Abstract
Transcatheter heart valve (THV) leaflet thrombosis in the neo-sinus and associated reduced leaflet motion is of clinical concern due to risks of embolism and worsened valve hemodynamics. Flow stasis in the neo-sinus (the space between the native and THV leaflets) is a known risk factor, but the role of proximal coronary flow is yet to be investigated. We tested two replicas of FDA approved commercial THVs—intra-annular and supra-annular (similar to the SAPIEN 3 and CoreValve families)—in a left heart simulator with coronary flow. Velocity fields in the left coronary cusp (LCC) and non (NCC) neo-sinus were quantified using high speed particle image velocimetry and particle residence times (PRT) were computed to evaluate flow stasis in the region. The supra-annular THV LCC neo-sinus had shorter PRT than its NCC neo-sinus (0.66 ± 0.00 vs. 0.76 ± 0.04, p = 0.038), while the intra-annular THV LCC neo-sinus had similar PRT to its NCC neo-sinus (1.93 ± 0.05 vs. 1.92 ± 0.03 cycles, p = 0.889). The supra-annular valve LCC and NCC neo-sinuses had shorter PRT than their intra-annular valve counterparts (p < 0.001). These results showed that coronary flow reduces flow stasis in the supra-annular THV neo-sinus and, ostensibly, thrombosis risk in the region. This effect was not significant in the intra-annular valve.







Similar content being viewed by others
Abbreviations
- THV:
-
Transcatheter heart valve
- LCC:
-
Left coronary cusp
- RCC:
-
Right coronary cusp
- NCC:
-
Non-coronary cusp
- PRT:
-
Particle residence time
- IAD:
-
Georgia Tech TAVR intra-annular device
- SAD:
-
Georgia Tech TAVR supra-annular device
- LCS:
-
Left-coronary sinus
- NCS:
-
Non-coronary sinus
- STJ:
-
Sino-tubular junction
- PIV:
-
Particle image velocimetry
References
Butnaru, A., J. Shaheen, D. Tzivoni, R. Tauber, D. Bitran, and S. Silberman. Diagnosis and treatment of early bioprosthetic malfunction in the mitral valve position due to thrombus formation. Am. J. Cardiol. 112:1439–1444, 2013.
Chakravarty, T., L. Søndergaard, J. Friedman, O. De Backer, D. Berman, K. F. Kofoed, H. Jilaihawi, T. Shiota, Y. Abramowitz, T. H. Jørgensen, T. Rami, S. Israr, G. Fontana, M. de Knegt, A. Fuchs, P. Lyden, A. Trento, D. L. Bhatt, M. B. Leon, R. R. Makkar, D. Ramzy, W. Cheng, R. J. Siegel, L. M. Thomson, G. Mangat, B. Hariri, F. J. Sawaya, and H. K. Iversen. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 389:2383–2392, 2017.
Dasi, L. P., H. Hatoum, A. Kheradvar, R. Zareian, S. H. Alavi, W. Sun, C. Martin, T. Pham, Q. Wang, P. A. Midha, V. Raghav, and A. P. Yoganathan. On the mechanics of transcatheter aortic valve replacement. Ann. Biomed. Eng. 45:310–331, 2017.
De Marchena, E., J. Mesa, S. Pomenti, C. Marin y Kall, X. Marincic, K. Yahagi, E. Ladich, R. Kutz, Y. Aga, M. Ragosta, A. Chawla, M. E. Ring, and R. Virmani. Thrombus formation following transcatheter aortic valve replacement. J. Am. Coll. Cardiol. Cardiovasc. Interv. 8:728–739, 2015.
Ducci, A., F. Pirisi, S. Tzamtzis, and G. Burriesci. Transcatheter aortic valves produce unphysiological flows which may contribute to thromboembolic events: an in-vitro study. J. Biomech. 49:4080–4089, 2016. https://doi.org/10.1016/j.jbiomech.2016.10.050.
Egbe, A. C., S. V. Pislaru, P. A. Pellikka, J. T. Poterucha, H. V. Schaff, J. J. Maleszewski, and H. M. Connolly. Bioprosthetic valve thrombosis versus structural failure. J. Am. Coll. Cardiol. 66:2285–2294, 2015.
