Abstract
When exposed to predation risk, some amphibian species show innate responses, while others recognize their predators by learning. To explore the role played by each mechanism in the assessment of predation risk, we investigated the effects of embryonic and larval exposure to predator chemical cues on tadpole defensive responses, including behavioural, morphological and life history traits. In the first experiment, agile frog (Rana dalmatina) embryos were exposed to the odour of either native (Aeshna cyanea larvae) or alien (Procambarus clarkii) predators each day from egg collection to hatchling (14 days). Body measures (mass, developmental stage, body length, tail length and tail depth) were recorded at hatching and a behavioural test was conducted to explore tadpole responses to predator cues and the potential interaction with their previous embryonic experience. In general, embryonic conditioning did not affect life history traits, except for a slight reduction in tail depth:length ratio for tadpoles exposed to odonate odours. Controls (embryos treated with water) after hatchling reduced their activity when exposed to gammarid-fed odonate cues, suggesting that responses were at least partially innate. Tadpoles exposed to odonate cues as embryos showed a strong defensive response when exposed to dragonfly kairomones. Tadpoles exposed to gammarid-fed crayfish as embryos showed clear behavioural responses towards the same cue (irrespectively of predator diet). Overall, our results suggest that embryonic exposure may tune the defensive responses of the larval stage and early exposure to naïve stimuli may promote their cautionary associations with predation risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To discriminate their potential predators, prey species can rely on two mechanisms: innate or learned responses (Chivers et al. 1996; Laurila 2000; Batabyal et al. 2014). Stable environments should promote the evolution of innate responses to predation-related stimuli, while in unpredictable environments individuals that are able to tune their behaviour by experience may have better chances of surviving and reproducing (Stephens 1991). Both mechanisms have pros and cons, and their evolution appears to be species-specific (Dunlap and Stephens 2016). As an example, while exposure to predatory cues during embryogenesis can prompt both morphological and behavioural responses which can enhance individual chances of escaping predation, the stress caused by perceived predation risk can also carry metabolic and immune costs, lowering the survival of later developmental stages (Sih and Moore 1993; Oulton et al. 2013).
Among amphibians, for which predator-induced behaviour has been largely investigated, some species show innate responses (e.g.: Bufo bufo and Rana temporaria, Laurila et al.1997; Bufo americanus, Gallie et al. 2001; Cryptobranchus alleganiensis, Gall and Mathis 2010), while others recognize their predators by learning (e.g.: Notophthalmus viridescens, Woody and Mathis 1998; Pelophylax perezi, Gonzalo et al. 2007; Pseudacris maculata, Ferrari and Chivers 2008; Euphlyctis cyanophlyctis, Supekar and Gramapurohit 2017).
As experience-mediated responses can be crucial in fine-tuning antipredatory responses, selection should favour the acquisition of reliable information about potential predators as early as possible. For species with aquatic eggs, such as most amphibians, individuals can achieve this information already at the embryonic stage (i.e.: prior to hatching) through chemosensory cues, which are widely available in the surrounding medium (Mathis et al. 2008; Ferrari et al. 2010; Ferrari and Chivers 2011; Garcia et al. 2017). For information to be effective, environmental predictability should be high (Stephens 1991). As, after hatching, tadpoles usually do not disperse a great distance, between-stage predictability—that is, paraphrasing Stephens (1991), the extent to which environmental conditions at egg stage predict larval stages—, is expected to be high for most anurans (except for species which lay terrestrial eggs: see the extensive work on Agalychnis callidryas embryos by Karen Warkentin (1995, 2005, 2011)). Thus, embryos exposed to water-borne predator cues may learn about the level of predation threat that they will face after hatching and display appropriate anti-predator responses (Ferrari and Chivers 2009; Mitchell and McCormick 2013; Polo-Cavia and Gomez-Mestre 2014).
Consistently, embryonic exposure to predator cues has been reported to affect hatching time (Sih and Moore 1993), growth rates (Orizaola and Brana 2005), and size at metamorphosis (Tarvin et al. 2015). Embryos exposed to chemical cues may learn to recognize predators if predator cues (also called kairomones) are coupled with prey-borne cues (i.e. alarm cues and tissue fragments from injured conspecifics) (Chivers et al. 1996; McCarthy and Fisher 2000; Mathis et al. 2008), suggesting that embryonic learning needs exposure to high predation risk scenarios (Garcia et al. 2017). The density of embryos is a further variable that may affect individual learning processes. While density has been reported to affect risk perception by tadpoles (Van Buskirk et al. 2011; Gazzola et al. 2021a; Guadin et al. 2021), seemingly no studies have been conducted on embryos.
