Abstract
In the context of the Hessian strict forest program, the reserve Hasenblick was set aside from forest management in 1988. The program aims to document the faunas of forest reserves in unprecedented detail, using a set of many different sampling methods. Here data on ground dwelling spiders and beetles that were sampled with pitfall traps in 2000 and 2001 and again in 2012 and 2013 are analyzed to assess temporal changes. In light of putative insect declines, we hypothesized a significant decrease in abundance, biomass, diversity, and trait composition of the arthropod communities. No substantial changes in community trait composition were observed in any species group. Abundance, biomass, and functional diversity of beetles and spiders were higher in the second survey, with exception of the beetle biomass, when Anoplotrupes stercorosus was excluded, and the functional diversity of spiders showed only a tendency to be higher in the second survey. Additionally, the extrapolated number of ground dwelling beetle species was higher during the second survey. However, in all tested measures, the observed differences between the surveys were not significantly higher than differences observed between consecutive years. Therefore, we are not able to detect directed long-term trends of (functional) diversity or biomass in our dataset. The results rather indicate high stability of the arthropod communities in this naturally developing forest, although short-term fluctuations in populations are high. And while climatic factors affected abundance and biomass of beetles and spiders overall, the timespan of about 10 years may be too short to clearly detect effects of climate change or changes in forest structure on the trait composition of the beetle or spider communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distribution and structure of German (European) forests were severely changed over the last centuries by human activities. Under natural conditions, most of Central Europe would presently be forested (Ellenberg and Leuschner 2010). In Germany, forests would cover more than 97% of the surface area (Knapp 2007). However, due to human activities today only about 31% of Germany are forested (BLE 2021a). In addition to this severe decline in forested area, nearly no primeval forests remain in Germany or Central Europe (Ellenberg and Leuschner 2010; Hampicke 2018). Many of today’s forests actually cover areas that once were completely deforested by humans (Leuschner and Immenroth 1994; Glaser and Hauke 2004). The decline in forested area goes hand in hand with a reduction in connectivity between individual forest stands, a loss of continuous forest tradition and very old growth stands, and limited availability of habitat structures typical of primeval forests. This strongly reduced occurrence frequency of primeval forest habitat structures, coupled with the introduction of structures that are typical to managed forests (e.g., introduced tree species, stumps, or logging paths), strongly affect the composition of forest animal communities (Assmann 1999; Schaefer 1999; Lindenmayer et al. 2000; Larsson 2001; Grove 2002; Buse 2012; Seibold et al. 2015; Flensted et al. 2016; Winter et al. 2016; Eckelt et al. 2017; Grodsky et al. 2018; Boggs et al. 2020).
Since 1988, strict forest reserves are designated in the federal state of Hesse (Germany) as both a contribution to nature conservation and a platform for investigating the impact of forest management on forest structure and biocoenoses (Althoff et al. 1991). Within these strict forest reserves, forest management is abandoned (Althoff et al. 1991). Since 1990, the fauna of Hessian strict forest reserves is surveyed in unprecedented detail (Dorow et al. 1992, 2001, 2004, 2009, 2010; Flechtner et al. 1999, 2000, 2006; Willig 2002; Dorow and Kopelke 2007; Blick et al. 2012, 2014; Meyer et al. 2021). The strict forest reserve Hasenblick (HB) was designated in 1988 (BLE 2021b). The arthropod community of HB was sampled between 2000 and 2001. Using the same sampling design, sampling was repeated in 2012 and 2013 to allow an analysis of the temporal changes in the arthropod community. As we sampled in two consecutive years, respectively, the relative importance of interannual variability in comparison with directed temporal trends can be assessed.
