Abstract
In a randomised trial, we found that integrated maternal HIV and infant health services through the end of breastfeeding were significantly associated with the primary outcome of engagement in HIV care and viral suppression at 12 months postpartum, compared to the standard of care. Here, we quantitatively explore potential psychosocial modifiers and mediators of this association. Our findings suggest that the intervention was significantly more effective among women experiencing an unintended pregnancy but did not improve outcomes among women reporting risky alcohol use. Although not statistically significant, our results suggest that the intervention may also be more effective among women experiencing higher levels of poverty and HIV-related stigma. We observed no definitive mediator of the intervention effect, but women allocated to integrated services reported better relationships with their healthcare providers through 12 months postpartum. These findings point to high-risk groups that may benefit the most from integrated care, as well as groups for whom these benefits are hampered and that warrant further attention in intervention development and evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increases in the availability and uptake of antiretroviral therapy (ART), including under guidelines of universal ART for pregnant and breastfeeding women, have led to massive reductions in new paediatric HIV infections [1,2,3]. However, poor retention in ART services remains a global concern, with particularly high levels of disengagement from HIV care documented among women during the postpartum period [4,5,6]. Pooled data from Option B + programs in Africa suggest that approximately 75% of women are retained in HIV care through 6–12 months postpartum [5]. In South Africa and many similar settings, prevention of mother-to-child transmission (PMTCT) services are integrated into antenatal care during pregnancy, but the timing of transfer out of antenatal services during the postpartum period differs across countries [7]. In South Africa, this transfer occurs immediately postpartum. Under these policies, women receive HIV services separate from routine infant care which may be provided in different locations. Across and within countries, policies related to the timing of transfer may be implemented differently in different health facilities [8], and transfer out of antenatal services has been highlighted as a major point of loss to follow-up in the maternal HIV care cascade [2, 9].
An approach which has been posited to reduce disengagement from care is the integration of HIV care into other routine health services, although the current evidence for effectiveness is mixed [10, 11]. In settings where integrated services have been shown to be effective, there is a need to examine factors that potentially modify intervention effects, and to explore the mechanisms of change leading to improved outcomes. This evidence is particularly important in low-resource settings to refine interventions and potentially target them to particular groups for whom they are most beneficial. In the context of a randomised controlled trial in Cape Town, South Africa [the “Maternal and Child Health – Antiretroviral Therapy” (MCH-ART) study], we compared the existing standard of care (control) to an integrated service (intervention), where maternal ART and infant health care were provided concurrently and in the same maternal and child health (MCH) clinic through the end of breastfeeding. We found that providing integrated services significantly increased the proportion of women who were engaged in HIV care and virally suppressed at 12 months postpartum [12]. We posited that the effectiveness of the intervention overall may be due to factors which include a lower burden of clinic visits due to co-located care; lower levels of HIV-related stigma through continued receipt of ART in the MCH clinic; and prolonged positive relationships with nurse-midwives in this clinic [13,14,15]. Further, we observed that the intervention was more effective among women initiating ART later in pregnancy, compared to those initiating ART earlier [12].
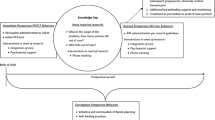
Given the promise of this model of care for postpartum women, there is a need to refine and target future implementation of this intervention. Critical to this refinement is the identification of factors that may modify intervention effects, and an exploration of mechanisms of change. A framework that may be beneficial to this is Social Action Theory (SAT). SAT delineates a model of contextual determinants as well as social interaction and individual self-regulatory processes, and the mechanisms by which these lead to health behaviours [16, 17]. Contextual factors known to be associated with poor ART outcomes include an individual’s background characteristics such as poverty [18,19,20], and internal contextual factors include mental health problems such as depression or psychological distress [18, 21, 22] as well as alcohol use [19, 21,22,23,24]. Among pregnant women specifically, other contextual determinants include late entry into antenatal care [18,19,20] and an unintended pregnancy [25, 26]. Social interaction processes that may negatively affect ART outcomes include negatives attitudes from or treatment by healthcare providers [18,19,20], HIV-related stigma [18,19,20, 27, 28], low levels of social support [18, 19, 22], and the fear or experience of intimate partner violence (IPV) [19, 20]. Finally, factors known to be associated with suboptimal ART behaviours at the level of individual self-regulation include poor knowledge about HIV and ART [19, 20, 29, 30], negative beliefs and concerns about ART use [19, 20, 31, 32], and low levels of adherence-self efficacy [20, 21, 33].
Following the conclusion of the MCH-ART randomised controlled trial, our aim here was to quantitatively examine (i) psychosocial modifiers of the intervention effect and (ii) potential mediators of the effect. The goal of examining modifiers was to identify groups for whom the intervention was most beneficial and to whom the intervention could be targeted, as well as groups for whom the intervention was not effective and that warrant further attention in intervention development. In parallel, the goal of examining mediators was to understand how integrated services lead to improved outcomes, with a view to refining interventions by identifying the most effective components.
