Summary
Background
(Analgo-) sedations for diagnostic and/or therapeutic procedures form part of the daily clinical routine for pediatric patients. National and international medical specialist associations have published guidelines indicating the general conditions of these procedures, yet the recommendations are not always consistent. Since anesthesiological activities are increasingly performed by nonanesthesiologists at our hospital, the Pediatric Clinic of the University Hospital of Saarland considered it necessary to develop an in-house standard.
Material and methods
On the basis of a standard dating back to 2005, which was developed and clinically applied by two of the authors of this article, we created our “Homburg standard”, taking into account the guidelines of the specialist associations and the international literature. This standard covers patient information, the consumption of food and drink, monitoring before, during and after the sedation as well as documentation. We will present the process of how our standard was established by analyzing protocols of the “old” standard—applied for a period of 18 months—and the application of our standard to two new studies performed at our hospital.
Results
In total, 159 sedations of the 18-month reference period could be evaluated; the two studies accounted for 72 sedations for diagnostic and/or interventional cardiac catheter examinations and 40 sedations for outpatient TEE examinations. None of the procedures was associated with complications endangering the safety of a patient. Whereas the documentation of the two studies was nearly complete, it varied considerably in the case of the 159 sedations, depending on how much time had passed since the most recent training.
Conclusion
Our standard is a practicable and safe method of performing sedations and analgosedations in pediatric patients. In addition, this standard allows clinical studies to be carried out and evaluated, taking into account certain organizational measures. The development of a specific guideline by the DGKJ and/or the GNPI is considered desirable.
Zusammenfassung
Grundlagen
(Analgo-)Sedierungen für diagnostische und/oder therapeutische Prozeduren gehören zum Alltag in der klinischen Versorgung von pädiatrischen Patienten. Nationale und internationale medizinische Fachgesellschaften haben mittels Leitlinien die Rahmenbedingungen für diese Prozeduren gesteckt, jedoch divergieren die Empfehlungen zum Teil voneinander. Aufgrund der zunehmenden Übernahme anästhesiologischer Tätigkeiten an unserer Klinik durch Nicht-Anästhesisten sahen wir uns veranlasst, einen hausinternen Standard an den Kliniken für Kinder- und Jugendmedizin des Universitätsklinikums des Saarlandes zu etablieren.
Material und Methoden
Basierend auf einem 2005 entwickelten Standard, an dessen Entwicklung und klinischer Anwendung ein Teil des Autorenteams beteiligt war, erarbeiteten wir aus den Leitlinien der Fachgesellschaften und der internationalen Literatur unseren „Homburger Standard“, der die Aufklärung, Nahrungs-/ Flüssigkeitsaufnahme, das Monitoring vor, während und nach einer Sedierung sowie die Dokumentation umfasst. Vorgestellt wird der Weg zur Etablierung unseres Standards mit der Analyse von Protokollen des „alten“ Standards – angewandt über einen Zeitraum von 18 Monaten – sowie die Anwendung unseres Standards im Rahmen zweier neuer Studien an unserer Klinik.
Ergebnisse
Insgesamt konnten aus den 18 Monaten 159 Sedierungen ausgewertet werden sowie aus den beiden Studien 72 (Sedierung bei diagnostischen und/oder interventionellen Herzkatheteruntersuchungen) bzw. 40 (Sedierung bei ambulanten TEE-Untersuchungen). Bei keiner Prozedur trat eine Komplikation auf, die eine Gefahr für die Sicherheit eines der Patienten dargestellt hätte. Während die Dokumentation im Rahmen der beiden Studien nahezu vollständig war, variierte sie bei den 159 Sedierungen zum Teil erheblich in Abhängigkeit vom zeitlichen Abstand zur letzten Schulung.