Insect populations are known to fluctuate greatly over time (Liebhold and Kamata 2000; Roy et al. 2001; Günther and Assmann 2004; Roth et al. 2021). These fluctuations usually are thought to be caused by climatic factors (Varley and Gradwell 1960; Courtney and Duggan 1983; Wallner 1987; Roy et al. 2001; Knape and de Valpine 2011) or to be community driven (predator prey interactions, diseases, etc.) (Morris 1959; Wallner 1987; Liebhold and Kamata 2000). Günther and Assmann (2004), e.g., found high overall fluctuations in carabid beetle communities of a forest stand in northern Germany and even higher fluctuations in individual species. Also Flechtner (2000) found twice as many beetle individuals in pitfall traps in the Hessian strict forest reserve “Niddahänge östlich Rudingshain” (NI) during the second year of a 2-year survey.
Several factors might cause long-term changes in the animal communities of unmanaged forests. The abandonment of forestry practices allows forests to recover a more primeval-forests-like structure and dynamic (Bengtsson et al. 2000). These changes in the forest structure also affect the forest animal communities (Bengtsson et al. 2000), although previous studies show no consistent difference between animal communities of managed and unmanaged forests (Paillet et al. 2009). While some studies found an increased arthropod diversity with lower management intensity (Paillet et al. 2009), others found a reduced diversity in unmanaged forests (de Warnaffe and Lebrun 2004; Lange et al. 2014). De Warnaffe and Lebrun (2004) state that some of these patterns might, however, be attributed to species that are not typical for forests but have high dispersal abilities and are able to colonize managed but not unmanaged forests. In addition to species diversity, the structural changes in the forests might also cause changes in the trait composition of the animal communities, e.g., threatened species that are negatively affected by forest management are likely to benefit from ceased forest management (Seibold et al. 2015).
Climate change also can be expected to increasingly affect the animal communities in Hessian (German) forests. For many animal species poleward or uphill shifts of their distribution ranges are reported (Konvicka et al. 2003; Hickling et al. 2006; Wilson et al. 2007; Feehan et al. 2009; Ott 2010; Köhler 2014; Kerr et al. 2015; Mason et al. 2015; McCain and Garfinkel 2021). Köhler (2014) found South-European-Mediterranean beetle species to be overrepresented among the beetle species that were newly recorded for Germany between 1998 and 2009. Therefore, an increasing number of species adapted to warm conditions can be expected to reach the Hessian forests as their distribution ranges expand northwards or uphill.
Climate change, urbanization or the conversion of suitable habitats for species rich insect communities to intensively used agricultural land, pollution, and the introduction of alien species are considered main drivers of insect decline (Sánchez-Bayo and Wyckhuys 2019; Wagner et al. 2021). However, Wagner et al. (2021) state that the relative importance and variability of these factors is still unclear. A general decline in insect populations is reported in several studies (e.g., Conrad et al. 2004, 2006; Franén and Johannesson 2007; Brooks et al. 2012; Bojková et al. 2014; Hallmann et al. 2017; Seibold et al. 2019; Roth et al. 2021). However, so far, only Homburg et al. (2019), Seibold et al. (2019), and Roth et al. (2021) included arthropods of German forests in their analyses, with differing results. Although all three studies reported declining species numbers of insect communities, Seibold et al. (2019) and Roth et al. (2021) found a decline in biomass in their forest sites that was not observed by Homburg et al. (2019). Roth et al. (2021) detected general declines in the abundance of the nocturnal macro moths in Bavarian forests. However, on the local scale patterns were less clear and they even detected a strong increase in species richness in one of their local datasets.
Here, data on the ground dwelling beetles and ground dwelling spiders caught with 15 pitfall-trap-triplets (three individual pitfall traps arranged on a straight line) in HB are presented in light of the discussion on insect decline in forests. Specifically, we would expect the same effect as in open landscapes: a decline in species richness, biomass and functional diversity associated with habitat loss and pest control measures (Sánchez-Bayo and Wyckhuys 2019; Wagner et al. 2021). For both animal groups the sampled species’ identity, abundance, and trait composition are described and compared between the sample years and survey periods. Additionally, they are assessed for temporal changes in biomass and functional diversity and the effects of species traits on changes in the probability of occurrence over time are analyzed.