Methods
This analysis draws on data from the MCH-ART trial, which evaluated integrated services during the postpartum period. The design and primary results of the trial have been previously reported [12, 34]. Briefly, the study was conducted in a primary care antenatal clinic in the community of Gugulethu, Cape Town, where an antenatal HIV prevalence of ~ 30% has been reported [35]. In this setting, women experience multiple overlapping risks, including poverty, unintended pregnancy, alcohol use, and IPV [36,37,38]. For the MCH-ART study, women living with HIV were enrolled when entering antenatal care, and women initiating ART were followed through delivery. Following enrolment, women attended up to three study measurement visits, coinciding with their 2nd antenatal visit; late 3rd trimester; and immediately postpartum.
MCH-ART Intervention
Women who attended the immediate postpartum study visit and were currently breastfeeding [471 (75%) of 628 women enrolled into antenatal follow-up] were enrolled into postpartum follow-up and randomly allocated. Random allocation was to the MCH-ART intervention (integrated concurrent and co-located maternal ART and paediatric care in the MCH clinic through the end of breastfeeding) or the standard of care (control), where women were referred as soon as possible postpartum to general HIV clinics, and their infants were referred to routine child health services. Although all postpartum women may benefit from integrated HIV and MCH care, the goal of this trial was to focus on breastfeeding women, given the ongoing risk of mother-to-child transmission (MTCT). Thus, only breastfeeding women were enrolled into the trial; a secondary aim of the trial was to explore the impact of the intervention on breastfeeding practices. Women subsequently attended up to five study measurement visits separate from any routine health services, at 6 weeks and 3, 6, 9 and 12 months postpartum. This analysis includes all women with primary outcome data available at 12 months postpartum [411 (87%) of 471 women enrolled]. All participants provided written informed consent prior to study enrolment. The study was approved by the Faculty of Health Sciences Human Research Ethics Committee at the University of Cape Town, and the Institutional Review Board of Columbia University Medical Centre.
Measures
Table 1 details the measures assessed at each study measurement visit, relevant to this analysis. All measures were administered by trained interviewers in participants’ home language, predominantly isiXhosa. Instruments were translated from English into isiXhosa and were back-translated using standard procedures [39]. Sociodemographic characteristics were assessed at enrolment, and a composite poverty score was created based on current employment status, type of housing (formal house or informal shack) and access to household assets (a flush toilet, piped water inside the home, electricity, a refrigerator, a telephone, and a television). Distribution-based cut-offs were used to categorize participants into three groups, representing the most disadvantaged (lowest scores), moderately disadvantaged (middle), and least disadvantaged women (highest scores) relative to other participants in the study based on this composite score. Women were asked using a single item whether they were trying to have a baby when they became pregnant, with pregnancy intentions categorised as planned versus unplanned.
At various antenatal and postpartum timepoints, a battery of psychosocial measures was administered. Measures were selected drawing on SAT, described above, and on factors known to be associated with adherence behaviours. We adapted previously used tools to assess HIV and HIV treatment knowledge [40,41,42], ART medication beliefs [43] and adherence self-efficacy [44]. Knowledge scores were summed, and we calculated mean scores for medication beliefs and self-efficacy; higher scores on each scale indicate higher levels of knowledge, beliefs about the necessity of taking ART, and adherence self-efficacy, respectively. We used items adapted from the Social Impact Scale to measure enacted and internalised HIV-related stigma [45], and from a measure of the perceived availability of social support to measure social support [46, 47]; both measures have been shown to perform well in this setting [48]. For these measures, higher scores indicate higher levels of HIV-related stigma and higher levels of perceived social support, respectively. Finally, women’s relationships with their routine healthcare providers were assessed using a 19-item Patient-Healthcare Provider Relationship Scale which was developed and validated among South African pregnant women [49], with higher scores indicating better patient-provider relationships. In analysis, we explored each of these constructs as continuous variables and using categories of above versus below the median score to classify participants according to relative levels.
Depression during the past week was assessed using the Edinburgh Postnatal Depression Scale (EPDS), which has been validated for use during pregnancy [50], with a score of ≥ 13 used to indicate possible depression [51]. Psychological distress during the past month was measured using the Kessler-10 (K-10) scale [52], with a threshold of ≥ 21.5 used to indicate psychological distress [53]. Alcohol use was measured using the Alcohol Use Disorders Identification Test - Consumption (AUDIT-C), with a score of ≥ 3 used to indicate risky drinking [54, 55]. At the 2nd antenatal study visit, women were asked to report alcohol use during the 12 months prior to pregnancy recognition; at the study visit conducted during the late 3rd trimester, women were asked about alcohol use during pregnancy. IPV was assessed using the World Health Organization Violence Against Women tool [56], with violence defined as any report of psychological, physical or sexual violence. Violence during the 12 months prior to pregnancy recognition was assessed at the 2nd antenatal study visit, and violence during pregnancy was assessed immediately postpartum.
At study visits, women provided 5ml of venous blood for HIV viral load (VL) testing, conducted by the National Health Laboratory Service (NHLS) using the Abbott Molecular RealTime HIV-1 assay (Abbott Molecular, Illinois, USA). Data on health service usage was requested from the Western Cape Provincial Health Data Centre, including data pertaining to HIV care visits; pharmacy dispensing; and laboratory results. The primary outcome for the MCH-ART trial was a binary composite endpoint of retention in care and viral suppression at 12 months postpartum. Retention in care was defined as any evidence of HIV-related clinical care received between 9 and 18 months postpartum, based on routinely collected records of HIV clinical care visits, ART dispensing, and HIV-related laboratory testing. Viral suppression was defined as VL < 50 copies/ml at the 12 month postpartum study visit. Women were considered as having achieved the primary endpoint if they had both evidence of retention in care and VL < 50 copies/mL [12].