The lack of responses recorded in prey animals exposed to predator cues alone during the embryonic stage may depend on a process known as latent inhibition (Lubow 1973). In the absence of prey-borne cues, embryos may fail to associate predator cues (in successive encounters) to an actual risk for their safety, preventing the display of adequate responses later in life. Repeated stimulation can also lead to habituation, that is a decrease in sensitivity toward the stimulus (Nisbet 2000). Habituation avoids costly responses when responding to a stimulus does not bring any benefit, even if it is innately perceived as dangerous (Blumstein 2016). These processes make discriminating between innate and learned responses not always a straightforward matter (Garcia et al. 2017).
We speculated that exposing tadpoles to a range of predator cues, both prior and after hatching may help to shed light on the role played by innate responses and learning in the assessment of predation risk. With this aim, we used as a model species the agile frog Rana dalmatina. The embryos of this species apparently do not modify the time of hatching in the presence of the cues of either native predators, such as the larvae of emperor dragonfly Anax imperator, or naïve ones, such as invasive red swamp crayfish Procambarus clarkii (Gazzola et al. 2018); nonetheless embryonic exposure may promote the elicitation of innate responses to predation threat at the larval stage (Gazzola et al. 2015). Otherwise, tadpoles may learn to discriminate predators by associating their odour to the alarm cues of preyed conspecifics (Garcia et al. 2017; Supekar and Gramapurohit 2017).
Therefore, firstly we conditioned agile frog embryos with the odours of two predators (one native and one alien) and, after hatching, exposed tadpoles to either the predator cues alone or paired with alarm cues. We recorded inter-treatment variation in life history traits of hatchlings and investigated the defensive behavioural responses of tadpoles. We expected possible innate responses to be shown only by tadpoles exposed to native predator odours or conspecific alarms cues. Tadpoles were expected to show a strong anti-predator response toward tadpole-fed native predators, while the response of tadpoles exposed to alien predators fed with conspecifics was expected to be less intense. As we deliberately avoided to expose embryos to conspecific alarm cues, we could not exclude processes such a habituation and latent inhibition to affect tadpole responses. Early exposure to chemical cues may also trigger a later neophobic behaviour and induce tadpoles to respond to naïve odours, or even increase the response towards the same cue (sensitization).
Materials and methods
Field collection and housing
The study was carried out with permission from the Italian Ministry of Environment (Prot. 0,035,817/PNM, validity 2013–2015) and in conformity with both the current Italian laws for amphibian collection and detention and Guidelines of the Animal Behaviour Society for the Use of Animals in Research.
Agile frogs breed in early spring in permanent or ephemeral ponds, laying up to 2000 eggs in a single clutch; only 18% of broods show multiple paternity (Lodé and Lesbarrères 2004). Eggs are vulnerable to predation by planarians, caddisfly larvae, leeches, crayfish and fishes, while tadpoles are threatened by a wider range of predators, including also dragonfly larvae, water beetles, snakes and birds (Wells 2007). Agile frog tadpoles have been recorded to show morphological (e.g.: deep and long tails, squat bodies) and behavioural (reduced activity, protean behaviour) responses to different aquatic predators (Teplitsky et al. 2005; Gazzola et al. 2018, 2021b).
On 18 February 2014 we collected ten egg masses from a permanent pond in San Colombano, Pavia, Italy (45°11′ - 9°26′), where Rana dalmatina is the only brown frog spawning. Until the onset of the experiment, clutches were maintained in different opaque plastic tanks (50 × 40 × 30 cm), containing 40 l of aged tap water and equipped with aerators. After hatching, tadpoles were transferred to a new set of tanks containing 40 l of aged tap water and fed with rabbit chow ad libitum. Every other day, 50% of the water was changed to keep adequate oxygen levels.
Dragonfly larvae and red swamp crayfish were taken from neighbouring waterbodies (n = 20 for each species), held individually in 800 ml plastic cups filled with 500 ml of aged water, and fed with live freshwater amphipod shrimps (Gammarus sp.) every other day.
All animals were maintained in an unheated room with open windows and under natural light conditions. Water temperature within tanks was checked every two days (between 10 a.m. and 2 p.m.) and ranged from 7° to 14 °C.
Experiment 1: effects of embryonic exposure to predation risk on hatchlings’ life history and morphological traits
We cautiously removed three samples of approximately 50 eggs from each clutch (n = 30) on the same day of collection, paying attention to keep the egg jelly intact. Egg samples were placed into separated plastic tanks (30 × 20 × 20 cm) containing 8 l of aged tap water and equipped with aerators, for a total of 30 tanks and 1500 eggs. Tanks were randomly distributed within the laboratory. Gosner’s developmental stage (GS) was assessed just after collection and ranged from 8 to 11 (7 clutches were at GS9).
