Summary
Background
Despite distinctive advances in the field of pancreatic cancer therapy over the past few years, patient survival remains poor. Fibroblast growth factors 8 (FGF8) and 18 (FGF18) both play a role in modulating the activity of malignant cells and have been identified as promising biomarkers in a number of cancers. However, no data exist on the expression of FGF8 and FGF18 in pancreatic ductal adenocarcinoma (PDAC).
Methods
Protein expression levels of FGF8 and FGF18 in postoperative specimens of neoadjuvantly treated and primarily resected patients were investigated using immunohistochemistry. Immunostaining scores were calculated as the products of the staining intensity and the staining rate. Scores exceeding the median score were considered as high expression.
Results
Specimens from 78 patients with PDAC were available and met the eligibility criteria for analysis of protein expression using immunohistochemistry. 15 (19.2%) patients had received neoadjuvant chemotherapy. High protein levels of FGF8 and FGF18 were detected in 40 (51.8%) and 33 (42.3%) patients, respectively. Kaplan–Meier analysis demonstrated significantly shorter overall survival in patients with high expression of FGF8 (p = 0.04). Multivariable Cox proportional hazard regression models revealed that high expression of FGF8 (Hazard ratio [HR] 0.53, 95% Confidence interval [CI] 0.32–0.89, p = 0.016) was an independent prognostic factor for diminished overall survival in patients with PDAC. By contrast, no statistical significance was found for FGF18 overexpression. In addition, the FGF8 protein level correlated with the factor resection margin (p = 0.042).
Conclusion
FGF8 is a promising target for new anticancer therapies using FGF inhibitors in pancreatic ductal adenocarcinomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
This is the first study of expression of FGF8 and FGF18 in pancreatic ductal adenocarcinoma
-
The patient cohort includes neoadjuvantly treated patients
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the 7th leading cause of cancer death, with more than 400,000 observed deaths annually worldwide [1, 2]. The incidence of PDAC is higher in more developed countries and in the elderly population [1, 3]. Despite the introduction of new therapeutic approaches combining multimodal treatment protocols and surgical resection, at only 8%, PDAC has the lowest 5‑year survival rate of all cancer types [4,5,6,7]. One of the most important causes of the devastating survival rate of PDAC lies in its late diagnosis. The majority of patients present with distant metastatic disease at the time of diagnosis and around one third of cases present with locally advanced disease [8]. Due to the increasing availability of diagnostic tools like computed tomography (CT), an increasing number of patients are diagnosed in an early locally advanced stage of disease and therefore qualify for neoadjuvant therapy, which has emerged as a standard treatment in borderline resectable PDAC [6, 9]. Although novel therapeutic combinations based on gemcitabine or FOLFIRINOX have shown promising results in all stages of disease, a large number of patients suffer from little to no efficacy based on the development of chemoresistance [10]. Patients with diabetes and chronic pancreatitis are more likely to suffer from PDAC. Furthermore, smoking, alcohol abuse, and a high fat and protein diet increase the risk of developing PDAC, underlining the crucial role of inflammation in pancreatic carcinogenesis which is characterized by desmoplasia-driven activation and differentiation of pancreatic stellate cells into pancreatic αSMA+/vimentin+ myofibroblasts [11,12,13]. Beside the systemic inflammatory response (SIR), emerging evidence determined that inflammation-associated vital genes, signaling pathways, and growth factors such as PI3K (phosphatidylinositol), Ras-MAPK (mitogen-activated protein kinase), STAT (signal and activator of transcription), Wnt, EGF (epidermal growth factor), and FGF (fibroblast growth factor) are involved in the carcinogenesis and progression of PDAC [14,15,16,17,18,19,20,21]. FGFs are peptide-like molecules which bind to their specific receptors (FGFR) on cell membranes to govern cell growth. FGFs can be found in numerous types of tissue and are known to promote fibroblast proliferation. Due to their high affinity for heparin, FGFs are also known as heparin-conjugate growth factors. The molecular structure of FGF proteins includes a heparin sulphate (HS) domain and a fibroblast growth factor receptor-binding domain. In mammals, 22 different FGFs are known today. Beside the biggest FGF subfamily, the canonical FGFs, one endocrine and one intracellular FGF subfamily exists and all FGFs mediate their cellular response via binding and activation of one of four FGFRs [14, 21]. A broad range of FGFs have been investigated in vitro and in vivo for their role and influence on PDAC, including the treatment response of pancreatic cancer cells to chemotherapy and the use of anti-FGF therapy, so called “ligand-traps” [14, 22,23,24,25,26,27,28,29].