Fuchs, A., O. De Backer, M. Brooks, M. C. De Knegt, G. Bieliauskas, M. Yamamoto, R. Yanagisawa, K. Hayashida, L. Søndergaard, and K. F. Kofoed. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 13:1067–1075, 2017.
Guyton, A. C., and J. E. Hall. TextBook in medical. Physiology 2006. https://doi.org/10.1038/sj.cr.7310053.
Hatoum, H., J. Dollery, S. M. Lilly, J. A. Crestanello, and L. P. Dasi. Implantation depth and rotational orientation effect on valve-in-valve hemodynamics and sinus flow. Ann. Thorac. Surg. 106:70–78, 2018.
Hatoum, H., P. Maureira, S. Lilly, and L. P. Dasi. Impact of leaflet laceration on transcatheter aortic valve-in-valve washout: BASILICA to solve neosinus and sinus stasis. JACC Cardiovasc. Interv. 12:1229–1237, 2019.
Hatoum, H., P. Maureira, S. Lilly, and L. P. Dasi. Impact of BASILICA on sinus and neo-sinus hemodynamics after valve-in-valve with and without coronary flow. Cardiovasc. Revasc. Med. 2019. https://doi.org/10.1016/j.carrev.2019.06.015.
Kasel, A. M., J. M. Khan, A. B. Greenbaum, and J. M. Michel. Intentional transcatheter laceration of the coronary cusp to prevent left main stem obstruction during transcatheter aortic valve implantation: first European experience with the BASILICA technique in a native aortic valve. Eur. Heart J. 40(17):1384–1385, 2018. https://doi.org/10.1093/eurheartj/ehy711.
Khan, J. M., D. Dvir, A. B. Greenbaum, V. C. Babaliaros, T. Rogers, G. Aldea, M. Reisman, G. B. Mackensen, M. H. K. Eng, G. Paone, D. D. Wang, R. A. Guyton, C. M. Devireddy, W. H. Schenke, and R. J. Lederman. Transcatheter laceration of aortic leaflets to prevent coronary obstruction during transcatheter aortic valve replacement: concept to first-in-human. JACC Cardiovasc. Interv. 11(7):677–689, 2018. https://doi.org/10.1016/j.jcin.2018.01.247.
Laschinger, J. C., C. Wu, N. G. Ibrahim, and J. E. Shuren. Reduced leaflet motion in bioprosthetic aortic valves-the FDA perspective. N. Engl. J. Med. 373:1996–1998, 2015.
Leetmaa, T., N. C. Hansson, J. Leipsic, K. Jensen, S. H. Poulsen, H. R. Andersen, J. M. Jensen, J. Webb, P. Blanke, M. Tang, and B. L. Norgaard. Early aortic transcatheter heart valve thrombosis: diagnostic value of contrast-enhanced multidetector computed tomography. Circ. Cardiovasc. Interv. 8:e001596, 2015.
Madukauwa-David, I. D., P. A. Midha, R. Sharma, K. McLain, R. Mitra, K. Crawford, S. H. Yoon, R. R. Makkar, and A. P. Yoganathan. Characterization of aortic root geometry in transcatheter aortic valve replacement patients. Catheter. Cardiovasc. Interv. 93:134–140, 2019.
Makkar, R. R., G. Fontana, H. Jilaihawi, T. Chakravarty, K. F. Kofoed, O. de Backer, F. M. Asch, C. E. Ruiz, N. T. Olsen, A. Trento, J. Friedman, D. Berman, W. Cheng, M. Kashif, V. Jelnin, C. A. Kliger, H. Guo, A. D. Pichard, N. J. Weissman, S. Kapadia, E. Manasse, D. L. Bhatt, M. B. Leon, and L. Søndergaard. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N. Engl. J. Med. 373:2015–2024, 2015.
Midha, P. A. A Parametric Investigation of Transcatheter Aortic Valve Replacement Performance. Dr. Thesis, 2017.
Midha, P. A., V. Raghav, I. Okafor, and A. P. Yoganathan. The effect of valve-in-valve implantation height on sinus flow. Ann. Biomed. Eng. 45:405–412, 2017.
Midha, P. A., V. Raghav, R. Sharma, J. F. Condado, I. U. Okafor, T. Rami, G. Kumar, V. H. Thourani, H. Jilaihawi, V. Babaliaros, R. R. Makkar, and A. P. Yoganathan. The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neosinus. Circulation 136:1598–1609, 2017.
