agresti A (2002) Categorical Data Analysis, 2nd edn. Wiley, Hoboken
Agrawal AA, Vala F, Sabelis MW (2002) Induction of preference and performance after acclimation to novel hosts in a phytophagous spider mite: adapt plasticity? Am Nat 159:553–565
Becerra JX (1997) Insects on plants: macroevolutionary chemical trends in host use. Science 276:253–256. https://doi.org/10.1126/science.276.5310.253
Blair CP, Abrahamson WG, Jackman JA, Tyrrell L (2005) Cryptic speciation and host-race formation in a purportedly generalist tumbling flower beetle. Evolution 59:304–316. https://doi.org/10.1111/j.0014-3820.2005.tb00991.x
Crawley MJ (2005) Statistics: An Introduction Using R. Wiley, West Sussex
Carroll SP, Boyd C (1992) Host race radiation in the soapberry bug: natural history with the history. Evolution 46:1052–1069. https://doi.org/10.1111/j.1558-5646.1992.tb00619.x
Coyne JA, Orr HA (2004) Speciation. Sinauer, Associates. Incorporated Publishers, Sunderland
Calatayud J, Hórreo JL, Madrigal-González J, Migeon A, Rodríguez M, Magalhães S, Hortal J (2016) Geography and major host evolutionary transitions shape the resource use of plant parasites. Proc Natl Acad Sci 113:9840–9845
Chae Y, Yokoyama N, Ito K, Fukuda T, Arakawa R, Zhang YX, Saito Y (2015) Reproductive isolation between Stigmaeopsis celarius and its sibling species sympatrically inhabiting bamboo (Pleioblastus spp.) plants. Exp Appl Acarol 66:11–23. https://doi.org/10.1007/s10493-014-9865-0
Chatzivasileiadis EA, Boon JJ, Sabelis MW (1999) Accumulation and turnover of 2-tridecanone in Tetranychus urticae and its consequences for resistance of wild and cultivated tomatoes. Exp Appl Acarol 23:1011–1021. https://doi.org/10.1023/a:1006394109643
Craig TP, Horner JD, Itami JK (1997) Hybridization studies on the host races of Eurosta solidaginis: implications for sympatric speciation. Evolution 51:1552–1560. https://doi.org/10.1111/j.1558-5646.1997.tb01478.x
de Boer R (1982) Laboratory hybridization between semi-incompatible races of the arrhenotokous spider mite Tetranychus urticae Koch (Acari: Tetranychidae). Evolution 36:553–560. https://doi.org/10.2307/2408100
Dobzhansky T (1946) Genetics of natural populations. Xiii. Recombination and variability in populations of Drosophila pseudoobscura. Genetics 31:269–290
Diehl S, Bush G (1984) An evolutionary and applied perspective of insect biotypes. Ann Rev Entomol 29:471–504
Drès M, Mallet J (2002) Host races in plant–feeding insects and their importance in sympatric speciation. Philos Trans R Soc Lond B Biol Sci 357:471–492
Dasmahapatra KK, Walters JR, Briscoe AD et al (2012) Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487:94–98. https://doi.org/10.1038/nature11041
Ehara S (1966) The tetranychoid mites of Okinawa island (Acarina: Prostigmata). J Fac Sci Hokkaido Univ Ser 6 Zool 16:1–22
Ehara S (1969) Three spider mites of the genus Eotetranychus infesting fruit trees in Japan: (Acarina: Tetranychidae). Appl Entomol Zool 4:16–22
Ehara S (1999) Revision of the spider mite family Tetranychidae of Japan (Acari, Prostigmata). Species Divers 4:63–141
Ehara S, Gotoh T (2009) Colored Guide to the Plant Mites of Japan. Zenkoku Noson Kyoiku Kyokai, Tokyo
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Fry JD (1989) Evolutionary adaptation to host plants in a laboratory population of the phytophagous mite Tetranychus urticae Koch. Oecologia 81:559–565