The prognostic role of the fibroblast growth factors (FGF) 8 and 18, both members of the FGF subfamily 8, were investigated in a number of malignancies [30,31,32,33,34,35]. Whereas the majority of studies describe diminished patient prognosis in cancers overexpressing FGF8 and FGF18, a protective effect of FGF18 has been found for gastroesophageal adenocarcinomas [36]. Furthermore, there are increasing data on the use of FGF ligand traps and fibroblast growth factor receptor (FGFR) inhibitors to overcome chemoresistance in various cancers, including pancreatic tumors [37,38,39]. However, no data on the expression of FGF8 and FGF18 in PADC are available to date. Therefore, the aim of this study was to investigate the expression rates and a possible prognostic role of FGF8 and FGF18 in a cohort of primarily resected and neoadjuvantly treated PDAC patients.
Materials and methods
Patients who underwent resection of PDAC between 1994 and 2012 at the Medical University of Vienna were identified from a prospectively maintained database. Patients with distant metastasis at the time of diagnosis or history of any other malignant disease were excluded. The study was approved by the ethics committee of the Medical University of Vienna, according to the declaration of Helsinki (EK 1518/2020). Patients with borderline resectable PDAC at the time of diagnosis received neoadjuvant treatment according to the recommendations of the interdisciplinary tumor board meetings at the Medical University of Vienna Tumor stage was determined according to the pathological tumor/node/metastasis (TNM) classification of the Union for International Cancer Control (UICC), 8th edition.
Immunohistochemistry (IHC) was performed as reported previously [36]. In brief, paraffin-embedded specimens fixed in 4% buffered formalin were used with 3‑µm thick histological sections. Expression of FGF8 and FGF18 was detected using polyclonal rabbit antibodies as follows: FGF8 antibody (Abcam®, Cambridge, UK, ab203030) in a dilution of 1:600 and FGF18 antibody (Assay Biotech®, Fremont, CA, USA, C12364) in a dilution of 1:500, respectively. Detection of the primary antibody was performed using a horseradish peroxidase (HRP)-labeled polymer system specific for mouse and rabbit IgG antibodies. The polymer complex was visualized using 3,3′-diaminobenzidine (DAB) and nuclei were counterstained using hematoxylin. All steps were performed according to the manufacturer’s protocols (Thermo Scientific™, Waltham, MA, USA; UltraVision LP Detection System HRP DAB). Stained sections were scanned using 3DHISTECH Ltd. (Budapest, Hungary) Pannoramic MIDI slides canner and analyzed and scored using Pannoramic Viewer 1.15.4 Software. Antibodies used in this study were optimized for PDAC on colorectal cancer and esophageal adenocarcinoma tissue with known expression from previously published studies [36,37,38].
Statistical analysis was performed using the R statistical software, Vienna, Austria (version 3.6) with the survival package. To determine the cut-off value for CA19‑9, the optimal cutpoints package was used as appropriate [40, 41]. Overall survival (OS) was defined as the time between primary surgery and the patient’s death. Death from causes other than PDAC or survival until the end of observation were considered as censored observations. Uni- and multivariable analyses were conducted using the Cox proportional hazard model as appropriate. The Kaplan–Meier estimator analysis was performed using the survminer package for R statistical software [42]. The log-rank test was used to determine the significance of differences in survival times. Potential significance of correlations between clinicopathological factors and FGF8 and FGF18 expression levels were analyzed with the χ2 test. Non-parametric Kendall’s rank correlation was performed to investigate potential statistical dependence between FGF8 and FGF18.