Data Analysis
Data were analysed using STATA V14.2 (Stata Corporation, College Station, Texas, USA) and R (R Foundation for Statistical Computing, Vienna, Austria). To assess modifiers of the intervention effect, we used stratified χ2 analyses and regression models including interaction terms. We also calculated stratum-specific risk differences (RD) for each potential effect modifier using additive binomial models and report coefficients with 95% confidence intervals (CI). For these analyses of effect modification, we used data from study visits prior to randomisation. For measures that were assessed multiple times prior to randomisation, consistent results were observed using later measures, thus we report results using the first measure of each construct. Consistent results were also observed when using sub-scales of the psychosocial measures and when exploring the effect of different forms of IPV. To assess mediation, we explored associations between trial arm and potential mediators measured after randomisation; and calculated the percentage change in the RD corresponding to the intervention effect when each potential mediator was added to the model. All analyses were exploratory in nature, thus we did not correct for multiple comparisons or use confirmatory analytic techniques such as structural equation modelling [57]. Rather than relying solely on p-values to indicate statistical significance, we focus on effect sizes and 95% CI to indicate the precision of our findings [58].
Results
Between March 2013 and April 2014, 628 pregnant women living with HIV were enrolled into antenatal follow-up. Of these women, 471 (75%) attended the immediate postpartum study visit and reported breastfeeding, and were enrolled into the MCH-ART trial. Compared to those enrolled, women who were excluded had entered antenatal care earlier (mean: 19.3 weeks versus 20.9 weeks; z: -2.36; p-value: 0.019) and had higher HIV treatment knowledge scores (mean score: 6.6 versus 6.5 out of a maximum score of 8; z: 1.93; p-value: 0.053) and higher social support scores (mean score: 4.4 versus 4.2 out of a maximum score of 5; z: 1.83; p-value: 0.068); no other demographic or psychosocial characteristics differed between women enrolled into the trial versus those not enrolled (Supplementary Table 1). Of the 471 women enrolled into the trial, 411 (87%) contributed primary outcome data (202 allocated to the intervention arm, and 209 to the control) and are included in this analysis. The mean age overall was 28.8 years; 25% had completed secondary education; and 38% were currently employed. Just over half of women (54%) were newly diagnosed HIV-positive; and 41% were married and/or cohabiting with their male partner. Baseline characteristics were similar across intervention and control, although women allocated to the control had entered antenatal care at a slightly later gestation compared to women allocated to the intervention (mean: 21.1 versus 19.9 weeks; z: -1.70; p-value: 0.090; Table 2).
Modifiers of the Intervention Effect
Psychosocial characteristics relevant to the analyses of effect modification are summarised in Table 2. These measures were administered prior to randomisation. Overall, we observed high levels of unintended pregnancy, risky alcohol use and IPV; high levels of adherence self-efficacy and social support; and low levels of HIV and treatment knowledge. No psychosocial characteristics differed appreciably across the intervention versus control group. In the overall sample, the RD for the primary outcome between intervention and control was 0.21 [95% CI: 0.12, 0.30]. We observed no evidence of effect modification by HIV or treatment knowledge; ART medication beliefs; adherence-self efficacy; social support; or patient-provider relationships during the antenatal period (Fig. 1; Table 3). In addition, depression, psychological distress, and IPV did not modify the intervention effect.
However, there was some evidence that the intervention was more effective in particular groups of women, although not all differences were statistically significant (Fig. 1; Table 3). Figure 2 graphically illustrates these interaction effects by showing the proportion of women allocated to the intervention and control who achieved the primary trial outcome across groups. First, although not statistically significant, the intervention effect was stronger among women experiencing higher levels of poverty. Among women experiencing the highest levels of disadvantage, 81% of those in the intervention arm achieved the trial outcome, compared to 49% of those in the control (RD: 0.31; 95% CI: 0.17, 0.46). In contrast, 76% and 64% of women experiencing moderate levels of disadvantage and allocated to the intervention and control, respectively, achieved the trial outcome (RD: 0.12; 95% CI: -0.03, 0.28; p-value for interaction: 0.077). Among women experiencing the lowest levels of disadvantage and allocated to the intervention and control, 72% and 56% achieved the trial outcome, respectively (RD: 0.16; 95% CI: 0.001, 0.32; p-value for interaction: 0.170). Second, although also not statistically significant, the intervention effect was stronger among women reporting higher levels of HIV-related stigma. Among women reporting high levels of stigma, 81% of those in the intervention arm achieved the trial outcome, versus 53% of those in the control (RD: 0.29; 95% CI: 0.16, 0.41). In comparison, 72% and 59% of women reporting low levels of stigma and allocated to the intervention and control achieved the trial outcome, respectively (RD: 0.13; 95% CI: 0.01, 0.26; p-value for interaction: 0.094).