Treatments began the day after collection. Ten tanks (500 eggs) served as control and were injected with 50 ml of water each, 10 tanks were injected with 50 ml of water containing the chemical odours (kairomones) collected from 5 gammarid-fed dragonfly larvae and the remaining 10 tanks were injected with 50 ml of water containing the kairomones collected from 5 gammarid-fed crayfish (Laurila 2000; Ferrari et al. 2010). Aliquots from the same treatment were poured into the same container and the resulting mixture was used as a chemical stimulus. During each day of the conditioning period, aliquots (150 ml) were get out of a subsample of five individual predators, which were randomly selected from the pool of 20 individuals. All treatments were provided daily by means of 60 ml sterile syringes and were stopped as soon as the first hatchling was observed in the experimental tank, an event that occurred 14 days after the onset of the experiment.
Hatching time was defined as when 50% of the embryos in each tank were completely detached from the yolk sac and lied on the bottom (Ireland et al. 2007; Gazzola et al. 2015). Just after hatching, ten tadpoles were carefully collected using a small fish net from each tank (n = 300), weighed (using a Sartorius R200D balance, Göttingen, Germany; accuracy ± 0.01 mg), and photographed in lateral view using a digital camera (Panasonic Lumix DMC FZ28, Kadoma, Osaka, Japan; sensor resolution: 10.1 megapixels, output images: 3.648 × 2.736 pixels), within a small glass chamber under standardized conditions (constant light, exposure, and distance of the subject). Pictures were analysed using ImageJ 1.48 (National Institutes of Health, Bethesda, MD, USA) to assess tadpole developmental stage, measure tail length, body length and maximum tail depth, and to calculate tail length:body depth and tail depth:length ratios. These body features were expected to affect swimming and antipredatory responses (Relyea 2001; Perotti et al. 2016). All measurements were taken three times per tadpole by the same person, who was blind to the embryonic treatment. These tadpoles were not involved in the second experiment.
Experiment 2: effects of embryonic exposure to predation risk on larval antipredator behavioural responses
After hatching (Gosner stage 25–26), we explored the effects of embryonic exposure on tadpole defensive behaviour. One hundred and ten tadpoles from each of the three embryonic treatments (11 per tank) were randomly selected for the behavioural experiment. The behavioural responses of conditioned tadpoles were tested against five different stimuli: 1) aged tap water (control), 2) gammarid-fed dragonfly larvae (i.e.: kairomones), 3) tadpole-fed dragonfly larvae (i.e.: kairomones + alarm cues), 4) gammarid-fed crayfish, 5) tadpole-fed crayfish. Each predator was fed with either tadpoles or gammarids (total weigh ca. 200 mg) at 6 p.m. the day before each experimental session, and odour cues were prepared following the procedure previously described. Consistently with previous studies, the cues of heterospecific prey (gammarids) were assumed to elicit no defensive response in tadpoles (Schoeppner and Relyea 2005; Hettyey et al. 2015; Gazzola et al. 2018).
Water was used as post-stimulus control treatment to assess whether the disturbance produced by the injection might affect tadpole behaviour and as reference level to evaluate the effect of the other treatments. Globally, we examined eleven treatment combinations, pairing the three embryonic treatments with three to four stimuli (Table 1). Tadpoles from all embryonic treatments received the neutral stimulus (water); individuals from the embryonic control treatment received both predator stimuli (gammarid-fed odonate and crayfish odours), those from the embryonic odonate group received the cues of dragonfly larvae fed with both types of diet and gammarid-fed crayfish cues. The group conditioned with crayfish received the cues of crayfish fed with both types of diet and also gammarid-fed odonate cues.
During four consecutive days, we conducted 10-min trials per tadpole, recording their activity before and after exposure to the chemical stimulus. Each tadpole was placed into a transparent plastic experimental tub (15 × 10 × 10 cm) filled with 250 ml of aged tap water, and left to acclimatize for 15 min. Each trial consisted of a 5 min pre-stimulus recording period, a 30 s infusion period (injection of the chemical stimulus), and a 5 min post-stimulus recording period. The stimulus was injected manually, using a 10 ml syringe, and consisted of 5 ml of predator-conditioned or control water. To minimize disturbance, it was slowly injected near the bottom of one side of the tub. All trials were performed inside the laboratory and tadpoles were video-recorded over the entire trial (JVC GZ-MG140E digital video camera, Milan, Italy). Because we recorded four tadpoles at a time, each experimental tub was surrounded by cardboard barriers to avoid disturbing. To measure activity, we drew two perpendicular lines across the center of the outer bottom of the tub and counted the number of lines crossed by tadpoles during the two observation periods (Gazzola et al. 2015, 2021a). The tested individual was considered to have crossed a line only when its whole body was on the other side of the line. Lowered activity was considered as a behavioural defensive response towards the perceived risk of predation (Relyea 2001; Van Buskirk 2001). Each tadpole was tested only once.
