Abstract
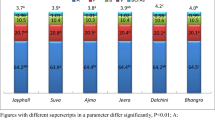
This study was taken up to assess the effect of supplementing tree leaves [Eucalyptus globules (Safeda), Populus tremula (Poplar), Ficus bengalensis (Banyan), Saraca asoca (Ashoka), Acacia nilotica (Kikar), Phoenix dactylifera (Khajoor), Aegle marmelos (Bael), Murraya koenigii (Curry), Cassia fistula (Amaltas), Bauhinia variegata (Kachnar), Mangifera indica (Mango) and Psidium guajava (Guava)] at 1–3% on fermentability and in vitro methane production from total mixed ration (TMR). The globulins content was highest in Aegle, while albumin was highest in Psidium leaves. Prolamin was highest in Ficus (17.3%), while glutelin was the highest in Phoenix (6.50%). Ficus (15.2%) and Psidium (10.7%) leaves contained highest level of condensed tannins. Eucalyptus and Mangifera leaves showed the highest antioxidant activity and flavonoid content respectively. Supplementation of TMR with leaves of Acacia, Psidium and Cassia resulted in higher (P < 0.05) DM digestibility, whereas NDF digestibility was highest in TMR supplemented with Mangifera and Acacia leaves. The VFAs concentration varied (P < 0.05) from 4.4 to 6.07 mM/dl in TMR supplemented with Eucalyptus and Saraca leaves. Bauhinia leaves supplementation resulted in the lowest (P < 0.05) methane production from TMR. It was concluded that TMR supplemented with leaves of M. indica, A. nilotica, P. guajava, C. fistula, E. globules and P. dactylifera at 1% has great potential to improve digestibility and or decrease methane production.




Similar content being viewed by others
References
AOAC (2000) Official methods of analysis, 7th edn. Association of Analytical Chemists, Gaithersburg
Aregheare EM, Abdulrazak SA (2005) Estimation of organic matter digestibility and metabolizable energy content of agro-industrial wastes using in vitro gas production. Nig J Anim Prod 31: 79–87 (CROSS REF Asian Aust J Anim Sci vol. 25, no. 10, pp 1404–1410 October 2012. http://www.ajas.info. http://dx.doi.org/10.5713/ajas.2012.12219. Effects of tropical high tannin non legume and low tannin legume browse mixtures on fermentation parameters and methanogenesis using gas production technique. Seresinhe T, Madushika SAC, Seresinhe Y, Lal PK, Ørskov ER)
Argos P, Pederson K, Marks MD, Larkins BA (1982) A structural model for maize zeinproteins. J Biol Chem 257:9984–9990
Baccou JC, Lambert F, Sanvaire Y (1977) Spectrophotometric method for the determination of total steroidal sapogenin. Analyst 102:458–466
Bakshi MPS, Wadhwa M (2004a) Effect of herbal feed additives on the nutrient utilization in buffalo calves, Bubalusbubalis. J Buffalo Sci Tech 10: 65–70. (CROSS REF: https://www.researchgate.net/publication/299737006 Modifying gut microbiomes in large ruminants: Opportunities in non-intensive husbandry systems Article April 2016. https://doi.org/10.2527/af.2016-0020)
Bakshi MPS, Wadhwa M (2004b) Evaluation of forest tree leaves of semi-hilly arid region as livestock feed. Asian Aust J Anim Sci 17:777–783
Bakshi MPS, Singh MP, Wadhwa M, Singh B (2011) Nutritional value of forest tree leaves as livestock feed in sub mountainous region of India. Indian J Anim Sci 81: 276–281. http://www.epubs.icar.org.in/ejournal/
Balabaa SI, Zake AY, Elshamy AM (1974) Total flavonols and rutin contents of the different organs of Sophora japonica. L. J Assoc Off Anal Chem 57: 752–755. https://doi.org/10.5958/j.2229-4473.26.2.103. https://arxiv.org/ftp/arxiv/papers/1703/1703.07992.pdf
Baran M, Zitnan R (2002) Effect of monensin sodium on fermentation efficiency in sheep rumen. Arch Tierz 45:181–185. https://doi.org/10.5194/aab-45-181-2002
Barry TN, Manley TR (1984) The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. 2. Quantitative digestion of carbohydrates and proteins. Br J Nutr 51:493
Blümmel M, Becker K (1997) The degradability characteristics of fifty-four roughages and roughage neutral-detergent fibre as described by in vitro gas production and their relationship to voluntary feed intake. Br J Nutr 77:757–786
Blümmel M, Makkar HPS, Becker K (1997) In vitro gas production: a technique revisited. J Anim Physiol Anim Nutr 77:24–34
Blümmel M, Vellaikumar S, Devulapalli R, Nigam SN, Upadhyaya HD, Khan A (2005) Preliminary observations on livestock productivity in sheep fed exclusively on haulms from eleven cultivars of groundnut. Int Arachis Newsl 25: 54–57. http://oar.icrisat.org/3210/1/IAN_25_54-57_2005.pdf
Budzinski IGF, Moon DH, Linden P, Moritz T, Labate CA (2016) Seasonal variation of carbon metabolism in the cambial zone of Eucalyptus grandis. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00932