In addition, the intervention effect differed significantly according to two characteristics: pregnancy intention and alcohol use. Among women reporting that their pregnancy was unintended, 76% of women in the intervention arm achieved the trial outcome, versus 49% of those in the control (RD: 0.27; 95% CI: 0.16, 0.38). In comparison, 79% and 73% of those reporting that their pregnancy was intended and allocated to the intervention and control achieved the trial outcome, respectively (RD: 0.06; 95% CI: -0.09, 0.21; p-value for interaction: 0.025). Finally, women who reported risky drinking in the 12 months prior to pregnancy recognition were significantly less likely to benefit from the intervention. In this group of women, 64% of those in the intervention arm and 60% of those in the control achieved the trial outcome (RD: 0.04; 95% CI: -0.15, 0.23), compared to 81% and 55% among those not reporting risky drinking, respectively (RD: 0.26; 95% CI: 0.16, 0.36; p-value for interaction: 0.040).
Mediators of the Intervention Effect
No appreciable differences were observed in HIV or treatment knowledge, ART medication beliefs or adherence self-efficacy between the intervention and control group at study measurement visits after randomisation (Table 4). Similarly, levels of HIV-related stigma and social support did not differ across groups over time. However, we observed a strong association between trial arm and postpartum patient-provider relationships, with women allocated to the intervention reporting significantly better relationships with their routine healthcare providers at 6, 9 and 12 months postpartum compared to women allocated to the control (Table 4). When each potential mediator was added to a model of the intervention effect, the percentage change in the RD for the intervention effect was negligible across potential mediators, ranging from − 2.1 to 4.3% (Table 4). When examining the effect of variables measured after randomisation on the primary outcome (combined binary endpoint of retention in HIV care and viral suppression), few significant associations were observed (Table 5). Women who achieved the primary outcome had slightly higher adherence self-efficacy and social support scores at 6 months postpartum, but these associations were not sustained at 12 months postpartum. As reported above, women allocated to the intervention reported significantly better relationships with their healthcare providers compared to those allocated to the control, but patient-provider relationships were not associated with the primary outcome.
Discussion
Using data from the MCH-ART study, which showed that integrated maternal and child health care during the postpartum period significantly increased retention in HIV care and viral suppression [12], this analysis examined psychosocial modifiers and mediators of the intervention effect. Here, we found evidence to suggest that the intervention was more effective among women experiencing higher levels of poverty, an unintended pregnancy, and higher levels of HIV-related stigma, while the intervention was less effective among women reporting risky drinking. None of the psychosocial constructs assessed mediated the association between trial arm and the primary outcome. Compared to women allocated to the standard of care, women allocated to the intervention reported significantly better relationships with their healthcare providers through 12 months postpartum, but better patient-provider relationships did not lead to improved outcomes.
Taken together, the intervention appeared to be most effective among women experiencing added vulnerability due to poverty, unintended pregnancy, and HIV-related stigma. We have previously demonstrated that unintended pregnancy is associated with elevated VL during the postpartum period [26]. Here, these data suggest that integrated care may be more beneficial for women reporting an unintended, compared to an intended, pregnancy. Unintended pregnancy is associated with delayed initiation of antenatal care [59,60,61], and consequently ART initiation later in pregnancy, and typically occurs in the context of multiple other vulnerabilities [62]. Consistent with the findings of this analysis, we observed stronger intervention effects among women initiating ART later in pregnancy, compared to those initiating earlier, as part of the primary trial analysis [12]. We hypothesise that the intervention may have provided a foundation for establishing and maintaining ART adherence. Remaining in the integrated service after delivery and having additional time during which to establish positive ART behaviours may have been more beneficial for women who had entered antenatal care later, compared to women who had entered care earlier and who had had more time to establish these behaviours prior to delivery [12].
Our finding regarding alcohol use is of particular concern. In South Africa, alcohol use during pregnancy is a major public health problem. High levels of problematic alcohol use have been observed among women living both with and without HIV in this setting [63,64,65,66,67], and substance use itself is a risk factor for poor ART outcomes [19, 24, 67,68,69,70]. Notably, alcohol use modified the intervention effect in this trial, with the intervention not associated with retention in HIV care and viral suppression among women reporting risky drinking in the 12 months prior to pregnancy recognition. Given that 26% of women in this study reported risky drinking during this time period, this is a large, high-risk group that warrants further attention. Psychological interventions have shown some effectiveness in promoting abstinence or reducing consumption among pregnant and postpartum women [71], and may be effective in reducing alcohol use and improving ART adherence among non-pregnant women living with HIV in South Africa [72]. Our findings suggest that more intensive interventions may be needed in place of or in conjunction with integrated HIV care to improve ART outcomes among pregnant women reporting a history of risky drinking.