Statistical analysis
To explore tadpoles’ responses, we used mixed models which allow to control for correlated data and include both fixed effects (which are related to the main hypothesis being tested; e.g.: treatment levels) and random effects (which represents a source of variation; e.g.: subject effects). The inclusion of random effects allows controlling this variation and obtaining better estimates of the main effects of interest. Hatching time (i.e.: the number of hours from the onset of the experiment to hatching) was considered as the response variable in a linear mixed model (LMM). The model included both embryonic chemical treatment (a factor with three levels: control, odonate cue and crayfish cue) and stage at collection as fixed effect and clutch of origin (n = 10) as a random effect. The effects of embryonic exposure on the mass and morphology of the hatchlings were analysed by means of LMMs, with embryonic treatment as a fixed factor and clutch as a random intercept effect. Genotype by environment interactions were tested, for each trait, by including a by-clutch random slope for the predator treatment effect in the LMMs of morphological traits. For all LMMs we used the Gaussian (i.e.: normal) distribution and identity link function. Significance of random slope- or fixed effects was tested by means of likelihood ratio tests, which follow a χ2 distribution, by computing the difference in − 2 loglikelihood of the model, both with or without random slope or treatment. Having detected violations of variance homogeneity and patterns in the distribution of the residuals, the Kruskal–Wallis test was used for comparing mean developmental stages at hatching for the different treatments.
Tadpole activity level was explored by using generalized linear mixed models (GLMMs) with negative binomial distribution, which is suitable for count data (Hilbe 2014). The number of lines crossed after exposure to stimuli was included as the response variable. Fixed effects included activity before stimulus (lines crossed before injection) and stimulus type, while clutch was included as random effect. We ran three different models, one for each embryonic treatment: this approach avoided convergence problems and increased the stability of the models. GLMMS were fitted using the negative binomial distribution and log link function. Confidence intervals, estimated means and planned comparisons with control groups (estimated differences or ratios) were obtained from fitted models using the R package emmeans (Lenth 2021). T-ratios were used to compare estimated means (Lenth 2021). Residual diagnostics and goodness of fit tests from the R package DHARMa (Hartig and Lohse 2020) were used to evaluate the assumptions of the models.
Results
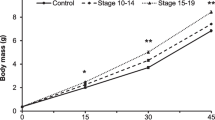
Hatching time was not affected by predator odour treatment (χ2 = 3.89, df = 2, P = 0.14) but depended on the embryonic developmental stage recorded when egg masses were collected (χ2 = 5.47, df = 1, P = 0.02). However, crayfish odour induced a slight, not significant reduction in hatching time in comparison with the control group (P = 0.12, Fig. 1). Individuals from control and treatment groups did not significantly differ in either mass at hatching (Fig. 2, Table 2), or Gosner’s developmental stage (χ2 = 1.35, df = 2, P = 0.51). We detected statistically significant genotype (clutch of origin) by environment (chemical stimulus) interactions for all morphological traits, but no difference was observed between control and predator-exposed groups, except for tail depth:length ratio, which was slightly lower in odonate-treated tadpoles respect to control tadpoles (P = 0.08; Table 2, Fig. 3).