Chandaramoni CMT, Haque NM, Murari-Lal JS, Khan MY (2002) Energy balance in faunated and defaunted sheep on a ration high in concentration to roughage (good quality) ratio. Pak J Nutr 1:31–33
Cottyn BG, Boucque CV (1968) Rapid methods for the gas chromatographic determination determination of volatile acids in rumen fluid. J Agric Food Chem 16:105–107
Czerkawski JW (1986) An introduction to rumen studies. Pergamon Press, Oxford, pp 221–222
Davidson PM, Naidu AS (2000) Phyto-phenols. In: Naidu AS (ed) Natural food antimicrobial systems. CRC Press, Boca Raton, pp 265–293
Demeyer DI (1991) Quantitative aspects of microbial metabolism in the rumen and hindgut. In: Jouany JP (ed) Rumen microbial metabolism and ruminant digestion. INRA Editions, Paris, pp 217–237
Dubois M, Gilles KN, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356
Dung DD, Godwin IR, Nolan JV (2011) Intake digestibility and rumen parameters of sheep fed commercial pellets or supplemented with Barley grain, or freeze-dried barley sprouts or fresh barley sprouts. In: Proceedings of 36th conference on Nigerian society for animal production University of Abuja, Nigeria, pp 526–528
FDA (2004) Food and drug administration of the US, Substances used as GRAS in food, 21 CFR 184
Garcia-Gonzalez R, Lopez S, Fernandez M, Gonzale JS (2006) Effect of addition of some medicinal plants on methane production in a stimulating fermenter (RUSITEC). Int Congr Ser 1293:172–175
Greathead H (2003) Plants and plant extracts for improving animal productivity. Proc Nutr Soc 62:279–290
Hancock KR, Ealing PM, White DWR (1994) Identification of sulphur rich proteins which resist rumen degradation and are hydrolyzed rapidly by intestinal proteases. Br J Nutr 72:855–863
Hegarty RS, Nolan JV (2007) Estimation of ruminal methane production from measurement of volatile fatty acid production. In: Makkar HPS, Vercoe PE (eds) Measuring methane production from ruminants. University of New England Publishing Unit, Armidale, pp 69–92
Hook SE, Northwood KS, Wright ADG, McBride BW (2009) Long-term monensin supplementation does not significantly affect the quantity or diversity of methanogenes in the rumen of the lactating dairy cow. Appl Environ Microbiol 75:374–380
Hundal JS, Wadhwa M, Bakshi MPS (2016a) Effect of supplementing essential oils on the in vitro methane production and digestibility of wheat straw. J Anim Nutr USA1 (3):14. http://animalnutrition.imedpub.com, https://doi.org/10.21767/2572-5459.100014
Hundal JS, Wadhwa M, Bakshi MPS (2016b) Methane mitigation potential of tannins and their impact on digestibility of nutrients in-vitro. Anim Nutr Feed Technol 16:505–513
IAEA (2000) Quantification of tannins in tree foliage a laboratory manual for the FAO/IAEA co-ordinated research project on ‘use of nuclear and related techniques to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants on tanniniferous. Tree Foliage’ pp 4–6
Jagota SK, Dani HM (1982) A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127(1):178–182
Jayanegara A, Leiber F, Kreuzer M (2012) Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J Anim Physiol Anim Nutr 96:365–375
Kumaran A, Karakumaran J (2007) In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci Technol 40:344–352
Lee SS, Hsu JT, Mantovani HC, Russell JB (2002) The effect of bovicin HC50, a bacteriocin from Strptococcusbovis HC50, on ruminal methane production in vitro. FEMS Microbiol Lett 217:51–55
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin–phenol reagent. J Biol Chem 193:265–275
Makkar HPS (2003) Effects and fate of tannins in ruminant animals, adaptation to tannins and strategies to overcome detrimental effects of feeding tannin rich feeds. Small Rumin Res 49:241–256
Makkar HPS, Blummel M, Borowy WK, Becker K (1993) Gravimetric determination of tannins and their correlations with chemical and precipitation methods. J Sci Food Agric 61:161–165
McAllister TA, Okine EK, Mathison GW, Cheng KJ (1996) Dietary, environmental and microbiological aspects of methane production in ruminants. Can J Anim Sci 76:231–243
Menke KH, Steingass H (1988) Estimation the energetic feed value obtained by chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28: 7–55. http://agris.fao.org/agris-search/search.do?recordID=DE88A022488
Menke KH, Raab L, Salweski A, Steingass H, Fritz D, Scheider W (1979) The estimation of digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they are incubated with rumen liquor in vitro. J Agric Sci Camb 93:217–222