Schlussfolgerung
Unser Standard stellt eine praktikable und sichere Möglichkeit zur Durchführung von Sedierungen pädiatrischer Patienten dar. Ebenso lassen sich im Rahmen dieses Standards klinische Studien unter Berücksichtigung bestimmter organisatorischer Maßnahmen durchführen und auswerten. Eine eigene Leitlinie der DGKJ und/oder der GNPI erscheint wünschenswert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly every physician working in in-patient pediatrics is faced with the challenge of carrying out sedations or analgosedations for certain diagnostic and/or therapeutic procedures. This is not only true for painful procedures, but also for examinations that do not cause pain, for example, computed tomography or magnetic resonance tomography scans, which often require deep sedation to immobilize the patients. Some medical associations such as the American Academy of Pediatrics (AAP), the American Society of Anesthesiologists (ASA), the German Society of Anesthesiology and Intensive Care (DGAI), and the Association of German Anesthesiologists (BDA) have developed guidelines describing the general conditions of sedation/analgosedation procedures [1–7]. Some of the recommendations differ from each other, for example, in terms of the required staff or the level of training of the person performing the sedataion/analgosedation. Against this background, we developed our own standard at the University Hospital and Polyclinic for Pediatrics and Adolescent Medicine of Martin Luther University Halle-Wittenberg (MLU HW) in 2005, taking into account the existing guidelines. The main objectives of the standard were, for one thing, the safety of our patients and, for another, practicability. The results of a test run were presented in a lecture at the Annual Meeting of the Society of Neonatology and Pediatric Intensive Care. The results of a 12-month application period were published in the “Klinische Pädiatrie” journal in 2008 [8]. We provided our standard to various children’s hospitals upon request. The ongoing debate during the past few years [9–12] and the increasing fulfillment of anesthesiologist tasks by nonanesthesiologists at our hospital prompted us to establish an updated in-house standard at the Clinic for Pediatrics and Adolescent Medicine of the University of Saarland. In addition to the practicability and safety for the patients, which had been proven by the application of the old standard, the implementation of a new standard was intended to allow studies about sedation/analgosedation procedures to be carried out. This paper will present our “Homburg standard” and show up the milestones of its development—ranging from literature review and data analysis extended to specific aspects between July 2005 and December 2006 to the application of our standard within the framework of two studies.
Material and methods
In addition to reviewing the literature, we performed an enhanced evaluation of the former retrospective data analysis, which could be extended to a period of 18 months. The retrospective data analysis, published in 2008, covered the period between July 2005 and June 2006 [8]. The extended retrospective data analysis, in turn, referred to the period between July 2005 and December 2006. Unfortunately, it was not possible to evaluate a longer period, owing to the lack of data records as the corresponding author changed his position in the spring of 2007.
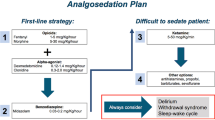
Our standard covers the entire management of sedations and analgosedations—ranging from history taking with an additional focus on anesthesia-relevant diseases, physical examination and patient information to the fasting period, monitoring before, during and after a sedation and the documentation in a specifically designed protocol (Fig. 1). A detailed description of the precise procedure can be found in Sauer et al. [8].
For the current version of our standard, we reviewed the existing guidelines and the scientific papers published since the publication of the previous version. The former standard was updated in accordance with the relevant findings. Table 1 provides an overview of our new standard.
As had been the case in the original version, we continued to summarize the definitions of sedation level, as declared by AAP and ASA [1, 13, 14], the risk profile according to the ASA classification [1, 15] and the in part rather diverging recommendations of the medical associations with regard to monitoring [see 8], the staff requirements and the qualification of the person [4, 15, 16] performing and supervising the sedation/analgosedation [8]. The organizational structures for emergency care, training and application of sedatives and analgetics, anamnesis and examination of patients as well as patient information have also been maintained [8].
The active substances used for (analgo-) sedations, applied either on their own or in combination, are listed in Table 2. For cardiac catheter examinations, we combined propofol und midazolam or 4-hydroxybutyric acid and midazolam; for analgesia, we additionally applied infiltration anesthesia using a local anesthetic in the area of the puncture site after deep sedation had been reached. We performed the outpatient TEE examinations in deep analgosedation, administering midazolam, S-ketamie, and disoprivan. In each case, the application was based on titration by effect.