In this secondary analysis, we observed no single definitive mediator of the intervention effect. We hypothesise that the intervention may be effective through a range of mechanisms that may operate differently within different groups of women, and should be explored in a larger trial. For example, integrated MCH services reduce the economic burden of attending separate visits and may thus be of most benefit to women experiencing higher levels of poverty [73]. In addition, we hypothesise that receiving HIV care concurrently with infant health care within the MCH clinic may lead to less inadvertent disclosure than attending a separate adult HIV clinic, and thus may be most beneficial to women who experience the highest levels of stigma [12]. However, care must be taken to avoid inadvertent HIV-status disclosure when providing integrated PMTCT and MCH services [13]. Although the MCH-ART trial was conducted in a single clinic, these findings may be of relevance to all contexts where levels of poverty and stigma are high. In this study, women allocated to the intervention reported significantly better relationships with their healthcare providers. Although we did not observe an association between better patient-provider relationships and improved engagement in care and viral suppression, continuity of care with the same provider may have other positive effects. However, these effects may not persist after transfer out of integrated services to new healthcare providers, and further work is needed to support women after the point of transfer [74, 75].
These findings should be considered in light of several limitations. They arise from a single antenatal clinic in a peri-urban area of South Africa and only women who were initiating ART during pregnancy were enrolled into the trial, thus the findings may not be generalisable to all postpartum women living with HIV. Although all postpartum women may benefit from integrated HIV and MCH care, only breastfeeding women were enrolled into the trial, thus limiting generalisability. Pregnant women were enrolled into the trial between March 2013 and April 2014 and were followed through January 2016, and changes in PMTCT and HIV care since that time may make our findings less generalisable to the current context. The psychosocial constructs explored were self-reported and are subject to recall and social desirability bias, which may be particularly relevant for behaviours such as alcohol use, although we used validated scales wherever possible. However, it is plausible that we did not observe hypothesised mediators of the intervention effect due to the challenges of measuring complex constructs, with the measures administered potentially not accurately representing the construct of interest. Although these measures were originally developed several decades ago in high-income countries, the constructs assessed and items included in these scales are still relevant and applicable to multiple settings. Although we attempted to collect patient-level data on direct and indirect costs to measure the economic burden of attending clinic visits, these data were only available for a subset of participants and it was not possible to accurately quantify costs at the individual level [73]. In addition, true modifiers and mediators of the intervention effect may not have been measured as part of the trial. As noted above, all analyses should be considered exploratory. The small number of women in some psychosocial subgroups led to wide confidence intervals when examining potential modifiers of the intervention effect and may have reduced our power to detect significant differences, while also limiting our ability to examine mechanisms within different subgroups.
Conclusions
Despite these limitations, this analysis provides novel and important findings related to modifiers and mediators of the effectiveness of integrated care for postpartum women living with HIV. Our findings suggest that integrated services are associated with significantly better patient-provider relationships over time. Further, women who face additional vulnerabilities due to poverty and unintended pregnancy may benefit the most from integrated care, and integrated services could potentially be targeted to settings where these characteristics are particularly prevalent. Notably, the benefits of integrated care appear to be hampered by alcohol use, suggesting that women experiencing problematic alcohol use warrant further attention in intervention development and evaluation.
Data Availability
All relevant data are within the paper.
Code Availability
N/A.
References
Abrams EJ, Myer L. Can we achieve an AIDS-free generation? Perspectives on the global campaign to eliminate new pediatric HIV infections. J Acquir Immune Defic Syndr. 2013;63:208–12. https://doi.org/10.1097/QAI.0b013e3182986f55.
Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 infection. N Engl J Med. 2016;374:761–70. https://doi.org/10.1056/NEJMra1505256.
Mandelbrot L, Tubiana R, Le Chenadec J, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015;61(11):1715–25. https://doi.org/10.1093/cid/civ578.
Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the option B + approach. Curr Opin HIV AIDS. 2013;8:474–89. https://doi.org/10.1097/COH.0b013e328363a8f2.
Knettel BA, Cichowitz C, Samwel Ngocho J, et al. Retention in HIV care during pregnancy and the postpartum period in the option B + era: systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr. 2018;77:427–38. https://doi.org/10.1097/QAI.0000000000001616.
Myer L, Phillips TK. Beyond “Option B+”: understanding antiretroviral therapy (ART) adherence, retention in care and engagement in ART services among pregnant and postpartum women initiating therapy in sub-saharan Africa. J Acquir Immune Defic Syndr. 2017;75(Suppl 2):115–22. https://doi.org/10.1097/QAI.0000000000001343.
Jones H, Wringe A, Todd J, et al. Implementing prevention policies for mother-to-child transmission of HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016. Bull World Health Organ. 2019;97(3):200–12. https://doi.org/10.2471/BLT.18.217471.
Phillips TK, Olsen H, Teasdale CA, et al. Uninterrupted HIV treatment for women: policies and practices for care transitions during pregnancy and breastfeeding in Côte d’Ivoire, Lesotho and Malawi. PLoS ONE. 2021;16(12):e0260530. https://doi.org/10.1371/journal.pone.0260530.
Phillips T, McNairy ML, Zerbe A, Myer L, Abrams EJ. Postpartum transfer of care among HIV-infected women initiating antiretroviral therapy during pregnancy. J Acquir Immune Defic Syndr. 2015;70(3):e102–9. https://doi.org/10.1097/QAI.0000000000000771.
Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Trop Med Int Health. 2018;23(2):136–48. https://doi.org/10.1111/tmi.13014.
Geldsetzer P, Yapa HM, Vaikath M, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc. 2016;19(1):20679. https://doi.org/10.7448/IAS.19.1.20679.