Fry JD (1990) Trade-offs in fitness on different hosts: evidence from a selection experiment with a phytophagous mite. Am Nat 136:569–580
Fry JD (1999) The role of adaptation to host plants in the evolution of reproductive isolation: negative evidence from Tetranychus urticae Koch. Exp Appl Acarol 23:379–387. https://doi.org/10.1023/a:1006245711950
Fry JD (2003) Detecting ecological trade-offs using selection experiments. Ecology 84:1672–1678
Futuyma DJ, Peterson SC (1985) Genetic variation in the use of resources by insects. Ann Rev Entomol 30:217–238
Feder JL, Chilcote CA, Bush GL (1988) Genetic differentiation between sympatric host races of the apple maggot fly Rhagoletis pomonella. Nature 336:61–64
Fellous S, Angot G, Orsucci M, Migeon A, Auger P, Olivieri I, Navajas M (2014) Combining experimental evolution and field population assays to study the evolution of host range breadth. J Evol Biol 27:911–919. https://doi.org/10.1111/jeb.12362
Filchak KE, Feder JL, Roethele JB, Stolz U (1999) A field test for host-plant dependent selection on larvae of the apple maggot fly, Rhagoletis pomonella. Evolution 53:187–200
Fujimoto H, Hiramatsu T, Takafuji A (1996) Reproductive interference between Panonychus mori Yokoyama and P. citri (McGregor) (Acari: Tetranychidae) in peach orchards. Appl Entomol Zool 31:59–65
Funk DJ, Filchak KE, Feder JL (2002) Herbivorous insects: model systems for the comparative study of speciation ecology. Genetica 116:251–267
Gotoh T (1986) Reproductive isolation between the two forms of Panonychus akitanus Ehara (Acarina: Tetranychidae). Exp Appl Acarol 2:153–160
Gould F (1979) Rapid host range evolution in a population of the phytophagous mite Tetranychus urticae Koch. Evolution 33:791–802
Gomi K, Gotoh T (1996) Host plant preference and genetic compatibility of the kanzawa spider mite, Tetranychus kanzawai Kishida (Acari: Tetranychidae). Appl Entomol Zool 31:417–425
Gotoh T, Bruin J, Sabelis MW, Menken SBJ (1993) Host race formation in Tetranychus urticae: genetic differentiation, host plant preference, and mate choice in a tomato and a cucumber strain. Entomol Exp Appl 68:171–178. https://doi.org/10.1007/bf02380535
Gotoh T, Oku H, Moriya K, Odawara M (1995) Nucleus–cytoplasm interactions causing reproductive incompatibility between two populations of Tetranychus quercivorus Ehara et gotoh (Acari: Tetranychidae). Heredity 74:405–414
Gotoh T, Suwa A, Kitashima Y, Rezk HA (2004) Developmental and reproductive performance of Tetranychus pueraricola Ehara and Gotoh (Acari: Tetranychidae) at four constant temperatures. Appl Entomol Zool 39:675–682
Greco NM, Pereyra PC et al (2006) Host-plant acceptance and performance of Tetranychus urticae (Acari, Tetranychidae). J Appl Entomol 130:32–36
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Kitashima Y, Gotoh T (1997) Electrophoretic differentiation of F1 hybrids of the sibling species Panonychus osmanthi Ehara et Gotoh and P. citri (McGregor) (Acari: Tetranychidae). Appl Entomol Zool 32:635–637. https://doi.org/10.1303/aez.32.635
Kobayashi N, Kumagai M, Minegishi D, Tamura K, Aotsuka T, Katakura H (2011) Molecular population genetics of a host-associated sibling species complex of phytophagous ladybird beetles (Coleoptera: Coccinellidae: Epilachninae). J Zool Syst Evol Res 49:16–24. https://doi.org/10.1111/j.1439-0469.2010.00581.x
Kumar S, Kumari R, Mishra S (2019) Pharmacological properties and their medicinal uses of cinnamomum: a review. J Pharm Pharmacol 71:1735–1761. https://doi.org/10.1111/jphp.13173