The fasting period was based on the recommendations of AAP and the anesthesiologist literature [1, 17–19], again specifying a fasting period of 4 h for clear liquids for the reasons of practicability.
The defined standard was presented during a hospital conference. In addition, the staff was trained in the following fields:
-
Patient and parent information
-
Clinical assessment of the sedation level
-
Patient classification according to the ASA categories
-
Application in terms of medical qualification, staff requirements, and monitoring
-
Application, dosage, effects, side-effects, and contraindications of the sedatives/analgetics
-
Documentation in the sedation protocol [8—basically identical].
The available protocols resulted in an 18-month evaluation period. This included all the sedations/analgosedations performed by consultants and pediatric residents at the Hospital of Pediatrics and Adolescent Medicine of Martin Luther University Halle-Wittenberg (MLU HW). Furthermore, our evaluation included the patients of two new studies. First of all, there is a prospective randomized study which has been approved by the ethics committee of the Medical Association of Saarland covering the period between April 2011 and March 2012 and comparing two different methods of analgosedation in the context of pediatric cardiac catheter examinations. Second, there is a retrospective study investigating all outpatient transesophageal echocardiographies (TEE examinations) in patients younger than 18 years of the year 2011 at the Clinic for Pediatric Cardiology of the University Hospital of Saarland. These sedations were exclusively performed by two physicians, one of whom was a specialist in pediatrics with the subspecialization in pediatric intensive care, while the other one was a specialist in pediatrics with subspecializations in pediatric intensive care and neonatology in addition to being a specialist in anesthesiology.
The minimum requirements for the working place, as described by Meyer and Kleinschmidt [20, 21] and specified in the updated recommendations of the DGAI and the BDA [7], are fulfilled, apart from a defibrillator, a ventilator (ventilation unit always at hand) and the end-expiratory CO2 monitoring. In the cardiac catheter laboratory and the pediatric cardiac outpatient clinic, there is always a defibrillator available. In addition, a defibrillator is at hand in the case of existing cardiac diseases; for all the other analgosedations, it is available within 2 min.
We distinguished three degrees of severity in the complications that occurred:
-
Minor complication: spontaneous recovery, no intervention necessary
-
Medium complication: intervention necessary, yet not administration of medication (apart from oxygen by means of nasal prongs or atropine to avoid hypersalivation) nor invasive measures (apart from suction in the nasal, oral, and pharyngeal cavities as well as placement of a nasopharyngeal tube)
-
Severe complication: intervention necessary, for example, invasive measures such as application of medication and/or infusion, mask ventilation, intubation, or resuscitation
Results
In the aforementioned 18-month period, a total of 159 sedations/analgosedations were carried out. These can be broken down as follows:
-
76 bone marrow punctures, either performed exclusively or in combination with bone punches or lumbar punctures: midazolam + S-ketamine in each case
-
30 joint punctures: midazolam + S-ketamine in 29 cases and 4-hydroxybutyric acid + S-ketamine in 1 patient
-
17 coloscopies: midazolam + S-ketamine in each case
-
12 gastroscopies: midazolam + S-ketamine in 8 cases, propofol and S-ketamine in 3 cases and propofol only in 1 case
-
5 placements of central venous catheters: midazolam + S-ketamine in each case, additional propofol in 1 case
-
3 echocardiographies: midazolam in each case
-
3 liver and kidney biopsies: midazolam + piritramid in each case
-
3 bronchoscopies: midazolam + S-ketamine + propofol in 2 cases, propofol + piritramid in 1 case
-
2 transesophageal echocardiographies: midazolam + S-ketamine in each case
-
2 angiographies: midazolam + S-ketamine in one case, midazolam + piritramid + propofol + phenobarbital in 1 case
-
1 radioscopy: midazolam + S-ketamine
-
1 pleuracentesis: midazolam + S-ketamine
-
4 undocumented interventions: midazolam + S-ketamine in each case.
