Abstract
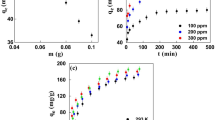
In this study, activated carbon (AC) obtained from biomass waste materials (sunflower seed shells -SSS) was synthesized by combining chemical (H3PO4 80% wt.) and thermal activation (at 544 °C). Synthesized AC exhibited a BET surface area of 1531 m2 g−1, and pore volume of 0.98 cm3 g−1. The material exhibited various surface functional groups, such as P2O7, C-O-P and -COOH, O = C, as well as a moderate graphitization degree (ID/IG < 1) and acidity caused by H3PO4 treatment. Moreover, its morphology and physicochemical features were evaluated by SEM–EDS, TGA, XPS, Raman, and FT-IR techniques. The material was used to study the adsorption of anti-inflammatory pharmaceutical compounds such as ibuprofen (IBF) and diclofenac (DIF) present in natural groundwater samples. The effects of parameters such as pH, activated carbon dose, temperature, and IBF or DIF initial concentration were optimized by using a central composite design (CCD). The results revealed that optimum conditions to remove DIF and IBF from natural groundwater samples were pH of 8.0 and 7.0, an AC dose of 0.79 and 1.0 g L−1, and a contact time of 60 min for DIF and IBF, respectively. A successful procedure to desorb both pollutants from adsorbent by using acetonitrile solutions was achieved, allowing the reuse study whose main results were that after four reusing cycles AC reduced its efficiency to remove DIF and IBF in 28 and 34%, respectively. Finally, the effect of ions, such as nitrate, bicarbonate, and sulfate at concentrations commonly found in natural groundwater on the adsorption of both pollutants onto AC was studied using deionized water. As a result, this study suggests considerable interest of AC in real applications due to its versatility and prolonged reuse to effectively remove anti-inflammatory compounds from natural aqueous solution.
Graphical Abstract

Highlights
-
Optimal conditions for the pollutants adsorption were found by an experimental design
-
Adsorption of pollutants on activated carbon was studied in groundwater samples
-
Good adsorbent properties of activated carbon remained even after 4 reusing cycles
-
Activated carbon exhibited several functional groups and high specific surface area
-
The effect of ions present in groundwater on pollutants adsorption was studied











Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Ahmed, M.J.: Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: Review. J. Environ. Manage. 190, 74–282 (2017). https://doi.org/10.1016/j.jenvman.2016.12.073
Álvarez-Torrellas, S., Rodríguez, A., Ovejero, G., García, J.: Comparative adsorption performance of ibuprofen and tetracycline from aqueous solution by carbonaceous materials. Chem. Eng. J. 283, 936–947 (2016). https://doi.org/10.1016/j.cej.2015.08.023
Alvear-Daza, J.J., Cánneva, A., Donadelli, J.A., Manrique-Holguín, M., Rengifo-Herrera, J.A., Pizzio, L.R.: Removal of diclofenac and ibuprofen on mesoporous activated carbon from agro-industrial wastes prepared by optimized synthesis employing a central composite design. Biomass Convers. Biorefin. (2022). https://doi.org/10.1007/s13399-021-02227-w
Antunes, M., Esteves, V.I., Guégan, R., Crespo, J.S., Fernandes, A.N., Giovanela, M.: Removal of diclofenac sodium from aqueous solution by Isabel grape bagasse. Chem. Eng. J. 192, 114–121 (2012). https://doi.org/10.1016/j.cej.2012.03.062
Baccar, R., Sarrà, M., Bouzid, J., Feki, M., Blánquez, P.: Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 211–212, 310–317 (2012). https://doi.org/10.1016/j.cej.2012.09.099
Bhadra, B.N., Ahmed, I., Kim, S., Jhung, S.H.: Adsorptive removal of ibuprofen and diclofenac from water using metal-organic framework-derived porous carbon. Chem. Eng. J. 314, 50–58 (2017). https://doi.org/10.1016/j.cej.2016.12.127
Bhadra, B.N., Seo, P.W., Jhung, S.H.: Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 301, 27–34 (2016). https://doi.org/10.1016/j.cej.2016.04.143