Langford SD, Boor PJ (1996) Oleander toxicity: an examination of human and animal toxic exposures. Toxicology 109:1–13. https://doi.org/10.1016/0300-483X(95)03296-R
Menken SB (1981) Host races and sympatric speciation in small ermine moths, Yponomeutidae. Entomol Exp Appl 30:280–292
Moriguchi M (2021) Sea animals at construction rituals in islands of Ryukyu archipelago. Reg Stud 27:69–79 (in Japanese)
Moriguchi M (2015) Report on gyodoku fishing in Ryukyu archipelago. J Hum Soc Sci (Proc Okinawa Univ) 17:69–75. https://doi.org/10.34415/00000254
Matsubayashi KW, Katakura H (2009) Contribution of multiple isolating barriers to reproductive isolation between a pair of phytophagous ladybird beetles. Evolution 63:2563–2580
Mori K, Saito Y (2013) Genetic basis of woven nest size in subsocial spider mites. Exp Appl Acarol 60:463–469
Murtaugh MP, Wrensch DL (1978) Interspecific competition and hybridization between twospotted and carmine spider mites. Ann Entomol Soc Am 71:862–864. https://doi.org/10.1093/aesa/71.6.862
Magalhães S, Fayard J, Janssen A, Carbonell D, Olivieri I (2007) Adaptation in a spider mite population after long-term evolution on a single host plant. J Evol biol 20:2016–2027. https://doi.org/10.1111/j.1420-9101.2007.01365.x
Marinosci C, Magalhães S, Macke E, Navajas M, Carbonell D, Devaux C, Olivieri I (2015) Effects of host plant on life-history traits in the polyphagous spider mite tetranychus urticae. Ecol Evol 5:3151–3158. https://doi.org/10.1002/ece3.1554
Matsuda T, Kozaki T, Ishii K, Gotoh T (2018) Phylogeny of the spider mite sub-family Tetranychinae (Acari: Tetranychidae) inferred from RNA-Seq data. PLOS ONE 13(9):e0203136
Matsuda T, Morishita M, Hinomoto N, Gotoh T (2014) Phylogenetic analysis of the spider mite sub-family Tetranychinae (Acari: Tetranychidae) based on the mitochondrial COI gene and the 18S and the 5′ end of the 28S rRNA genes indicates that several genera are polyphyletic. PLOS ONE 9(10):e108672
Mavárez J, Salazar CA, Bermingham E, Salcedo C, Jiggins CD, Linares M (2006) Speciation by hybridization in Heliconius butterflies. Nature 441:868–871
Nagatomo S (1973) Biological observation on the six-spotted spider mite, Eotetranychus sexmaculatus Riley. Chagyo Kenkyu Hokoku (Tea Research Journal) 40:31–36
Navajas M (1998) Host plant associations in the spider mite tetranychus urticae (Acari: Tetranychidae): insights from molecular phylogeography. Exp Appl Acarol 22:201–214
Navajas M, Gutierrez J, Williams M, Gotoh T (2001) Synonymy between two spider mite species, Tetranychus kanzawai and T. Hydrangeae (Acari: Tetranychidae), shown by ribosomal ITS2 sequences and cross-breeding experiments. Bull Entomol Res 91:117–123
Nishi N, Miyaji K, Fukumoto T, Hamashima A, Uchino K, Kisaki K, Kumamoto O, Sakaue T, Inamori H, Sakamaki Y (2019) Arthropod pests of the avocado plant in Kagoshima prefecture, Japan. Kyushu Plant Prot Res 65:24–29. https://doi.org/10.4241/kyubyochu.65.24
Nosil P, Crespi BJ, Sandoval P (2002) Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature 417:440–443
Ohshima I (2010) Host-associated pre-mating reproductive isolation between host races of Acrocercops transecta: mating site preferences and effect of host presence on mating. Ecol Entomol 35:253–257
Osakabe MH (2002) Which predatory mite can control both a dominant mite pest, Tetranychus urticae, and a latent mite pest: Eotetranychus asiaticus, on strawberry? Exp Appl Acarol 26:219–230