Myer L, Phillips TK, Zerbe A, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: a randomised controlled trial. PLoS Med. 2018;15(3):e1002547. https://doi.org/10.1371/journal.pmed.1002547.
Berlacher M, Mercer T, Apondi EO, et al. Integrating prevention of mother-to-child transmission of HIV care into general maternal child health care in western Kenya. Int J MCH AIDS. 2021;10(1):19–28. https://doi.org/10.21106/ijma.429.
Duffy MH. Practical information and guidance for integration of MNCH and HIV programs within a continuum of health and social services. Arlington, VA: USAID’s AIDS Support and Technical Assistance Resources, AIDSTAR-One, Task Order 1. 2013.
Kiragu K, Collins L, Von Zinkernagel D, Mushavi A. Integrating PMTCT into maternal, newborn, and child health and related services: experiences from the global plan priority countries. J Acquir Immune Defic Syndr. 2017;75(Suppl 1):36–S42. https://doi.org/10.1097/QAI.0000000000001323.
Ewart CK. Social action theory for a public health psychology. Am Psychol. 1991;46(9):931–46. https://doi.org/10.1037//0003-066x.46.9.931.
Traube DE, Holloway IW, Smith L. Theory development for HIV behavioral health: empirical validation of behavior health models specific to HIV risk. AIDS. 2011;23(6):663–70. https://doi.org/10.1080/09540121.2010.532532.
Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588. https://doi.org/10.7448/IAS.16.1.18588.
Hodgson I, Plummer ML, Konopka SN, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS ONE. 2014;9(11):e111421. https://doi.org/10.1371/journal.pone.0111421.
Omonaiye O, Kusljic S, Nicholson P, Manias E. Medication adherence in pregnant women with human immunodeficiency virus receiving antiretroviral therapy in sub-saharan Africa: a systematic review. BMC Public Health. 2018;18:805. https://doi.org/10.1186/s12889-018-5651-y.
Nel A, Kagee A. Common mental health problems and antiretroviral therapy adherence. AIDS Care. 2011;23(11):1360–5. https://doi.org/10.1080/09540121.2011.565025.
Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-saharan Africa: a systematic review. BMJ Glob Health. 2016;1:e000125. https://doi.org/10.1136/bmjgh-2016-000125.
Ramlagan S, Peltzer K, Ruiter RAC, Barylski NA, Weiss SM, Sifunda S. Prevalence and factors associated with fixed-dose combination antiretroviral drugs adherence among HIV-positive pregnant women on option B treatment in Mpumalanga province, South Africa. Int J Environ Res Public Health. 2018;15:161. https://doi.org/10.3390/ijerph15010161.
Rotheram-Borus MJ, Weichle TW, Wynn A, et al. Alcohol, but not depression or IPV, reduces HIV adherence among south african mothers living with HIV over 5 years. AIDS Behav. 2019;23(12):3247–56. https://doi.org/10.1007/s10461-019-02617-2.
Brittain K, Remien RH, Mellins CA, et al. Determinants of suboptimal adherence and elevated HIV viral load in pregnant women already on antiretroviral therapy when entering antenatal care in Cape Town, South Africa. AIDS Care. 2018;30(12):1517–23. https://doi.org/10.1080/09540121.2018.1503637.
Brittain K, Phillips TK, Zerbe A, Abrams EJ, Myer L. Long-term effects of unintended pregnancy on antiretroviral therapy outcomes among south african women living with HIV. AIDS. 2019;33:885–93. https://doi.org/10.1097/QAD.0000000000002139.
Sweeney SM, Vanable PA. The association of HIV-related stigma to HIV medication adherence: a systematic review and synthesis of the literature. AIDS Behav. 2016;20:29–50. https://doi.org/10.1007/s10461-015-1164-1.
Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(Suppl 2):18640. https://doi.org/10.7448/IAS.16.3.18640.
Jones D, Cook R, Rodriguez A, Waldrop-Valverde D. Personal HIV knowledge, appointment adherence and HIV outcomes. AIDS Behav. 2013;17(1):242–9. https://doi.org/10.1007/s10461-012-0367-y.
Wawrzyniak AJ, Ownby RL, McCoy K, Waldrop-Valverde D. Health literacy: impact on the health of HIV-infected individuals. Curr HIV/AIDS Rep. 2013;10(4):295–304. https://doi.org/10.1007/s11904-013-0178-4.
Gonzalez JS, Penedo FJ, Llabre MM, et al. Physical symptoms, beliefs about medications, negative mood, and long-term HIV medication adherence. Ann Behav Med. 2007;34(1):46–55. https://doi.org/10.1007/BF02879920.
Kalichman S, Kalichman MO, Cherry C. Medication beliefs and structural barriers to treatment adherence among people living with HIV infection. Psychol Health. 2016;31(4):383–95. https://doi.org/10.1080/08870446.2015.1111371.
Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. https://doi.org/10.1186/PREACCEPT-1453408941291432.
Myer L, Phillips TK, Zerbe A, et al. Optimizing antiretroviral therapy (ART) for maternal and child health (MCH): rationale and design of the MCH-ART study. J Acquir Immune Defic Syndr. 2016;72:189–96. https://doi.org/10.1097/QAI.0000000000001056.