Bibi, M., Rashid, J., Malik, M., Iqbal, A., Xu, M.: Physicochemical analysis and detection of exceptionally high diclofenac concentration in the pharmaceutical wastewaters collected from the production units of national industrial zone, Rawat, Pakistan. Appl. Water Sci. 13 (2023). https://doi.org/10.1007/s13201-023-01954-x
Bourassi, M., Kárászová, M., Pasichnyk, M., Zazpe, R., Herciková, J., Fíla, V., Macak, J.M., Gaálová, J.: Removal of ibuprofen from water by different types membranes. Polymers (Basel). 13 (2021). https://doi.org/10.3390/polym13234082
Chang, C.F., Chen, T.Y., Chin, C.J.M., Kuo, Y.T.: Enhanced electrochemical degradation of ibuprofen in aqueous solution by PtRu alloy catalyst. Chemosphere 175, 76–84 (2017). https://doi.org/10.1016/j.chemosphere.2017.02.021
Chen, H., Akhtar, L.: Chapter 3 - Fate and transport of pharmaceuticals and personal care products in soils and groundwater. In: Gao, B. (ed.) Emerging Contaminants in Soil and Groundwater Systems, pp. 49–82. Elsevier (2022). https://doi.org/10.1016/B978-0-12-824088-5.00004-5
Chen, M., He, F., Hu, D., Bao, C., Huang, Q.: Broadened operating pH range for adsorption/reduction of aqueous Cr(VI) using biochar from directly treated jute (Corchorus capsularis L.) fibers by H3PO4. Chem. Eng. J. 381, 122739 (2020). https://doi.org/10.1016/j.cej.2019.122739
Chu, G., Zhao, J., Huang, Y., Zhou, D., Liu, Y., Wu, M., Peng, H., Zhao, Q., Pan, B., Steinberg, C.E.W.: Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 240, 1–9 (2018). https://doi.org/10.1016/j.envpol.2018.04.003
Czech, B., Oleszczuk, P.: Sorption of diclofenac and naproxen onto MWCNT in model wastewater treated by H2O2 and/or UV. Chemosphere 149, 272–278 (2016). https://doi.org/10.1016/j.chemosphere.2015.12.057
Díez, E., Gómez, J.M., Rodríguez, A., Bernabé, I., Sáez, P., Galán, J.: A new mesoporous activated carbon as potential adsorbent for effective indium removal from aqueous solutions. Microporous and Mesoporous Mater. 295 (2020). https://doi.org/10.1016/j.micromeso.2019.109984
di Lorenzo, T., Cifoni, M., Baratti, M., Pieraccini, G., di Marzio, W.D., Galassi, D.M.P.: Four scenarios of environmental risk of diclofenac in European groundwater ecosystems. Environ. Pollut. 287, (2021). https://doi.org/10.1016/j.envpol.2021.117315
Iqbal, J., Mohamed Al Hajeri, B., Shah, N.S., Wilson, K., Xavier, C., Shaalan, J., Al-Taani, A.A., Howari, F., Nazzal, Y.: Preparation of H3PO4 modified Sidr biochar for the enhanced removal of ciprofloxacin from water. Int. J. Phytoremediation. 24, 1231–1242 (2022). https://doi.org/10.1080/15226514.2021.2025038
Jiang, M., Yang, W., Zhang, Z., Yang, Z., Wang, Y.: Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. J. Environ. Sci. (China) 31, 226–234 (2015). https://doi.org/10.1016/j.jes.2014.09.035
Jodeh, S., Abdelwahab, F., Jaradat, N., Warad, I., Jodeh, W.: Adsorption of diclofenac from aqueous solution using Cyclamen persicum tubers based activated carbon (CTAC). J. Assoc. Arab Univ. Basic Appl. Sci. 20, 32–38 (2016). https://doi.org/10.1016/j.jaubas.2014.11.002
Kumar, M., Jaiswal, S., Sodhi, K.K., Shree, P., Singh, D.K., Agrawal, P.K., Shukla, P.: Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance, (2019)
Kundu, A., sen Gupta, B., Hashim, M.A., Redzwan, G.: Taguchi optimization approach for production of activated carbon from phosphoric acid impregnated palm kernel shell by microwave heating. J. Clean. Prod. 105, 420–427 (2015). https://doi.org/10.1016/j.jclepro.2014.06.093
Larous, S., Meniai, A.H.: Adsorption of Diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrogen Energy 41, 10380–10390 (2016). https://doi.org/10.1016/j.ijhydene.2016.01.096
Lonappan, L., Rouissi, T., Kaur Brar, S., Verma, M., Surampalli, R.Y.: An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 249, 386–394 (2018). https://doi.org/10.1016/j.biortech.2017.10.039
Ma, L., Liu, Y., Yang, Q., Jiang, L., Li, G.: Occurrence and distribution of Pharmaceuticals and Personal Care Products (PPCPs) in wastewater related riverbank groundwater. Sci. Total Environ. 821, (2022). https://doi.org/10.1016/j.scitotenv.2022.153372