Osakabe M, Komazaki S (1996) Host range segregation and reproductive incompatibility among Panonychus citri populations infesting osmanthus trees and other host plants. Appl Entomol Zool 31:397–406
Okuzaki Y, Takami Y, Sota T (2010) Resource partitioning or reproductive isolation: the ecological role of body size differences among closely related species in sympatry. J Anim Ecol 79:383–392
Ottenburghs J, Ydenberg RC, Van Hooft P, Van Wieren SE, Prins HHT (2015) The avian hybrids project: gathering the scientific literature on avian hybridization. Ibis 157:892–894. https://doi.org/10.1111/ibi.12285
R Core Team (2022) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Roff DA (1997) Evolutionary Quantitative Genetics. Chapman & Hall, New York
Rioja C, Zhurov V, Bruinsma V, Grbic M, Grbic V (2017) Plant-herbivore interactions: a case of an extreme generalist, the two-spotted spider mite tetranychus urticae. Mol Plant Microbe Interact 30:935–945
Saito Y (1983) The concept of “life types” in Tetranychinae. An attempt to classify the spinning behaviour of Tetranychinae. Acarologia 24:377–391
Saito Y (1985) Life types of spider mites. In: Helle W, Sabelis MW (eds) Spider Mites Their Biology, Natural Enemies and Control 1A. Elsevierm, Amsterdam, pp 253–264
Saito Y (1987) Extraordinary effects of fertilization status on the reproduction of an arrhenotokous and sub-social spider mite (Acari: Tetranychidae). Res Popul Ecol 29:57–71
Smith JW (1975) Spider mites: Population suppression by interspecific hybridization. Environ Entomol 4:588–590
Stearns SC (1992) The Evolution of Life Histories. Oxford University Press, Oxford
Saito Y, Lin JZ, Zhang YX, Ito K, Liu Q, Chittenden AR (2016) Two new species and four new life types in Tetranychidae. Ann Entomol Soc Am 109:463–472. https://doi.org/10.1093/aesa/sav158
Sangeet L, Fan H, Matthew TW, Leif A, Grant BR, Peter RG (2018) Rapid hybrid speciation in darwin’s finches. Science 359:224–228. https://doi.org/10.1126/science.aao4593
Sato Y, Saito Y, Mori K (2000a) Patterns of reproductive isolation between two groups of Schizotetranychus miscanthi Saito (Acari: Tetranychidae) showing different male aggression traits. Appl Entomol Zool 35:611–618
Sato Y, Saito Y, Mori K (2000b) Reproductive isolation between populations showing different aggression in a subsocial spider mite, Schizotetranychus miscanthi Saito (Acari: Tetranychidae). Appl Entomol Zool 35:605–610
Sato Y, Alba JM, Sabelis MW (2014) Testing for reproductive interference in the population dynamics of two congeneric species of herbivorous mites. Heredity 113:495–502
Sato Y, Breeuwer JAJ, Egas M, Sabelis MW (2015) Incomplete premating and postmating reproductive barriers between two parapatric populations of a social spider mite. Exp Appl Acarol 65:277–291
Sato Y, Sakamoto H, Gotoh T, Saito Y, Chao JT, Egas M, Mochizuki A (2018) Patterns of reproductive isolation in a haplodiploid–strong postmating, prezygotic barriers among three forms of a social spider mite. J Evol Biol 31:866–881
Sato Y, Fujiwara S, Egas M, Matsuda T, Gotoh T (2021) Patterns of reproductive isolation in a haplodiploid mite, Amphitetranychus viennensis: prezygotic isolation, hybrid inviability and hybrid sterility. BMC Ecol Evol 21:177. https://doi.org/10.1186/s12862-021-01896-5
Sousa VC, Zélé F, Rodrigues LR, Godinho DP, Charlery de la Masselière M, Magalhães S (2019) Rapid host-plant adaptation in the herbivorous spider mite tetranychus urticae occurs at low cost. Curr Opin Insect Sci 36:82–89