Moore, B. L., and L. P. Dasi. Coronary flow impacts aortic leaflet mechanics and aortic sinus hemodynamics. Ann. Biomed. Eng. 43:2231–2241, 2015.
Oliver, J. M., P. Gallego, A. Gonzalez, F. J. Dominguez, C. Gamallo, and J. M. Mesa. Bioprosthetic mitral valve thrombosis: clinical profile, transesophageal echocardiographic features, and follow-up after anticoagulant therapy. J. Am. Soc. Echocardiogr. 9:691–699, 1996.
Pache, G., P. Blanke, W. Zeh, and N. Jander. Cusp thrombosis after transcatheter aortic valve replacement detected by computed tomography and echocardiography. Eur. Heart J. 34:3546, 2013.
Rashid, H. N., A. Nasis, R. P. Gooley, J. D. Cameron, and A. J. Brown. The prevalence of computed tomography-defined leaflet thrombosis in intra- versus supra-annular transcatheter aortic valve prostheses. Catheter. Cardiovasc. Interv. 92:1414–1416, 2018.
Sakamoto, S., S. Takahashi, A. U. Coskun, M. I. Papafaklis, A. Takahashi, S. Saito, P. H. Stone, and C. L. Feldman. Relation of distribution of coronary blood flow volume to coronary artery dominance. Am. J. Cardiol. 111(10):1420–1424, 2013. https://doi.org/10.1016/j.amjcard.2013.01.290.
Swanson, W. M., and R. E. Clark. Dimensions and geometric relationships of the human aortic valve as a function of pressure. Circ. Res. 35:871–882, 1974.
Trepels, T., S. Martens, M. Doss, S. Fichtlscherer, and V. Schächinger. Thrombotic restenosis after minimally invasive implantation of aortic valve stent. Circulation 120:e23–e24, 2009.
Trusty, P., V. Sadri, I. D. Madukauwa-David, N. Kamioka, R. Sharma, R. Makkar, V. Babaliaros, and A. P. Yoganathan. Neosinus flow stasis correlates with thrombus volume post-TAVR: a patient-specific in vitro study. JACC. Cardiovasc. Interv. 12(13):1288–1290, 2019. https://doi.org/10.1016/j.jcin.2017.10.017.
Acknowledgments
The authors would like to acknowledge the members of the Cardiovascular Fluid Mechanics Laboratory for their assistance and feedback.
Funding
The work at the Cardiovascular Fluid Mechanics Laboratory at the Georgia Institute of Technology was funded through the BME Gurley Foundation and the Mary and James Wesley Fellowship Endowment, as well as discretionary funds made available to the Principal Investigator, such as the Wallace H Coulter Endowed Chair.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Lakshmi Prasad Dasi oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10439_2019_2324_MOESM1_ESM.tiff
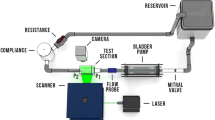
Supplementary material 1 Supplementary figure 1: Schematic of the coronary flow module. Components included a piston pump, two check valves, a flow probe, and variable resistance. (TIFF 12383 kb)
10439_2019_2324_MOESM2_ESM.tiff
Supplementary material 2 Supplementary figure 2: IAD flow streamlines and velocities for the LCC and NCC neo-sinuses and native sinuses at select time points in systole. (TIFF 19832 kb)
10439_2019_2324_MOESM3_ESM.tiff
Supplementary material 3 Supplementary figure 3: SAD flow streamlines and velocities for the LCC and NCC neo-sinuses and native sinuses at select time points in systole. (TIFF 19909 kb)
10439_2019_2324_MOESM4_ESM.tiff
Supplementary material 4 Supplementary figure 3: Representative snapshots of particle positions for the LCC of the supra-annular valve. (TIFF 0 kb)
Supplementary material 5 Video 1: Velocity contours and streamlines for the supra-annular valve LCC. (MP4 37794 kb)
Supplementary material 6 Video 2: Velocity contours and streamlines for the intra-annular valve LCC. (MP4 45106 kb)
Supplementary material 7 Video 3: Velocity contours and streamlines for the supra-annular valve NCC. (MP4 25784 kb)
Supplementary material 8 Video 4: Velocity contours and streamlines for the intra-annular valve NCC. (MP4 41932 kb)
Appendix 1
Appendix 1
Intra-annular Valve
Velocity fields and instantaneous streamlines for LCC and NCC of the intra-annular valve at select time points in the cardiac cycle are presented in and Fig. 4 (diastole) and Supplementary Fig. 1 (systole). Key flow characteristics in the neo-sinuses and sinuses at these time points are outlined as follows.