Estimated means (large points) and 95% CI (vertical bars) for hatching time of Rana dalmatina eggs exposed to the kairomones of either native dragonfly larvae or alien crayfish (LMMs). The top of the plot shows the estimated effects (difference) as comparison with the control treatment (water); estimates not overlapping with the vertical-dashed line (i.e. difference = 0) indicate significant differences
Estimated means (large points) and 95% CI (vertical bars) of Rana dalmatina hatchlings’ mass (LMMs). The top of the plot shows the estimated effects (difference) as comparison with the control treatment (water); estimates not overlapping with the vertical-dashed line (i.e.: difference = 0) indicate significant differences
Estimated mean ratio (large points) and 95% CI (vertical bars) of tail depth:length ratios of Rana dalmatina hatchlings (LMMs). The top of the plot shows the estimated effects (difference) as comparison with the control treatment (water); estimates not overlapping with the vertical-dashed line (i.e. difference = 0) indicate significant differences
The behaviour of tadpoles exposed at the embryonic stage to either odonate or crayfish cues was strongly affected by both pre-stimulus activity (χ2 = 78.18, df = 1, P < 0.0001 and χ2 = 89.13, df = 1, P < 0.0001, respectively) and chemical stimuli (χ2 = 15.79, df = 3, P = 0.001 and χ2 = 18.01, df = 3, P = 0.0002, respectively). For tadpoles belonging to the embryonic control group, the effect of chemical treatment was not significant (pre-exposure activity: χ2 = 65.82, df = 1, P < 0.0001; chemical stimuli: χ2 = 3.79, df = 2, P = 0.15). Anyway, they showed a weak decrease in activity (P = 0.057), as respect to controls, when exposed to odonate kairomones but not to crayfish cues (Fig. 4). Tadpoles exposed to odonate kairomones as embryos showed a stronger defensive response than the embryonic control group when the dragonfly cue was provided (P = 0.004 vs P = 0.057, respectively; Fig. 4) and an even stronger decrease in activity when exposed to paired odonate kairomones and conspecifics’ alarm cues. Tadpoles also responded to crayfish kairomones (Fig. 4). Indeed, exposure to crayfish cues during embryonic development induced clear defensive responses when tadpoles received the same cue (gammarid-fed crayfish; Fig. 4), and the response increased further with tadpole-fed crayfish cues. In the crayfish conditioned group tadpoles responded to gammarid fed-odonate cue significantly decreasing their movements in comparison with control.
Estimated means (large points) and 95% CI (vertical bars) of post-stimulus activity of Rana dalmatina tadpoles from the negative binomial mixed model. The top of the plot shows the estimated effects (ratio) as comparison with the control treatment (water); estimates not overlapping with the vertical-dashed line (i.e. ratio = 1) indicate significant differences. Predator diet is also indicated (g: gammarid-fed, t: tadpole-fed)
Discussion
When exposed to either odonate or crayfish cues, agile frog embryos did not modify either the time of hatching or other life history traits. Tadpoles which were exposed to a predator cue as embryos responded to the same stimulus by reducing their level of activity; the reduction was stronger when conspecific alarm cues were paired with predator cues. Both predator-conditioned groups also responded to the kairomones of the other predator species.
Since death has unquestionable consequences on individual fitness, predation is widely recognized as a major selective pressure. Both innate and learned anti-predator responses have been demonstrated to improve survival and thus should be adaptive, notwithstanding it is often difficult to disentangle the effects of inheritance and early experience (Magurran et al. 1993). Although we cannot exclude a priori that tadpole responses were experience-mediated in a very early phase of the embryonic development (e.g.: before the collection of egg masses), the anti-predator responses shown toward dragonfly cues by tadpoles which had not been conditioned as embryos (controls) suggests that their shift in behaviour was at least partly inherited. This means that, if we assume early embryonic learning, agile frogs were capable of associating environmental signals to predation threat only for the predator with which they shared a long coevolution history. This result was expected, having been previously observed in several species, including other brown frogs (Rana temporaria, Hettyey et al. 2015; Rana latastei, Scribano et al. 2020), Pelodytes ibericus and Bufo bufo (Nunes et al. 2013).
Embryonic exposure confirmed that learning can play a major role in shaping post-hatching, behavioural defensive responses. It increased the intensity of the response of odonate-exposed individuals and, most of all, elicited a sharp response in tadpoles exposed to alien crayfish cues after having been conditioned with the same predator’s kairomones. As first postulated by Hepper and Waldman (1992), olfactory experiences before hatching may confer several adaptive advantages, providing information on the surrounding environment and shaping behaviour throughout ontogeny. Contrary to previous reports (Nunes et al. 2013; Belda et al. 2016), embryonic learning may allow tadpoles to recognize non-native predators as potential threats and elicit defensive behaviours even in the absence of conspecific alarm cues, increasing their chances of survival. Further studies are needed to ascertain if the environmental concentrations of chemical signals are usually sufficient to promote olfactory learning by embryos.
Unexpectedly, tadpoles exposed as embryos to odonate cues responded also to crayfish odour, although to a lower degree than those exposed to the cue of the native predator. This result may depend on cross-sensitization, that is a state of hyper-responsiveness to novel stressors consequent to a prior history of cute stress (Fanselow et al. 1993; Belda et al. 2016). Long-term exposure to odonate cue may have caused either a sensitization of neurochemical alarm pathways or a generalized state of anxiety, which increased tadpole susceptibility to novel stimuli. Accordingly, Gazzola et al. (2015) demonstrated that embryonic exposure to predation risk has long-term effects on the activity of olfactory bulb’s neurons, enhancing the elicitation of behavioural defenses in agile frog tadpoles.