Results
Tissue of 78 patients with pancreatic ductal adenocarcinoma was available for analysis. Mean age was 65 (22–85) years, 34 patients (43.6%) were females and 44 (56.4%) males. The most frequent tumor differentiation was (y)G2 in 53 (68.0%) patients. Most patients (53, 68.0%) showed (y)pT3 stage, 63 (80.8%) patients showed nodal involvement ((y)pN1), and 15 (19.2%) patients had received neoadjuvant chemotherapy. Of the 78 eligible cases, 40 (51.3%) and 45 (57.7%) cases showed high expression of FGF8 and FGF18, respectively (Fig. 1). Significant correlation was only observed between high expression of FGF8 and the factor resection margin (p = 0.042). Correlations between the expression of FGF8 and FGF18 and clinicopathological parameters are compiled in Table 1. Among all samples tested, high expression of FGF8 and FGF18 was found in 22 (28.2%) cases. Analysis of a potential correlation of high FGF8 and FGF18 expression found statistical significance (p = 0.036).
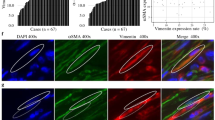
Specimen of pancreatic ductal adenocarcinomas stained for fibroblast growth factor (FGF) 8 (a and c) and FGF18 (b and d). a, b High expression levels of FGF8 and FGF18, respectively. c, d Low/absent expression of FGF18, respectively. The red bar corresponds to 50 µm. Original magnification × 400 all
Median survival time was 30 months (range 0–153 months) and disease recurrence occurred in 70 (88.9%) patients. Survival analysis using Kaplan–Meier curves for visualization found significantly shorter OS rates for patients with high FGF8 expression (p = 0.04; Fig. 2). No significance could be found for high FGF18 expression and OS (Fig. 2). Three- and 5‑year OS were 15 (16.7%) months and 2 (2.2%) months, respectively. Univariable Cox proportion hazard regression analysis revealed that FGF8 (HR 0.62, 95% CI 0.38–0.99, p = 0.04), pain (HR 1.79, 95% CI 1.15–2.76, p = 0.009), and surgical procedure (HR 4.41, 95% CI 1.32–14.70, p = 0.02) were significantly associated with OS (Table 2). In multivariable analysis including patients’ age and gender, UICC staging, tumor differentiation, resection margin, and neoadjuvant and adjuvant treatment, FGF8 (HR 0.53, 95% CI 0.32–0.89, p = 0.016) expression remained the only independent prognostic factor for OS (Table 3). In a separate multivariable Cox regression model for FGF18, using the same cofactors as for FGF8, no statistical significance was observed for any of the included factors (Table 4).
Discussion
Pancreatic ductal adenocarcinoma is one of the leading causes of cancer-related deaths worldwide. The majority of patients suffering from PDAC are diagnosed with advanced or metastatic disease and only 15 to 20% of patients are suitable to receive primary resection [5]. Even though the combination of surgery and multimodal therapies has resulted in improvements in the treatment of patients with PDAC over recent decades, survival rates remain poor. Therefore, better understanding of the pathogenesis of PDAC is urgently needed to develop new diagnostic and improved treatment approaches.