Malhotra, M., Suresh, S., Garg, A.: Tea waste derived activated carbon for the adsorption of sodium diclofenac from wastewater: adsorbent characteristics, adsorption isotherms, kinetics, and thermodynamics. Environ. Sci. Pollut. Res. 25, 32210–32220 (2018). https://doi.org/10.1007/s11356-018-3148-y
Martin, R.J., Ng, W.J.: Chemical regeneration of exhausted activated carbon-I. Water Res. 18, 59–73 (1984)
Mestre, A.S., Hesse, F., Freire, C., Ania, C.O., Carvalho, A.P.: Chemically activated high grade nanoporous carbons from low density renewable biomass (Agave sisalana) for the removal of pharmaceuticals. J. Colloid Interface Sci. 536, 681–693 (2019). https://doi.org/10.1016/j.jcis.2018.10.081
Mestre, A.S., Pires, J., Nogueira, J.M.F., Parra, J.B., Carvalho, A.P., Ania, C.O.: Waste-derived activated carbons for removal of ibuprofen from solution: Role of surface chemistry and pore structure. Bioresour. Technol. 100, 1720–1726 (2009). https://doi.org/10.1016/j.biortech.2008.09.039
Mondal, S., Aikat, K., Halder, G.: Biosorptive uptake of ibuprofen by chemically modified Parthenium hysterophorus derived biochar: Equilibrium, kinetics, thermodynamics and modeling. Ecol. Eng. 92, 158–172 (2016a). https://doi.org/10.1016/j.ecoleng.2016.03.022
Mondal, S., Bobde, K., Aikat, K., Halder, G.: Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: Equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J. Environ. Manage. 182, 581–594 (2016b). https://doi.org/10.1016/j.jenvman.2016.08.018
Pap, S., Taggart, M.A., Shearer, L., Li, Y., Radovic, S., Turk Sekulic, M.: Removal behaviour of NSAIDs from wastewater using a P-functionalised microporous carbon. Chemosphere. 264, (2021). https://doi.org/10.1016/j.chemosphere.2020.128439
Patel, M., Kumar, R., Kishor, K., Mlsna, T., Pittman, C.U. Jr., Mohan, D.: Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 119, 3510–3673 (2019). https://doi.org/10.1021/acs.chemrev.8b00299
Salvador, F., Martin-Sanchez, N., Sanchez-Hernandez, R., Sanchez-Montero, M.J., Izquierdo, C.: Regeneration of carbonaceous adsorbents. Part II: Chemical, microbiological and vacuum regeneration. Micropor. Mesopor. Mat. 202, 277–296 (2015). https://doi.org/10.1016/j.micromeso.2014.08.019
Saucier, C., Adebayo, M.A., Lima, E.C., Cataluña, R., Thue, P.S., Prola, L.D.T., Puchana-Rosero, M.J., Machado, F.M., Pavan, F.A., Dotto, G.L.: Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J. Hazard. Mater. 289, 18–27 (2015). https://doi.org/10.1016/j.jhazmat.2015.02.026
Shin, J., Kwak, J., Kim, S., Son, C., Lee, Y.G., Baek, S., Park, Y., Chae, K.J., Yang, E., Chon, K.: Facilitated physisorption of ibuprofen on waste coffee residue biochars through simultaneous magnetization and activation in groundwater and lake water: Adsorption mechanisms and reusability. J. Environ. Chem. Eng. 10, (2022). https://doi.org/10.1016/j.jece.2022.107914
Shin, J., Kwak, J., Lee, Y.G., Kim, S., Son, C., Cho, K.H., Lee, S.H., Park, Y., Ren, X., Chon, K.: Changes in adsorption mechanisms of radioactive barium, cobalt, and strontium ions using spent coffee waste biochars via alkaline chemical activation: Enrichment effects of O-containing functional groups. Environ. Res. 199, (2021). https://doi.org/10.1016/j.envres.2021.111346
Shirani, Z., Song, H., Bhatnagar, A.: Efficient removal of diclofenac and cephalexin from aqueous solution using Anthriscus sylvestris-derived activated biochar. Sci. Total Environ. 745, (2020). https://doi.org/10.1016/j.scitotenv.2020.140789
Show, S., Karmakar, B., Halder, G.: Sorptive uptake of anti-inflammatory drug ibuprofen by waste biomass–derived biochar: experimental and statistical analysis. Biomass Convers. Biorefin. 12, 3955–3973 (2022a). https://doi.org/10.1007/s13399-020-00922-8
Show, S., Karmakar, B., Halder, G.: Sorptive uptake of anti-inflammatory drug ibuprofen by waste biomass–derived biochar: experimental and statistical analysis. Biomass Convers. Biorefin. 12, 3955–3973 (2022b). https://doi.org/10.1007/s13399-020-00922-8