The maximum dosage indicated in Table 2 was not exceeded. However, a gastroscopy had to be cancelled and repeated in a general anesthesia supervised by an anesthesiologist because it was not possible to achieve adequate reflex reduction using the combination of propofol and S-ketamine and the saturation dropped sharply several times when the endoscope was inserted.
In line with our classification of the degrees of severity, no severe complications occurred in the 159 (analgo-) sedations. The same holds true for the TEE study. In the cardiac catheter study, by contrast, two patients of the 4-hydroxybutyric acid group suffered from severe complications requiring suction, oxygen administration, and drug application because of an allergic response to the contrast medium. Both procedures could be performed and completed smoothly.
The data evaluation focused on those parameters which had not been documented sufficiently in the analysis published in 2008, in particular ASA classification, sufficient sedation, sufficient vital parameters at the end of the monitoring and time intervals. Additional parameters assessed were weight, duration of the sedation, vital parameters (heart rate, blood pressure, saturation), and potential complications. It is the careful documentation of the aforementioned parameters that allows randomized prospective studies with various sedation regimes for identical procedures to be performed. The same holds true for the retrospective analysis of a single standardized procedure. Table 3 provides a brief overview of the three analyzed groups of patients.
Table 4 and 5 show the complete documentation of the aforementioned parameters. After the second training session, which was held after 4 months (the first training session was held before the standard was introduced), the results varied. While the complete documentation had increased after the second training session for all parameters, the documentation behavior both improved and deteriorated—in particular in the area of ASA classification and time intervals for recording the vital parameters (10 instead of 5 min) in the remaining 6 months (see Table 4).
As Table 5 demonstrates, the documentation behavior in both studies was nearly impeccable, showing consistently positive rates of over 90 %. Only the “sedation sufficient” parameter had not been ticked in nine cases each in the two groups of the cardiac catheter study. However, all procedures could be performed without any problems. The three missing ASA classifications of the propofol group could be completed on the basis of the medical file. All complications or sedation incidents in the cardiac catheter study were documented appropriately on the reverse side of the protocol. It was only in one patient of the 4-hydroxybutyric acid group and two patients in the propofol group that the corresponding notes were missing on the front page of the protocol.
Since the TEE study included outpatients only, we did not record the ASA classification. When we evaluated the data, we realized that this was a drawback and modified our procedure for outpatient (analgo-) sedations.
Discussion
In line with the national [including 7] and international recommendations [including 1, 22], we think that it is indispensable to have a standardized procedure for sedations and analgosedations of pediatric patients which takes into account the structural and organizational conditions of each hospital.
The assessment of the sedation depth continues to be based on the AAP definitions, outlined in the guidelines of 1992 and 2002 [13] and reaffirmed in 2012 [23]. They are also applied or recommended in other publications or guidelines [7, 22, 24–26]. For reasons of practicability, the assessments of “minimal sedation” (formerly called anxiolysis) and “moderate sedation” (formerly called conscious sedation or sedation/analgesia) were again summarized by the term of “moderate sedation”, which resulted in three differentiations of the sedation depth. We consider “general anesthesia” to form part of the organizational domain of an anesthesiologist department, which is also in line with the recommendations of the aforementioned specialist associations [including 6].
In spite of the aforementioned restrictions [8, 15], we consider the application of the ASA classification to asses a patient’s current health condition to be positive.
As far as the fasting periods are concerned, we adhere to our old standard; in addition to the aforementioned considerations [8], we have defined a minimum fasting period of 4 h for clear liquids, breast-milk, liquid dairy products, and industrial infant milk for reasons of simplification. We have adjusted the age categories in line with the German recommendations [7]. For reasons of safety, we think it is indispensable to enquire for and check the patient’s latest intake of food and liquids—even though this aspect might have been discussed in detail in the context of the patient information. We are very skeptical about the recommendations of the NICE (National Institute for Health and Care Excellence) clinical guideline 112 [22] to have no fasting period in case of “minimal sedation”, “sedation with nitrous oxide (in oxygen)” and “moderate sedation during which children or young person will maintain verbal contact with the healthcare professional”. For one thing, the abstract of this guideline [22] points out to rather weak evidence—“based on moderate quality observational studies”—for another, any food or liquid that remains in the digestive system might result in severe complications if, accidentally, the sedation is deeper than initially intended.