While previous studies stressed the need for predator odours to be paired to conspecific alarm cues (Mathis et al. 2008; Ferrari and Chivers 2009, 2011), our results suggest that embryonic learning can be triggered by the predator stimulus alone and does not always imply the occurrence of processes such as latent inhibition or habituation.
Life history traits were not affected by olfactory conditioning, confirming the higher plasticity of behavioural responses (Gazzola et al. 2018). The weak reduction in tail depth of tadpoles exposed to odonate cues during the embryonic phase had been previously observed in another study on the agile frog (Gazzola et al. 2015), where both lowered tadpole size and delayed hatching time had also been recorded. Morphological responses seem to show high inter-population variability and are probably affected by several variables, including environmental conditions and predator density (Lee et al. 2020; Gazzola et al. 2021a).
Conclusions
The diversity and relative impact of predators are expected to vary among life stages (e.g. embryos, larvae and adults). Anyway, the first two stages of anuran ontogeny usually develop in very similar environmental conditions, making it possible to assess the effects of early predator environments on the traits and behaviour of individuals after the “ontogenetic niche shift” (Relyea 2005). By exposing the same individuals to predation threat in both stages we could point out both innate and learned responses and demonstrate that embryonic learning may tune the defensive responses of the larval stage, at least at laboratory cue concentrations. The effectiveness of predator cues unpaired with conspecific alarm cues suggests that embryonic exposure to even naïve stressors may promote some kind of sensitization and their cautionary associations with predation risk, with indisputable benefits whenever the cue actually belongs to a potential predator, but costs when it does not. In the latter case, further studies carried out in an ontogenetic perspective may assess whether and to which extent behavioural shifts are reversible.
Data availability
Data are available on reasoned request from the corresponding author.
References
Batabyal A, Gosavi SM, Gramapurohit NP (2014) Determining sensitive stages for learning to recognise predators in larval bronzed frogs: importance of alarm cues in learning. J Biosci 39:701–710. https://doi.org/10.1007/s12038-014-9455-7
Belda X, Nadal R, Armario A (2016) Critical features of acute stress-induced cross-sensitization identified through the hypothalamic-pituitary-adrenal axis output. Sci Rep 6:1–12. https://doi.org/10.1038/srep31244
Blumstein DT (2016) Habituation and sensitization: new thoughts about old ideas. Anim Behav 120:255–262. https://doi.org/10.1016/j.anbehav.2016.05.012
Chivers SL, Brown GE, Smith RJF (1996) The evolution of chemical alarm signals: attracting predator’s benefits alarm signal senders. Am Nat 148:649–659. https://doi.org/10.1086/285945
Dunlap AS, Stephens DW (2016) Reliability, uncertainty, and costs in the evolution of animal learning. Curr Opin Behav Sci 12:73–79. https://doi.org/10.1016/j.cobeha.2016.09.010
Fanselow MS, DeCola JP, Young SL (1993) Mechanisms responsible for reduced contextual conditioning with massed un-signaled unconditional stimuli. J Exp Psychol Anim Behav Proc 19:121–137. https://doi.org/10.1037/0097-7403.19.2.121
Ferrari MCO, Chivers DP (2008) Cultural learning of predator recognition in mixed-species assemblages of frogs: the effect of tutor-to-observer ratio. Anim Behav 75:1921–1925. https://doi.org/10.1016/j.anbehav.2007.10.037
Ferrari MCO, Chivers DP (2009) Latent inhibition of predator recognition by embryonic amphibians. Biol Lett 5:160–162. https://doi.org/10.1098/rsbl.2008.0641
Ferrari MCO, Chivers DP (2011) Learning about non-predators and safe places: the forgotten elements of risk assessment. Anim Cognit 14:309–316. https://doi.org/10.1007/s10071-010-0363-4
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator prey interactions in aquatic ecosystems: a re-view and prospectus. Can J Zool 88:698–724. https://doi.org/10.1139/Z10-029
Gall BG, Mathis A (2010) Innate predator recognition and the problem of introduced trout. Ethology 116:47–58. https://doi.org/10.1111/j.1439-0310.2009.01718.x