Sugasawa J, Kitashima Y, Gotoh T (2002) Hybrid affinities between the green and the red forms of the two-spotted spider mite tetranychus urticae (Acari: Tetranychidae) under laboratory and semi-natural conditions. Appl Entomol Zool 37:127–139. https://doi.org/10.1303/aez.2002.127
Suzuki H, Yasuda K, Ohashi K, Takahashi H, Fukaya M, Yano S, Osakabe M (2011) Kanzawa spider mites acquire enemy-free space on a detrimental host plant, oleander. Entomol Exp et Appl 138:212–222. https://doi.org/10.1111/j.1570-7458.2010.01092.x
Takafuji A, Fujimoto H (1985) Reproductive compatibility between populations of the citrus red mite, Panonychus citri (McGregor)(Acarina: Tetranychidae). Popul Ecol 27:361–372
Tanabe T, Sota T (2008) Complex copulatory behavior and the proximate effect of genital and body size differences on mechanical reproductive isolation in the millipede genus parafontaria. Am Nat 171:692–699
Tajima R, Ohashi K, Takafuji A (2007) Specific adaptation of sympatric populations of the kanzawa spider mite, Tetranychus kanzawai (Acari: Tetranychidae) to three host plants. J Acarol Soc Jpn 16:21–27
Thompson KA, Rieseberg LH, Schluter D (2018) Speciation and the city. Trends Ecol Evol 33:815–826
Turissini DA, McGirr JA, Patel SS, David JR, Matute DR (2018) The rate of evolution of postmating-prezygotic reproductive isolation in drosophila. Mol Biol Evol 35:312–334
Via S (2001) Sympatric speciation in animals: the ugly duckling grows up. Trends Ecol Evol 16:381–390
Venables WN, Ripley BD (2002) Modern Applied Statistics with S, 4th edn. Springer, New York
Villacis-Perez E, Snoeck S, Kurlovs AH, Clark RM, Breeuwer JAJ, Van Leeuwen T (2021) Adaptive divergence and post-zygotic barriers to gene flow between sympatric populations of a herbivorous mite. Commun Biol 4:853
Yano S, Takabayashi J, Takafuji A (2001) Trade-offs in performance on different plants may not restrict the host plant range of the phytophagous mite, Tetranychus urticae. Exp Appl Acarol 25:371–381
Zar JH (2010) Biostatistical Analysis, 5th edn. Prentice Hall, Hoboken
Zeileis A, Kleiber C, Jackman S (2008) Regression models for count data in R. J Stat Softw 27:8. http://www.jstatsoft.org/v27/i08/
Acknowledgements
I thank Drs. Tetsuo Gotoh, Hidenari Kishimoto, and Yasuki Kitashima for their valuable information concerning E. asiaticus. I thank Mr. Ren Iwasa for his support in collecting supplemental data and preparing this manuscript.
Funding
This work was supported by a Cabinet Office grant-in-aid and the Advanced Next-Generation Greenhouse Horticulture by IoP (Internet of Plants), Japan.
Author information
Authors and Affiliations
Contributions
KI: planning, data collection and analysis, investigation, discussion, writing, funding acquisition; KT: planning, data collection and analysis, investigation, discussion.
Corresponding author
Ethics declarations
Competing interests
We have no pecuniary or other personal interest, direct or indirect, in any matter that raises or may raise a conflict with our duties. The authors have no financial or non-financial interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ito, K., Takatsuki, K. Hybridisation between host races broadens the host range of offspring in Eotetranychus asiaticus (Acari: Tetranychidae). Exp Appl Acarol 90, 227–245 (2023). https://doi.org/10.1007/s10493-023-00811-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00811-5