Looking back on the 18-month period and the 159 analgosedations and the 2 studies with a total of 113 patients, the combination of the parameters of risk profile, sedation depth, medical qualification, staff requirements, and monitoring as described by Sauer et al. [8] has proved to be effective.
It should be a matter of course to implement monitoring and documentation—on the basis of anesthesiologist standards. Several publications explicitly point out to this requirement [1, 3, 4, 5, 7, 22, 27, 28]. We also emphasize the need for medical qualification, ensuring adequate training when performing anesthesiologist activities, and the staff requirements for analgosedations. In addition to the examiner, there must be one physician exclusively in charge of the analgosedation. To ensure the safety of the patients entrusted to us, it has to be avoided that physicians perform both activities. As the DGAI and the BDA sum up “in the case of severely ill children (ASA status III-IV) and all deep sedations, an additional physician with training in anesthesiology or intensive care must be available in addition to the examiner; this physician must not be identical with the examiner, and must solely be in charge of continually monitoring the vital parameters” [7]. Whether there should be a second physician in the case of moderate sedations of children with ASA status I and II, as required by our standard for the sake of the safety of our patients, has not been examined sufficiently yet and is also being debated at our hospital. Since the majority of our analgosedations can be classified as deep, we cannot draw any conclusion from our data. As far as the qualification of the physicians is concerned, we think that it is indispensable that they are able to perform a safe sedation procedure on the basis of a profound knowledge of the applied analgetics and sedatives as well as adequate monitoring. In addition, they have to be fully competent of airway and emergency management including resuscitation measures. If sedations are performed by physicians in specialty training, we think that there has to be a specialist or staff physician readily available who is experienced in emergency care—as is the case in anesthesiologist organizational structures. It remains to be said that several guidelines and publications (see above) deal with documentation, medical qualification, and staff requirements, which are fulfilled by our standard. Whether the presence of a second nurse (one nurse assisting the examiner, one assisting in the sedation) is compulsory for all deep sedations or whether one nurse might be enough, depending on the kind of examination/procedure, has not been investigated sufficiently yet. However, we think that it is absolutely necessary that the assisting nurse has sufficient training and experience in emergency care of sedated patients.
We were astonished by the varying completeness of the parameters to be documented in the 18-month period. There was an initial training session before the standard had been introduced, a second one followed 4 months after the introduction of the standard. After the second training session, documentation improved in part significantly for all parameters. Since the documentation behavior showed both improving and deteriorating levels of completeness in months 13 through 18, further training sessions seem to be appropriate. Against this background, we suggest annual training sessions for a start; depending on the re-evaluation of the documentation behavior, these intervals could later on be reduced to 6-month intervals or extended to 2 years.
The nearly perfect documentation in the context of the two studies probably results from the fact that only two physicians were in charge of the analgosedations here. For clinical studies in the area of diagnostic and/or therapeutic procedures, it could therefore make sense to limit the number of physicians involved. In addition, we think that it is a matter of course to ensure a complete documentation, including the full chronology of the administered drugs and infusions and the recorded vital parameters. We believe that a sedation protocol as described by Sauer et al. [8], for example, is most appropriate to fulfill these requirements.
Finally, it remains to be said that there are also some points of weakness. First of all, the experience of the old standard was based on a small number of cases, which could be increased by 56 patients only. Second, the analysis of the protocols from the old standard was based on data obtained retrospectively. Third, the described experience with the new standard was again gathered from a moderate number of patients. On the other hand, we summarized the national and international recommendations to form a feasible standard, which can be easily part of the daily routine in a children’s hospital.