The family of FGFs consists of seven subfamilies, categorized by their way of secretion, sequence similarities, and functional properties [43]. Physiologically, FGFs are involved in cell proliferation and angiogenesis. The canonical FGF8 subfamily consists of FGF8, FGF17, and FGF18, which bind and activate FGFRs with heparin/heparin sulfate as a cofactor [44]. The FGF8 gene is encoded on chromosome 10q24.32 and participates in embryonic development, mediating the epithelial to mesenchymal and mesenchymal to epithelial transitions. Furthermore, FGF8 is involved in craniopharyngeal and cardiovascular development. The FGF18 gene is encoded on chromosome 5q35.1 and like FGF8 it is involved in embryonic development and morphogenesis of blood vessels. Furthermore, it acts as a pleiotropic growth factor for upper gastrointestinal organs. Alterations of FGF signaling might be followed by FGFR gene amplification and fusion, mutation, or by FGF and/or FGFR overexpression. Due to these characteristics, overexpression of FGFs can promote carcinogenesis and distant metastasis, and therefore have a fundamental role in cancer [21]. In a number of malignancies, including gastric cancer, gastroesophageal adenocarcinoma, and PDAC, the prognostic role of aberrant FGF expression was investigated [30, 35, 36, 38, 39]. However, to date, only few data on the expression of FGF8 and FGF18 in PDAC are available [23]. Even though promising data on the preclinical use of anti-FGF therapies (ligand traps) exist, hardly any data exist on the blockage of FGF8 and FGF18 [13, 45, 46].
Due to the introduction and routine use of chemotherapeutic drugs, such as gemcitabine, nab-paclitaxel, oxaliplatin, irinotecan, leucovorin, and fluorouracil, in both the neoadjuvant and adjuvant settings, improved patient survival rates could be seen over the past few decades. Over the past decade, gemcitabine-treated and modified FOLFIRINOX (oxaliplatin, irinotecan, leucovorin, and fluorouracil)-treated patients showed satisfying improvements in survival rates. Therefore, these two chemotherapy regimens were established as standard for patients with PDAC [47]. However, patients still suffer from severe side effects and frustrating outcomes related to poor therapeutic results, often based on the development of chemical resistance. Particularly the development of resistance to chemotherapy has emerged as one of the biggest challenges in PDAC therapy. Causing disease recurrence in the majority of patients with PDAC, the investigation of the molecular mechanisms by which PDAC cells develop resistance to chemotherapeutic agents became the aim of a number of studies. Multiple mechanisms and factors play an important role in the development of chemical resistance; however, the development of new and durable treatment options still lags behind [10]. So far, research on molecularly targeted therapies for PDAC have focused on EGFR, VEGF, and RAS pathways, using agents such as erlotinib, cetuximab, and panitumumab. However, no clear benefit in terms of patients’ overall or disease-free survival could be achieved to date [20, 48, 49].
To the best of our knowledge, this is the first study investigating the prognostic role of FGF8 and FGF18 in PDAC using IHC on tumor tissue. Based on our findings that high expression of FGF8 is independently associated with diminished overall survival, one can hypothesize that FGF8 represents a promising target for further investigations in anti-PDAC therapy, especially to overcome the nascent problem of chemoresistance.
References
Are C, Chowdhury S, Ahmad H, Ravipati A, Song T, Shrikandhe S, et al. Predictive global trends in the incidence and mortality of pancreatic cancer based on geographic location, socio-economic status, and demographic shift. J Surg Oncol. 2016;114(6):736–42.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140–1.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Djanani A, Schmiderer A, Niederreiter L, Niederreiter M, Tilg H. Management of ductal pancreatic cancer: the oncologists view: systemic treatment options in 2018. Eur Surg. 2019;51(3):135–8.
Klaiber U, Roth S, Hackert T, Neoptolemos JP. Evolution of oncosurgical management of pancreatic cancer. Eur Surg. 2019;51(3):165–73.
Sahakyan MA, Labori KJ, Primavesi F, Soreide K, Stattner S, Edwin B. Minimally invasive pancreatic surgerywhere are we going? Eur Surg. 2019;51(3):98–104.
Huang L, Jansen L, Balavarca Y, Molina-Montes E, Babaei M, van der Geest L, et al. Resection of pancreatic cancer in Europe and USA: an international large-scale study highlighting large variations. Gut. 2019;68(1):130–9.