Gallie JA, Mumme RL, Wissinger SA (2001) Experience has no effect on the development of chemosensory recognition of predators by tadpoles of the American toad, Bufo americanus. Herpetologica 57:376–383
Garcia TS, Urbina JC, Bredeweg EM, Ferrari MCO (2017) Embryonic learning and developmental carry-over effects in an invasive anuran. Oecologia 184:623–631. https://doi.org/10.1007/s00442-017-3905-5
Gazzola A, Brandalise F, Rubolini D, Rossi P, Galeotti P (2015) Fear is the mother of invention: anuran embryos exposed to predator cues alter life-history traits, post-hatching behaviour, and neuronal activity patterns. J Exp Biol 218:3919–3930. https://doi.org/10.1242/jeb.126334
Gazzola A, Russo G, Balestrieri A (2018) Embryonic and larval defensive responses of agile frog (Rana dalmatina) to alien crayfish. Ethology 124:347–356. https://doi.org/10.1111/eth.12737
Gazzola A, Balestrieri A, Brazzale G, Pellitteri-Rosa D (2021a) Effects of conspecific density on tadpole risk assessment and defensive behaviour. Behaviour 159:21–37. https://doi.org/10.1163/1568539X-bja10114
Gazzola A, Balestrieri A, Scribano G, Fontana A, Pellitteri-Rosa D (2021b) Contextual behavioural plasticity in Italian agile frog (Rana latastei) tadpoles exposed to native and alien predator cues. J Exp Biol. https://doi.org/10.1242/jeb.240465
Gonzalo A, Lopez P, Martin J (2007) Iberian green frog tadpoles may learn to recognise novel predators from chemical alarm cues of conspecifics. Anim Behav 74:447–453. https://doi.org/10.1016/j.anbehav.2006.11.032
Guadin B, Gazzola A, Balestrieri A, Scribano G, Martín J, Pellitteri-Rosa D (2021) Effects of a group-living experience on the antipredator responses of individual tadpoles. Anim Behav 180:93–99. https://doi.org/10.1016/j.anbehav.2021.08.009
Hartig F, Lohse L (2020) Package ‘DHARMa'.–<http://florianhartig.github.io/DHARMa
Hepper PG, Waldman B (1992) Embryonic olfactory learning in frogs. Quart J Exp Psycol Sec B Comp Physiol Psychol 44:179–197. https://doi.org/10.1080/02724999208250611
Hettyey A, Tòth Z, Thonhauser KE, Frommen JG, Penn DJ, Van Buskirk J (2015) The relative importance of prey-borne and predator borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179:699–710. https://doi.org/10.1007/s00442-015-3382-7
Hilbe JM (2014) Modelling count data. Cambridge University Press, New York
Ireland DH, Wirsing AJ, Murray DL (2007) Phenotypically plastic responses of green frog embryos to conflicting predation risk. Oecologia 152:162–168
Laurila A (2000) Behavioural responses to predator chemical cues and local variation in antipredator performance in Rana temporaria tadpoles. Oikos 88:159–168. https://doi.org/10.1034/j.1600-0706.2000.880118.x
Laurila A, Kujasalon J, Ranta E (1997) Different antipredatory behavior in two anuran tadpoles: effects of predator diet. Behav Ecol Sociobiol 40:329–336. https://doi.org/10.1007/s002650050349
Lee YC, Darmaillacq AS, Dickel L, Chiao CC (2020) Effects of embryonic exposure to predators on the postnatal defensive behaviors of cuttlefish. J Exp Mar Biol Ecol 524:151–288. https://doi.org/10.1016/j.jembe.2019.151288
Lenth VR et al 2021. Package ‘emmeans’ R topics documented. <https://github.com/rvlenth/emmeans>
Lodé T, Lesbarrères D (2004) Multiple paternity in Rana dalmatina, a monogamous territorial breeding anuran. Naturwissenschaften 91:44–47. https://doi.org/10.1007/s00114-003-0491-7
Lubow RE (1973) Latent inhibition. Psychol Bull 79:398–407. https://doi.org/10.1037/h0034425
Magurran AE, Seghers BH, Carvalho GR, Shaw PW (1993) Evolution of adaptive variation in antipredator behaviour. Mar Freshw Behav Phy 23:29–44. https://doi.org/10.1080/10236249309378855
Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP (2008) Learning by embryos and the ghost of predation future. Proc Roy Soc B Biol Sci 275:2603–2607. https://doi.org/10.1098/rspb.2008.0754
McCarthy TM, Fisher WA (2000) Multiple predator-avoidance behaviours of the freshwater snail Physella heterostropha pomila: responses vary with risk. Freshw Biol 44:387–397. https://doi.org/10.1046/j.1365-2427.2000.00576.x
Mitchell MD, McCormick MI (2013) Ontogenetic differences in chemical alarm cue production determine antipredator responses and learned predator recognition. Behav Ecol Sociobiol 67:1123–1129. https://doi.org/10.1007/s00265-013-1537-2
Nisbet IC (2000) Disturbance, habituation, and management of waterbird colonies. Waterbirds 23:312–332