Conclusion
Owing to the relatively low number of just 271 analyzed patients, it is difficult to make a statement based on broad evidence. On the other hand, we can look back on nearly 10 years of experience with the old and new standard. We are deeply convinced that our “Homburg standard” provides a feasible and safe regulation of the preparation and monitoring of sedation and analgosedation in pediatric patients. It is also suitable for performing clinical studies, which are urgently needed in view of the rather scarce data about analgosedation for diagnostic and therapeutic procedures in pediatric patients. Regular training should be ensured—for a start at intervals of not more than 12 months after the standard has been established, as our experience has shown. In view of scarcer and scarcer human resources and the increasing density of the activities of physicians and nurses, the safety of the children and adolescents entrusted to us calls for sufficient provision of qualified staff. This requirement can be summarized by a quote by Gozal and Mason: “The challenge facing sedation care providers moving forward in the 21st century will be to determine how to apply the local, regional and national guidelines to the individual sedation practices. A greater challenge, perhaps impossible, will be to determine whether the sedation community can come together worldwide to develop standards, guidelines and recommendations for safe sedation practice” [9].
Ethical standard statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of interest
Harald Sauer, Laura Gruenzinger, Jochen Pfeifer, Ulla Lieser, and Hashim Abdul-Khaliq declare that there is no conflict of interest.
References
American Academy of Pediatrics and the American Academy of Pediatric Dentistry ( 2006, Reaffirmed 2011). Guideline for monitoring and managment of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 2012;129:e1103.
American Academy of Pediatrics. Work Group on Sedation. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587–602.
American Society of Anesthesiologists. Advisory on granting privileges for deep sedation to non-anesthesiologists sedation practioners; Approved by the ASA House of Delegates on October 20, 2010.
American Society of Anesthesiologists. Standard for basic anesthetic monitoring; Committee of Origin: Standards and Practise Parameters; Approved by the ASA House of Delegates on October 21, 1986, and last amended on October 20, 2010 with effective date of July 1, 2011.
American Society of Anesthesiologists. Statement on granting privileges for administration of moderate sedation to practioners who are not anesthesia professionals; Approved by the ASA House of Delegates on October 25, 2005, and last amended on October 19, 2011.
Beschluss des Engeren Präsidiums der DGAI und des BDA vom 17.01.2011. Narkosen durch pädiatrische Intensivmediziner; Anästh. Intensivmed. 2010;51:603–14. (Decision of the Executive Committee of the DGAI and the BDA on January 1, 2011. Anesthesia performed by Pediatric Intensivists; Anästh. Intensivmed. 51 (2010) S603-614)
Beschluss des Engeren Präsidiums der DGAI und des BDA vom 07.05.2010. Analgosedierung für diagnostische und therapeutische Maßnahmen im Kindesalter; Anästh. Intensivmed. 2010;51:603–14. (Decision of the Executive Committee of the DGAI and the BDA on May 7, 2010. Analgosedation for diagnostic and therapeutic procedures in Childhood; Anästh. Intensivmed. 51 (2010) S603-614).
Sauer H, Haase R, Lieser U, Horneff G. Vorbereitung und Monitoring im Rahmen von Sedierungen und Analgosedierungen durch Fachärzte und Weiterbildungsassistenten in der Kinder- und Jugendmedizin. Klin Padiatr. 2008;220(3):189–95. (Sauer H, Haase R, Lieser U, Horneff G. Preparation and Monitoring of Sedation and Analgosedation Carried out by Pediatricians and Pediatric Training Assistants).
Gozal D, Mason K. Pediatric sedation: a global challenge. Int J Pediatr. 2010;2010:701257. (15 pages).
Leroy P, Schipper D, Knape H. Professional skills and competence for safe and effective procedural sedation in children: recommendations based on a systemic review of the literature. Int J Pediatr. 2010;2010:934298.(16 pages).
Neuhäuser C, Wagner B, Heckmann M, Weigand M, Zimmer KP. Analgosedierung für schmerzhafte Eingriffe bei Kindern und Jugendlichen. Dtsch Arztebl Int. 2010;107(14):241–7. (Neuhäuser C, Wagner B, Heckmann M, Weigand M, Zimmer KP. Analgosedation for Painful Procedures in Pediatrics and Adolescent; Dtsch Arztebl Int 2010; 107(14): 241-247 Klin Padiatr 2008; 220(3): 189-195).