Gassner EM, Poskaite P. Imaging response evaluation after novel neoadjuvant treatments of pancreatic cancer. Eur Surg. 2019;51(3):146–52.
Zeng S, Pottler M, Lan B, Grutzmann R, Pilarsky C, Yang H. Chemoresistance in pancreatic cancer. Int J Mol Sci. 2019;20(18):4504.
Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112(9):1366–72.
Zhang JJ, Jia JP, Shao Q, Wang YK. Diabetes mellitus and risk of pancreatic cancer in China: a meta-analysis based on 26 case-control studies. Prim Care Diabetes. 2019;13(3):276–82.
Presta M, Chiodelli P, Giacomini A, Rusnati M, Ronca R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol Ther. 2017;179:171–87.
Kang X, Lin Z, Xu M, Pan J, Wang ZW. Deciphering role of FGFR signalling pathway in pancreatic cancer. Cell Prolif. 2019;52(3):e12605.
Gruber ES, Jomrich G, Kaider A, Gnant M, Sahora K, Schindl M. The prognostic index independently predicts survival in patients with pancreatic ductal adenocarcinoma undergoing resection. Ann Surg Oncol. 2020;27(6):2017–24.
Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, et al. Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. 2020;24(3):610–8.
Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, et al. Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treatment of pancreatic cancer. Curr Med Chem. 2017;24(13):1321–31.
Hao C, Li Z, Zhang X, Zhang H, Shang H, Bao J, et al. Expression and clinical significance of EGF and TGF-alpha in chronic pancreatitis and pancreatic cancer. Minerva Endocrinol. 2018;43(3):253–8.
Javadinia SA, Shahidsales S, Fanipakdel A, Joudi-Mashhad M, Mehramiz M, Talebian S, et al. Therapeutic potential of targeting the Wnt/beta-catenin pathway in the treatment of pancreatic cancer. J Cell Biochem. 2018; https://doi.org/10.1002/jcb.27835.
Soreide K, Primavesi F, Labori KJ, Watson MM, Stattner S. Molecular biology in pancreatic ductal adenocarcinoma: implications for future diagnostics and therapy. Eur Surg. 2019;51(3):126–34.
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116–29.
Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, et al. KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF‑I signaling in pancreatic cancer. Clin Cancer Res. 2011;17(13):4254–66.
Kornmann M, Beger HG, Korc M. Role of fibroblast growth factors and their receptors in pancreatic cancer and chronic pancreatitis. Pancreas. 1998;17(2):169–75.
Leung HY, Gullick WJ, Lemoine NR. Expression and functional activity of fibroblast growth factors and their receptors in human pancreatic cancer. Int J Cancer. 1994;59(5):667–75.
Liu B, Wang Z, Li HY, Zhang B, Ping B, Li YY. Pim‑3 promotes human pancreatic cancer growth by regulating tumor vasculogenesis. Oncol Rep. 2014;31(6):2625–34.
Niu J, Chang Z, Peng B, Xia Q, Lu W, Huang P, et al. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J Biol Chem. 2007;282(9):6001–11.
Torres C, Perales S, Alejandre MJ, Iglesias J, Palomino RJ, Martin M, et al. Serum cytokine profile in patients with pancreatic cancer. Pancreas. 2014;43(7):1042–9.
Vickers SM, MacMillan-Crow LA, Green M, Ellis C, Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch Surg. 1999;134(3):245–51.
Yamanaka Y, Friess H, Buchler M, Beger HG, Uchida E, Onda M, et al. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993;53(21):5289–96.
Dorkin TJ, Robinson MC, Marsh C, Bjartell A, Neal DE, Leung HY. FGF8 over-expression in prostate cancer is associated with decreased patient survival and persists in androgen independent disease. Oncogene. 1999;18(17):2755–61.
Gauglhofer C, Sagmeister S, Schrottmaier W, Fischer C, Rodgarkia-Dara C, Mohr T, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53(3):854–64.