Nunes AL, Richter-Boix A, Laurila A, Rebelo R (2013) Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia 171:115–127. https://doi.org/10.1007/s00442-012-2389-6
Orizaola G, Brana F (2005) Plasticity in newt metamorphosis: the effect of predation at embryonic and larval stages. Freshw Biol 50:438–446. https://doi.org/10.1111/j.1365-2427.2005.01332.x
Oulton LJ, Haviland V, Brown C (2013) Predator recognition in rainbowfish, Melanotaenia duboulaui embryos. PLoS ONE 8:e76061. https://doi.org/10.1371/journal.pone.0076061
Perotti MG, Pueta M, Jara FG, Ubeda CA, Moreno Azocar DL (2016) Lack of functional link in the tadpole morphology induced by predators. Curr Zool 62:227–235. https://doi.org/10.1093/cz/zow014
Polo-Cavia N, Gomez-Mestre I (2014) Learned recognition of introduced predators determines survival of tadpole prey. Funct Ecol 28:432–439. https://doi.org/10.1111/1365-2435.12175
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2005) Constraints on inducible defenses: phylogeny, ontogeny, and phenotypic trade-offs. In: Barbosa P, Castellanos I (eds) The ecology of predator-prey interactions. Oxford University Press, Oxford, pp 189–207
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Scribano G, Balestrieri A, Gazzola A, Pellitteri-Rosa D (2020) Strong behavioural defensive responses of endemic Rana latastei tadpoles induced by a native predator’s odour. Ethology 126:922–930. https://doi.org/10.1111/eth.13072
Sih A, Moore RD (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am Nat 142:947–960. https://doi.org/10.1086/285583
Stephens D (1991) Change, regularity, and value in the evolution of animal learning. Behav Ecol 2:77–89. https://doi.org/10.1093/beheco/2.1.77
Supekar SC, Gramapurohit NP (2017) Can embryonic skipper frogs (Euphlyctis cyanophlyctis) learn to recognise kairomones in the absence of a nervous system? J Biosci 42:459–468
Tarvin RD, Silva Bermúdez C, Briggs VS, Warkentin KM (2015) Carry-over effects of size at metamorphosis in red-eyed treefrogs: higher survival but slower growth of larger metamorphs. Biotropica 47:218–226. https://doi.org/10.1111/btp.12198
Teplitsky C, Plénet S, Léna JP, Mermet N, Malet E, Joly P (2005) Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole. J Evol Biol 18:180–190. https://doi.org/10.1111/j.1420-9101.2004.00790.x
Van Buskirk J (2001) Specific induced responses to different predator species in anuran larvae. J Evol Biol 14:482–489
Van Buskirk J, Ferrari M, Kueng D, Näpflin K, Ritter N (2011) Prey risk assessment depends on conspecific density. Oikos 120:1235–1239
Warkentin KM (1995) Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc Natl Acad Sci USA 92:3507–3510. https://doi.org/10.1073/pnas.92.8.3507
Warkentin KM (2005) How do embryos assess risk? Vibrational cues in predator-induced hatching of red-eyed treefrogs. Anim Behav 70:59–71. https://doi.org/10.1016/j.anbehav.2004.09.019
Warkentin KM (2011) Plasticity of hatching in amphibians: evolution, trade-offs, cues and mechanisms. Integr Comp Biol 51:111–127. https://doi.org/10.1093/icb/icr046
Wells KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago, USA
Woody DR, Mathis A (1998) Acquired recognition of chemical stimuli from an unfamiliar predator: associative learning by adult newts Notophthalmus viridescens. Copeia 4:1027–1031. https://doi.org/10.2307/1447352
Acknowledgements
We thank Matteo Spagnolo and Maurizio Florio for their help with laboratory work.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, AG and DPR; formal analysis, AG; investigation, AG and DPR.; data curation, AG; writing—original draft preparation, AG and AB; writing—review and editing, AG, AB, and DPR; visualization, AG, AB, and DPR All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All procedures followed institutional guidelines and ethics, and were reviewed and approved by the Societas Herpetologica Italica (SHI), the national Institute for Environmental Protection and Research (ISPRA), and the Italian Ministry of Environment, Land and Sea (MATTM; permits code: 0006075e23/03/2018dPNM).
Additional information
Handling Editor: Télesphore Sime-Ngando.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gazzola, A., Balestrieri, A. & Pellitteri-Rosa, D. Embryonic exposure to native and alien predator cues tunes tadpole defensive behaviour. Aquat Ecol 57, 421–431 (2023). https://doi.org/10.1007/s10452-023-10019-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-023-10019-9