Song, C., Chen, K., Chen, M., Jin, X., Liu, G., Du, X., Chen, D., Huang, Q.: Sequential combined adsorption and solid-phase photocatalysis to remove aqueous organic pollutants by H3PO4-modified TiO2 nanoparticles anchored on biochar. J Water Process Eng. 45, 102467 (2022). https://doi.org/10.1016/j.jwpe.2021.102467
Torrellas, S.Á., GarcíaLovera, R., Escalona, N., Sepúlveda, C., Sotelo, J.L., García, J.: Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem. Eng. J. 279, 788–798 (2015). https://doi.org/10.1016/j.cej.2015.05.104
Tran, H.N., You, S.J., Chao, H.P.: Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 4, 2671–2682 (2016). https://doi.org/10.1016/j.jece.2016.05.009
Tran, H.N., You, S.J., Hosseini-Bandegharaei, A., Chao, H.P.: Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 120, 88–116 (2017). https://doi.org/10.1016/j.watres.2017.04.014
Trovó, A.G., Nogueira, R.F.P.: Diclofenac abatement using modified solar photo-fenton process with Ammonium Iron(III) citrate. J. Braz. Chem. Soc. 22, 1033–1039 (2011). https://doi.org/10.1590/S0103-50532011000600005
Vergili, I.: Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources. J. Environ. Manage. 127, 177–187 (2013). https://doi.org/10.1016/j.jenvman.2013.04.036
Vogna, D., Marotta, R., Napolitano, A., Andreozzi, R., D’Ischia, M.: Advanced oxidation of the pharmaceutical drug diclofenac with UV/H 2O2 and ozone. Water Res. 38, 414–422 (2004). https://doi.org/10.1016/j.watres.2003.09.028
Xie, J., Liu, M., He, M., Liu, Y., Li, J., Yu, F., Lv, Y., Lin, C., Ye, X.: Ultra-efficient adsorption of diclofenac sodium on fish-scale biochar functionalized with H3PO4 via synergistic mechanisms. Environ. Pollut. 322, (2023). https://doi.org/10.1016/j.envpol.2023.121226
Yang, H., Chen, P., Chen, W., Li, K., Xia, M., Xiao, H., Chen, X., Chen, Y., Wang, X., Chen, H.: Insight into the formation mechanism of N, P co-doped mesoporous biochar from H3PO4 activation and NH3 modification of biomass. Fuel Process. Technol. 230, 107215 (2022). https://doi.org/10.1016/j.fuproc.2022.107215
Yin, R., Guo, W., Wang, H., Du, J., Wu, Q., Chang, J.S., Ren, N.: Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 357, 589–599 (2019). https://doi.org/10.1016/j.cej.2018.09.184
Zeng, H., Zeng, H., Zhang, H., Shahab, A., Zhang, K., Lu, Y., Nabi, I., Naseem, F., Ullah, H.: Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar. J. Clean. Prod. 286, (2021). https://doi.org/10.1016/j.jclepro.2020.124964
Zhang, S., Dong, Y., Yang, Z., Yang, W., Wu, J., Dong, C.: Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem. Eng. J. 304, 325–334 (2016). https://doi.org/10.1016/j.cej.2016.06.087
Acknowledgements
Authors thank to “Consejo Nacional de Investigaciones Científicas y Técnicas”-CONICET Argentina for the economic support (grant PIP 0449 and 1492) and National University of La Plata-UNLP and CONICET for the economic support (grant PIO 024).
Funding
“Consejo Nacional de Investigaciones Científicas y Técnicas”-CONICET Argentina for the economic support (grant PIP 1492). National University of La Plata-UNLP and CONICET for the economic support (grant PIO 024).
Author information
Authors and Affiliations
Contributions
JJAD: [Conceptualization, Methodology, Investigation, Formal Analysis, Visualization, Writing-original draft]. JARH: [Conceptualization, Methodology, writing review & editing, Supervision, Project Administration, and Funding Acquisition]. LRP: [Conceptualization, Methodology, writing review & editing, Supervision, Project Administration, and Funding Acquisition].
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to participate and consent to publish
All authors have read and approved the manuscript and they agree with the order of authors listed in the text.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alvear-Daza, J.J., Rengifo-Herrera, J.A. & Pizzio, L.R. Performance and optimization of diclofenac and ibuprofen adsorption onto activated carbon synthesized from sunflower seed shell (Helianthus annuus) in natural groundwater samples. Adsorption (2024). https://doi.org/10.1007/s10450-024-00461-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00461-y