Systolic acceleration (22 ms, 50 ms, Supplementary Fig. 1): During early acceleration in the LCC neo-sinus, streamlines originating from the leaflet and extending toward the STJ suggested fluid washout of the region (maximum velocity: 0.26 m/s). A similar trend was seen in the NCC neo-sinus, with a maximum velocity of 0.25 m/s recorded. Fluid streamlines into the coronary artery from the LCC sinus were observed at velocities below 0.04 m/s; a region of closed streamlines was seen in the NCC sinus. As acceleration progressed, velocities in the neo-sinuses dropped significantly (maximum velocity LCC: 0.13 m/s, NCC: 0.12 m/s). Rotating flow was seen between the native leaflets and the STJ and streamlines from the sinus suggested fluid washout toward the central jet.
Peak systole (150 ms, Supplementary Fig. 1): Counterclockwise rotating flow was seen in the LCC neosinus but was not evident in the NCC neo-sinus (maximum velocity LCC: 0.13 m/s, NCC: 0.10 m/s). Streamlines at the top of the LCC sinus suggested fluid wash-into the coronary artery.
Systolic deceleration (250 ms, Supplementary Fig. 1): Counterclockwise rotating flow remained in the LCC neo-sinus at lower velocities than peak systole; streamlines in the NCC neo-sinus suggested fluid wash-into the neo-sinus (maximum velocity LCC: 0.07 m/s, NCC: 0.12 m/s). A zone of closed streamlines was seen in both LCC and NCC sinuses, with relatively high velocities toward the bottom of the sinuses (maximum velocity LCC: 0.14 m/s, NCC: 0.13 m/s).
Early diastole (450 ms, Fig. 4): In both LCC and NCC neo-sinuses, counterclockwise rotating flow was seen, indicating potential fluid recirculation behind the leaflets after valve closure (maximum velocity LCC: 0.12 m/s, NCC: 0.09 m/s). Streamlines in the LCC sinus suggested wash-into the coronary artery (maximum velocity: 0.09 m/s). A region of closed streamlines with low velocities was seen at the center of the NCC sinus (maximum velocity: 0.03 m/s).
Mid diastole (550 ms, Fig. 4): The region of counterclockwise rotating flow in the LCC and NCC neosinuses remained but appeared stronger in the LCC neo-sinus (maximum velocity LCC: 0.06 m/s, NCC: 0.04 m/s). Streamlines from the LCC sinus into the coronary artery remained present (maximum velocity: 0.09 m/s). The region of closed streamlines in the NCC sinus remained present (maximum velocity: 0.02 m/s).
Late diastole (650 ms, Fig. 4): The region of counterclockwise rotating flow in the LCC and NCC neosinuses remained present (maximum velocity LCC: 0.05 m/s, NCC: 0.03 m/s). Streamlines from the top of the LCC neo-sinus into the coronary artery suggested washout of fluid in this region. Streamlines from the LCC sinus into the coronary artery remained present (maximum velocity: 0.05 m/s). The region of closed streamlines in the NCC sinus remained present (maximum velocity: 0.02 m/s).
End diastole (750 ms, Fig. 4): A weaker region of counterclockwise rotating flow in the LCC and NCC neosinuses remained present (maximum velocity LCC: 0.05 m/s, NCC: 0.03 m/s). Streamlines from the top of the LCC neo-sinus into the sinus suggested entrainment of fluid from the top of the LCC neo-sinus into the sinus. Streamlines from the LCC sinus into the coronary artery remained present (maximum velocity: 0.03 m/s). Flow in the NCC sinus was quiescent.
Supra-annular Valve
Velocity fields and instantaneous streamlines for LCC and NCC of the supra-annular valve at select time points in the cardiac cycle are presented in Fig. 5 (diastole) and Supplementary Fig. 2 (systole). Key flow characteristics of the neo-sinuses and sinuses at these time points are outlined as follows.