Tanaka A, Kamiakito T, Takayashiki N, Sakurai S, Saito K. Fibroblast growth factor 8 expression in breast carcinoma: associations with androgen receptor and prostate-specific antigen expressions. Virchows Arch. 2002;441(4):380–4.
Sonvilla G, Allerstorfer S, Stattner S, Karner J, Klimpfinger M, Fischer H, et al. FGF18 in colorectal tumour cells: autocrine and paracrine effects. Carcinogenesis. 2008;29(1):15–24.
Wei W, Mok SC, Oliva E, Kim SH, Mohapatra G, Birrer MJ. FGF18 as a prognostic and therapeutic biomarker in ovarian cancer. J Clin Invest. 2013;123(10):4435–48.
Zhang J, Zhou Y, Huang T, Wu F, Pan Y, Dong Y, et al. FGF18, a prominent player in FGF signaling, promotes gastric tumorigenesis through autocrine manner and is negatively regulated by miR-590-5p. Oncogene. 2019;38(1):33–46.
Jomrich G, Hudec X, Harpain F, Winkler D, Timelthaler G, Mohr T, et al. Expression of FGF8, FGF18, and FGFR4 in Gastroesophageal Adenocarcinomas. Cells. 2019; https://doi.org/10.3390/cells8091092.
Ahmed MA, Selzer E, Dorr W, Jomrich G, Harpain F, Silberhumer GR, et al. Fibroblast growth factor receptor 4 induced resistance to radiation therapy in colorectal cancer. Oncotarget. 2016; https://doi.org/10.18632/oncotarget.12099.
Harpain F, Ahmed MA, Hudec X, Timelthaler G, Jomrich G, Mullauer L, et al. FGF8 induces therapy resistance in neoadjuvantly radiated rectal cancer. J Cancer Res Clin Oncol. 2019;145(1):77–86.
Zhou Y, Wu C, Lu G, Hu Z, Chen Q, Du X. FGF/FGFR signaling pathway involved resistance in various cancer types. J Cancer. 2020;11(8):2000–7.
R Development Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018.
Therneau T. A package for survival analysis. 2015. p. 2.38.
Kassambara AKM, Biecek P, Fabian S. survminer: Drawing Survival Curves using “ggplot2”. 2019.
Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281(23):15694–700.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–66.
Tanaka A, Furuya A, Yamasaki M, Hanai N, Kuriki K, Kamiakito T, et al. High frequency of fibroblast growth factor (FGF) 8 expression in clinical prostate cancers and breast tissues, immunohistochemically demonstrated by a newly established neutralizing monoclonal antibody against FGF 8. Cancer Res. 1998;58(10):2053–6.
Wang L, Park H, Chhim S, Ding Y, Jiang W, Queen C, et al. A novel monoclonal antibody to fibroblast growth factor 2 effectively inhibits growth of hepatocellular carcinoma xenografts. Mol Cancer Ther. 2012;11(4):864–72.
de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8–16.
Sheahan AV, Biankin AV, Parish CR, Khachigian LM. Targeted therapies in the management of locally advanced and metastatic pancreatic cancer: a systematic review. Oncotarget. 2018;9(30):21613–27.
Liu H, Zhang B, Sun Z. Spectrum of EGFR aberrations and potential clinical implications: insights from integrative pan-cancer analysis. Cancer Commun. 2020;40(1):43–59.
Acknowledgements
The authors would like to thank Prof. Brigitte Marian for supporting this study with her enormous knowledge on fibroblast growth factors and their receptors.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Jomrich, L. Wilfing, S. Radosavljevic, A. Parak, D. Winkler, G. Timelthaler, M. Schindl, S.F. Schoppmann, and B. Klösch declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jomrich, G., Wilfing, L., Radosavljevic, S. et al. Fibroblast growth factor 8 overexpression is predictive of poor prognosis in pancreatic ductal adenocarcinoma. Eur Surg 52, 282–289 (2020). https://doi.org/10.1007/s10353-020-00669-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-020-00669-6