Myer L, Phillips TK, Hsiao N-Y, et al. Plasma viraemia in HIV-positive pregnant women entering antenatal care in South Africa. J Int AIDS Soc. 2015;18:20045. https://doi.org/10.7448/IAS.18.1.20045.
Iyun V, Brittain K, Phillips TK, et al. Prevalence and determinants of unplanned pregnancy in HIV-positive and HIV-negative pregnant women in Cape Town, South Africa: a cross-sectional study. BMJ Open. 2018;8:e019979. https://doi.org/10.1136/bmjopen-2017-019979.
Brittain K, Remien RH, Phillips T, et al. Factors associated with alcohol use prior to and during pregnancy among HIV-infected pregnant women in Cape Town, South Africa. Drug Alcohol Depend. 2017;173:69–77. https://doi.org/10.1016/j.drugalcdep.2016.12.017.
Bernstein M, Phillips T, Zerbe A, et al. Intimate partner violence experienced by HIV-infected pregnant women in South Africa: a cross-sectional study. BMJ Open. 2016;6:e011999. https://doi.org/10.1136/bmjopen-2016-011999.
Preciago J, Henry M. Linguistic barriers in health education and services. In: Garcia JG, Zea MC, editors. Psychological interventions and research with latino populations. Boston: Allyn and Bacon; 1997.
Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counselling and testing in a black township in Cape Town, South Africa. Sex Transm Infect. 2003;79(6):442–7. https://doi.org/10.1136/sti.79.6.442.
Wagner GJ, Remien RH, Carballo-Diéguez A, Dolezal C. Correlates of adherence to combination antiretroviral therapy among members of HIV-positive mixed status couples. AIDS Care. 2002;14(1):105–9. https://doi.org/10.1080/09540120220097973.
Wagner GJ, Kanouse DE, Koegel P, Sullivan G. Correlates of HIV antiretroviral adherence in persons with serious mental illness. AIDS Care. 2004;16(4):501–6. https://doi.org/10.1080/09540120410001683420.
Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. https://doi.org/10.1080/08870449908407311.
Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12(3):255–66. https://doi.org/10.1080/09540120050042891.
Fife BL, Wright ER. The dimensionality of stigma: a comparison of its impact on the self of persons with HIV/AIDS and cancer. J Health Soc Behav. 2000;41(1):50–67.
Barrera MA. A method for assessing social support networks in community survey research. Connections. 1980;3(3):8–13.
Arnsten JH, Li X, Mizuno Y, et al. Factors associated with antiretroviral therapy adherence and medication errors among HIV-infected injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl 2):64–71. https://doi.org/10.1097/QAI.0b013e31815767d6.
Brittain K, Mellins CA, Phillips T, et al. Social support, stigma and antenatal depression among HIV-infected pregnant women in South Africa. AIDS Behav. 2017;21:274–82. https://doi.org/10.1007/s10461-016-1389-7.
Barry OM, Bergh A-M, Makin JD, Etsane E, Kershaw TS, Forsyth BWC. Development of a measure of the patient-provider relationship inantenatal care and its importance in PMTCT. AIDS Care. 2012;24:680–6. https://doi.org/10.1080/09540121.2011.630369.
Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99–107.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150(6):782–6. https://doi.org/10.1192/bjp.150.6.782.
Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76. https://doi.org/10.1017/s0033291702006074.
Spies G, Stein DJ, Roos A, et al. Validity of the Kessler 10 (K-10) in detecting DSM-IV defined mood and anxiety disorders among pregnant women. Arch Womens Ment Health. 2009;12:69–74. https://doi.org/10.1007/s00737-009-0050-0.
Bush K, Kivlahan DR, Mcdonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–95. https://doi.org/10.1001/archinte.158.16.1789.
Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821–9. https://doi.org/10.1001/archinte.163.7.821.
Garcia-Moreno CJ, Jansen H, Ellsberg M, et al. WHO multi-country study on women’s health and domestic violence against women. World Health Organization; 2005.
Ullman JB, Bentler PM. Structural Equation Modeling. In: Weiner I, Schinka JA, Velicer WF, editors. Handbook of Psychology, Second Edition. 2012. https://doi.org/10.1002/9781118133880.hop202023.
Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat. 2016;70(2):129–33. https://doi.org/10.1080/00031305.2016.1154108.
Gipson JD, Koenig MA, Hindin MJ. The effects of unintended pregnancy on infant, child, and parental health: a review of the literature. Stud Fam Plann. 2008;39:18–38. https://doi.org/10.1111/j.1728-4465.2008.00148.x.
Cheng D, Schwarz EB, Douglas E, Horon I. Unintended pregnancy and associated maternal preconception, prenatal and postpartum behaviors. Contraception. 2009;79:194–8. https://doi.org/10.1016/j.contraception.2008.09.009.
Dibaba Y, Fantahun M, Hindin MJ. The effects of pregnancy intention on the use of antenatal care services: a systematic review and meta-analysis. Reprod Health. 2013;10:50. https://doi.org/10.1186/1742-4755-10-50.
Santelli J, Rochat R, Hatfield-Timajchy K, et al. The measurement and meaning of unintended pregnancy. Perspect Sex Reprod Health. 2003;35:94–101. https://doi.org/10.1363/3509403.