Systolic acceleration (25, 50 ms, Supplementary Fig. 2): During early acceleration, streamlines in both LCC and NCC neo-sinuses suggested fluid washout of the regions; in the LCC neo-sinus, streamlines pointed toward the sinus while NCC neo-sinus streamlines pointed toward the STJ (maximum velocity LCC: 0.14 m/s, NCC: 0.14 m/s). As acceleration progressed, streamlines from the sinus into the neo-sinus suggested fluid wash-into both the NCC and LCC neo-sinuses (maximum velocity LCC: 0.07 m/s, NCC: 0.05 m/s). A counterclockwise vortex was present at both the LCC and native leaflet tips. Throughout acceleration, streamlines from the LCC sinus into the coronary artery suggested fluid washout of the region, while flow was slower in the NCC sinus and toward the STJ (maximum velocity LCC: 0.09 m/s, NCC: 0.04 m/s).
Peak systole (150 ms, Supplementary Fig. 2): Streamlines from both LCC and NCC neo-sinuses suggested washout of fluid from the regions toward the STJ (maximum velocity LCC: 0.07 m/s, NCC: 0.08 m/s). Streamlines from the LCC sinus suggested flow toward both the coronary artery and the STJ, while streamlines from the NCC sinus suggested flow toward the STJ (maximum velocity LCC: 0.08 m/s, NCC: 0.06 m/s).
Systolic deceleration (250 ms, Supplementary Fig. 2): Streamlines from the LCC and NCC neo-sinuses suggested flow toward the THV leaflets as they closed (maximum velocity LCC: 0.07 m/s, NCC: 0.06 m/s). Furthermore, streamlines from the LCC sinus suggested flow toward both the coronary artery and the THV leaflets, while streamlines from the NCC sinus suggested flow toward the THV leaflets (maximum velocity LCC: 0.07 m/s, NCC: 0.05 m/s).
Early diastole (450 ms, Fig. 5): As coronary flow reached its peak, streamlines from the LCC neo-sinus to the STJ, sinus, and coronary artery suggested fluid washout due to coronary flow; streamlines from the NCC neo-sinus to the STJ suggested fluid washout at lower velocities (maximum velocity LCC: 0.06 m/s, NCC: 0.03 m/s). Streamlines from the LCC sinus into the coronary artery suggested fluid washout of the region, while a region of closed streamlines was apparent in the NCC sinus (maximum velocity LCC: 0.11 m/s, NCC: 0.06 m/s).
Mid diastole (550 ms, Fig. 5): As coronary flow remained at a maximum, flow characteristics were similar to those of early diastole. Streamlines from the LCC neo-sinus to the sinus and coronary artery suggested fluid washout from the region due to coronary flow; streamlines from the NCC neo-sinus to the STJ and sinus suggested fluid washout at lower velocities (maximum velocity LCC: 0.06 m/s, NCC: 0.03 m/s). Streamlines from the LCC sinus into the coronary artery suggested fluid washout of the region, while the region of closed streamlines in the NCC sinus remained present (maximum velocity LCC: 0.12 m/s, NCC: 0.04 m/s).
Late diastole (650 ms, Fig. 5): Flow trends for the LCC and NCC cusps were similar to mid diastole. Streamlines suggested fluid washout of the LCC neo-sinus directly into the coronary artery, and out of the NCC neo-sinus into the sinus (maximum velocity neo-sinus, LCC: 0.03 m/s, NCC: 0.02 m/s). Streamlines suggested fluid washout from the LCC sinus into the coronary artery, while a region of closed streamlines in the NCC sinus remained present (maximum velocity LCC: 0.08 m/s, NCC: 0.04 m/s).
End diastole (750 ms, Fig. 5): As coronary flow waned, flow patterns were similar to late diastole. Streamlines suggested fluid washout of the LCC neo-sinus directly into the coronary artery, and out of the NCC neo-sinus into the sinus (maximum velocity neo-sinus, LCC: 0.02 m/s, NCC: 0.01 m/s). Streamlines suggested fluid washout from the LCC sinus into the coronary artery, while there was fluid wash-into the NCC sinus (maximum velocity LCC: 0.06 m/s, NCC: 0.03 m/s).
Rights and permissions
About this article
Cite this article
Madukauwa-David, I.D., Sadri, V., Midha, P.A. et al. An Evaluation of the Influence of Coronary Flow on Transcatheter Heart Valve Neo-Sinus Flow Stasis. Ann Biomed Eng 48, 169–180 (2020). https://doi.org/10.1007/s10439-019-02324-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02324-y