Mishra AK, Sahu N, Mishra A, Ghosh AK, Jha S, Chattopadhyay P (2010) Phytochemical screening and antioxidant activity of essential oil of Eucalyptus leaf. Pharmacogn J 2:25–28. https://doi.org/10.1016/S0975-3575(10)80045-8
Mohamed H, Ons M, Yosra ET, Rayda S, Neji G, Moncef N (2009) Chemical composition and antioxidant and radical scavenging activites of periplocalaevigata root bark extracts. J Sci Food Agric 89:897–905
Monteiro PV, Tirupaksha TK, Rao DR (1982) Proteins of Italian millet: amino acid composition solubility, fractionation and electrophoresis of protein fractions. J Sci Fd Agric 33:1072–1079
Moss AR, Jouany JP, Newbold J (2000) Methane production by ruminants: its contribution to global warming. Ann Zootech 49:231–253
Neelam Rani, Wadhwa M, Kaushal S, Bakshi MPS (2006) Herbal feed additives and performance of buffalo calves. Anim Nutr Feed Technol 6: 147–151. http://www.indianjournals.com/anft-6-1-018
NRC (1989) Nutrient requirements of dairy cattle, 6th edn. National Research Council, National academy of sciences, Washington, DC
Ørskov ER (1975) Manipulation of rumen fermentation for highest food utilization. World Rev Nutr Diet 22: 153–182. https://doi.org/10.1159/000397977. https://www.ncbi.nlm.nih.gov/pubmed/1103481
Patra AK, Saxena J (2009) Dietary phytochemicals as rumen modifiers: a review of the effects on microbial population. J Antonie van Leewenhock 96:363–375
Patra AK, Saxena J (2011) Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J Sci Food Agric 91:24–37
Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyaniding and delphinidin. Phytochem 25:223–230
Rana KK, Wadhwa M, Bakshi MPS (2006) Seasonal variations in tannin profile of tree leaves. Asian Aust J Anim Sci 19:1134–1138
Reed JD, Soller H, Woodward A (1990) Fodder tree and straw diets for sheep: intake, growth, digestibility and the effects of phenolics on nitrogen utilization. Anim Feed Sci Technol 30:39–50
Santra A, Saikia A, Baruah KK (2012) Scope of rumen manipulation using medicinal plants to mitigate methane production. J Pharm 3: 115–120. https://bioinfopublication.org/files/articles/3_2_19_JP.pdf
Shon MY, Kim TH, Sung NJ (2003) Antioxidant and free radical scavenging activity of phillinusbaumil (phillinus of Hymens Chaeloceae) extract. Food Chem 82:593–597
Snedecor GW, Cochran WG (1994) Statistical methods. Oxford and IBH Publications, New Delhi
Soltan YA, Morsy AS, Sallam SMA, Louvandini H, Abdalla AL (2012) Comparative in vitro evaluation of forage legumes (Prosopis, Acacia, Atriplex, and Leucaena) on ruminal fermentation and methanogenesis. J Anim Feed Sci 21:759–772
SPSS (2007) Statistical packages for social sciences, version 16. SPSS Inc, Chicago
Systat (1996) Systat 6.0.1 for windows: statistics. SPSS Inc., Chicago
Tan HY, Sieo CC, Abdullah N, Liang JB, Huang XD, Ho YW (2011) Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim Feed Sci Technol 169:185–193
UNFCCC (2014) Global warming potentials. United Nations Framework Convention on Climate Change. http://unfccc.int/ghg_data/items/3825.php
Ungerfeld EM, Rust SR, Boone DR, Liu Y (2004) Effect of several inhibitors on pure cultures of ruminal methanogenes. J Appl Microbiol 97:520–526
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Wadhwa M, Bakshi MPS (2006) Herbal feed additives-impact on the rumen environment in buffaloes. Indian J Anim Nutr 23: 102–109. http://www.indianjournals.com/ijor.aspx?target=ijor:ijan&volume=23&issue=2&article=008
Wadhwa M, Kaur N, Bakshi MPS (2010) Degradability of protein fractions of conventional and non-conventional protein supplements. Anim Nutr Feed Technol 10: 235–243. http://www.indianjournals.com/anft-10-2-011
Widiawati Y, Thalib A (2009) Comparison of fermentation kinetics (in vitro) of grass and shrub legume leaves: the pattern of VFA concentration, estimated CH4 and microbial biomass production. Indones J Agric 2: 21–27. http://pustaka.litbang.pertanian.go.id/publikasi/ja021094.pdf
Williams YJ, Popovaski S, Rea SM, Skillmam LC, Toovey AF, Northwood KS, Wright ADG (2009) Avaccine against rumen methanogens can alter the composition of archeal population. Appl Environ Microbiol 75:1860–1866
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deuri, P., Sood, N., Wadhwa, M. et al. Screening of tree leaves for bioactive components and their impact on in vitro fermentability and methane production from total mixed ration. Agroforest Syst 94, 1455–1468 (2020). https://doi.org/10.1007/s10457-019-00374-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-019-00374-8