Davis EC, Rotheram-Borus MJ, Weichle TW, Rezai R, Tomlinson M. Patterns of alcohol abuse, depression, and intimate partner violence among township mothers in South Africa over 5 years. AIDS Behav. 2017;21:174–82. https://doi.org/10.1007/s10461-017-1927-y.
Peltzer K, Pengpid S. Maternal alcohol use during pregnancy in a general national population in South Africa. S Afr J Psychiat. 2019;25:a1236. https://doi.org/10.4102/sajpsychiatry.v25i0.1236.
Petersen Williams P, Jordaan E, Mathews C, Lombard C, Parry CD. Alcohol and other drug use during pregnancy among women attending midwife obstetric units in the Cape metropole, South Africa. Adv Prev Med. 2014;871427. https://doi.org/10.1155/2014/871427.
Raggio GA, Psaros C, Fatch R, et al. High rates of biomarker-confirmed alcohol use among pregnant women living with HIV in South Africa and Uganda. J Acquir Immune Defic Syndr. 2019;82:443–51. https://doi.org/10.1097/QAI.0000000000002156.
Ramlagan S, Rodriguez VJ, Peltzer K, Ruiter RAC, Jones DL, Sifunda S. Self-reported long-term antiretroviral adherence: a longitudinal study among HIV infected pregnant women in Mpumalanga, South Africa. AIDS Behav. 2019;23:2576–87. https://doi.org/10.1007/s10461-019-02563-z.
Adeniyi OV, Ajayi AI, Ter Goon D, Owolabi EO, Eboh A, Lambert J. Factors affecting adherence to antiretroviral therapy among pregnant women in the Eastern Cape, South Africa. BMC Infect Dis. 2018;18:175. https://doi.org/10.1186/s12879-018-3087-8.
Mellins CA, Chu C, Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20:958–68. https://doi.org/10.1080/09540120701767208.
Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26:2039–52. https://doi.org/10.1097/QAD.0b013e328359590f.
Samawi L, Petersen Williams P, Myers B, Fuhr DC. Effectiveness of psychological interventions to reduce alcohol consumption among pregnant and postpartum women: a systematic review. Arch Womens Ment Health. 2021;24:557–68. https://doi.org/10.1007/s00737-020-01100-5.
Wechsberg WM, Browne FA, Ndirangu J, et al. Outcomes of implementing in the real world the women’s Health CoOp intervention in Cape Town, South Africa. AIDS Behav. 2021;25(Suppl 3):276–89. https://doi.org/10.1007/s10461-021-03251-7.
Cunnama L, Abrams EJ, Myer L, et al. Provider- and patient-level costs associated with providing antiretroviral therapy during the postpartum phase to women living with HIV in South Africa: a cost comparison of three postpartum models of care. Trop Med Int Health. 2020;25(12):1553–67. https://doi.org/10.1111/tmi.13493.
Pellowski JA, Weber AZ, Phillips TK, et al. You must leave but I didn’t want to leave”: qualitative evaluation of the integration of ART into postnatal maternal and child health services in Cape Town, South Africa. AIDS Care. 2020;32(4):480–5. https://doi.org/10.1080/09540121.2019.1659913.
Phillips TK, Mogoba P, Brittain K, et al. Long-term outcomes of HIV-infected women receiving antiretroviral therapy after transferring out of an integrated maternal and child health service in South Africa. J Acquir Immune Defic Syndr. 2020;83:202–9. https://doi.org/10.1097/QAI.0000000000002236.
Acknowledgements
The authors would like to thank the women who participated in this study, as well as the study staff for their support of this research. This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the National Institute of Child Health and Human Development (NICHD), grant numbers 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation. Dr. Pellowski was supported by a career development grant from the National Institute of Mental Health (NIMH K01MH112443). Drs. Remien and Mellins are supported by a grant from NIMH to the HIV Center for Clinical and Behavioral Studies (P30-MH45320).
Funding
Open access funding provided by the University of Cape Town. This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the National Institute of Child Health and Human Development (NICHD), grant numbers 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation. Drs. Remien and Mellins are supported by a grant from NIMH to the HIV Center for Clinical and Behavioral Studies (P30-MH45320). Dr. Pellowski was supported by a career development grant from the National Institute of Mental Health (NIMH K01MH112443).
Author information
Authors and Affiliations
Contributions
LM and EJA conceived of and designed the parent study, with input from RHR and CAM. KBrittain, TP, and AZ implemented the research, data collection and data management. KBrittain and KBrown performed the data analysis. KBrittain wrote the first draft of the manuscript. All authors critically reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics Approval
This study was performed in line with the principles of the 1964 Declaration of Helsinki and its later amendments. The study was approved by the Faculty of Health Sciences Human Research Ethics Committee at the University of Cape Town, and the Institutional Review Board of Columbia University Medical Centre.
Consent to Participate
All participants provided written informed consent prior to enrolment.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brittain, K., Brown, K., Phillips, T. et al. Why do Integrated Maternal HIV and Infant Healthcare Services work? A Secondary Analysis of a Randomised Controlled Trial in South Africa. AIDS Behav 27, 3831–3843 (2023). https://doi.org/10.1007/s10461-023-04097-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-023-04097-x