Strauß J, van Aken H, Becke K, Philippi-Höhne C, Sorgatz H. Nicht abgestimmt. Dtsch Arztebl Int. 2010;107(44):785.
American Academy of Pediatrics, Committee on Drugs. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: addendum. Pediatrics. 2002;110:836–8.
American Society of Anesthesiologists. Continuum. of Depth of Sedation—Definition of General Anesthesia and Levels of Sedation/Analgesia. ( ASA House of Delegates on October 13, 1999. www.asahq.org/publicationsAndServices/standards/20.pdf www.asahq/publicationsAndServices/standards/02.pdf#2).
Danner K, Madler C. (2001) Perioperatives Risiko und Patientenvorbereitung. In: Kochs E, Krier C, Buzello W, Adams HA, editors. Anästhesiologie ains Band. 1. Stuttgart: Thieme; 2001. S. 555–7.
Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatric: analysis of medications used for sedation. Pediatrics. 2000;106:633–44.
American Society of Anesthesiologists. Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Practice guidelines for Preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;96:896–905.
Brady M, Kinn S, O´Rourke K, Randhawa N, Stuart P. Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst Rev. 2005;18(2):CD005285.
Hoffman GM, Nowakowski R, Troshynski TJ, Berens RJ, Weisman SJ. Risk reduction in pediatric procedural sedation by application of an American academy of pediatrics/American society of anesthesiologists process model. Pediatrics. 2002;109:236–43.
American Society of Anesthesiologists. Standard on nonoperating room anesthetizing locations; Committee of Origin: Standard and Practice Parameters; Approved by the ASA House of Delegates on October 19, 1994, and last amended on October 16, 2013.
Meyer S, Kleinschmidt S. Diagnostische und therapeutische Prozeduren—Sedierung und Analgesie im Kindesalter. Monatsschrift Kinderheilkd. 2005;153:291–303. (Meyer S, Kleinschmidt S. Diagnostic and therapeutic procedures—sedation and analgesia in Childhood; Monatsschrift Kinderheilkd 2005—153: 291-303).
Sury M, Bullock I, Raber S, DeMott K. Sedation for diagnostic and therapeutic procedures in children and young people: summary of NICE guidance. BMJ. 2010;341:c6819. (NICE National Institute for Health and Care Excellence (2010). Sedation in children and young people — Sedation for diagnostic and therapeutic procedures in children and young people. NICE clinical guideline 112; guidance.nice.org.uk/cg112).
American Acadamy of Pediatrics. Policy Statement—AAP Publications Reaffirmed and Retired. Pediatrics. 2012. www.pediatrics.org/cgi/doi/10.1542/peds.2012-0163.
Assmann A, Heinrichs W, Landauer B, Radke J, Riphaus A, Wehrmann T, Van Aken H, Martin J. Zusammenfassung der S3-Leitlinie “Sedierung in der gastrointestinalen Endoskopie”. Anästh Intensivmed. 2009;50:000–0.
van Beek E, Leroy P. Safe and effective procedural sedation for gastrointestinal endoscopy in children. J Pediatr Gastroenterol Nutr. 2012;54:171–185.
Lee MC. Sedation for pediatric endoscopy. Pediatr Gastroenterol Hepatol Nutr. 2014;17(1):6–12.
American Society of Anesthesiologists. Statement on documentation of anesthesia care; Committee of Origin: Quality Management and Departmental Administration; Approved by the ASA House of Delegates on October 15, 2003, and last amended on October 16, 2013.
Mahajan C, Dash H. Procedural sedation and analgesia in pediatric patients. J Pediatr Neurosci. 2014;9(1):1–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sauer, H., Grünzinger, L., Pfeifer, J. et al. Sedation and analgosedation performed by pediatricians—experience made with the implementation of an in-house sedation standard. Wien Med Wochenschr 166, 54–61 (2016). https://doi.org/10.1007/s10354-015-0400-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-015-0400-7