Material and methods
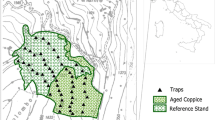
The strict forest reserve Hasenblick (HB) is situated in the North of the German federal state of Hesse (center coordinates (WGS 84): 51.05856, 8.63562) and covers an area of 46 hectares. Forest management ceased in 1988 when the reserve was designated (BLE 2021b). The reserve is mostly covered by beech forests (Luzulo-Fagetum) (Neckermann and Neckermann-Achterholt 2014).
Two faunistic surveys were conducted in HB, as part of the “Hessian strict forest reserves program” (Althoff et al. 1991; Dorow et al. 1992). Data on the years 2000 and 2001 are available from the first survey and on the years 2012 and 2013 from the second survey. Both surveys are based on the samples collected with 15 pitfall trap triplets that were operated at the same locations during both surveys. The traps were distributed to cover the various forest floor habitat structures of the reserve. The habitat structure at these 15 trap locations did not change significantly among surveys. An individual pitfall trap is assembled of a plastic pipe of 20 cm length and an inner diameter of 10 cm that is vertically inserted in the forest floor. The upper end of the pipe is even with the surface of the forest floor, to allow for animals that are walking on the forest floor to fall into the pipe. A twist-off-glass (350 ml) with preservative liquid (2 parts 70% ethanol, 1 part 99% glycerol, 1 ml liquid detergent), as well as a plastic funnel, are inserted in the pipe. The funnel fits tightly in the pipe and is meant to lead specimens that fell into the pipe in the collecting glass. A 30 × 30 cm zinc sheet was used to cover the trap from rain and falling leaves. Three individual pitfall traps, arranged in a straight line, with a distance of 5 m between adjacent traps, form a triplet (Dorow et al. 1992). The traps were emptied monthly. Here data on the caught beetles and spiders are presented for three consecutive months each year (18.4.–18.7.2000; 24.4.–19.7.2001; 25.4.–25.7.2012; 16.4.–31.7.2013). All adult beetles and spiders were identified to species level. The beetles caught during the first survey were mostly identified by Frank Köhler, the beetles caught during the second survey were identified mostly by staff members of the ÖKOTEAM-Institute. The spiders were identified by Theo Blick.
Information on the forest affinity of individual species is taken from the forest affinity lists of beetles (Köhler et al. 2019) and spiders (Blick et al. 2019) published in Dorow et al. (2019a) (see Schneider et al. (2021b) for an assignment of German and English abbreviations). The body length of beetle species is taken from Assing and Schülke (2011), Freude et al. (1967), Freude et al. (1969), Freude et al. (1974), Freude et al. (1976), Freude et al. (1979), and Müller-Motzfeld (2006). The body length of spider species is taken from Nentwig et al. (2021). The Red List status of beetle species is taken from the recently published German Red lists of beetles (Schmidt et al. 2016; Bense et al. 2021; Bussler and Bense 2021; Esser 2021; Schaffrath 2021; Schmidl et al. 2021a, 2021b, 2021c; Sprick et al. 2021). The Red List status of spider species is taken from the recently published German Red list of spiders (Blick et al. 2016). For statistical analyses the Red List status of each species found during the two surveys is assigned to one of three higher level categories [“*”, “D”, and “nb” sensu Ludwig et al. (2009) = level 1 (not threatened); “V” sensu Ludwig et al. (2009) = level 2 (near threatened); “G” sensu Ludwig et al. (2009) = level 3 (threatened)]. The remaining traits of the species are either taken from the private database of Frank Köhler (Coleoptera; Köhler unpublished), or the private database of Theo Blick (Araneae; Blick unpublished). The species traits categories are given in Table 1. Complete species lists including the information that was taken from the private databases of Frank Köhler and Theo Blick are given in the supplementary material (Online Resource: Tables. S7, S8). A distribution type, as given for the beetle species (basing on Horion (1941), Horion (1949), Horion (1953), Horion (1955), Horion (1956), Horion (1958), Horion (1960), Horion (1961), Horion (1963), Horion (1965), Horion (1967), Horion (1974)), was not available for spider species. Food preference of beetles follows Köhler unpubl. (see Online Resource Table S7), whereas the zoophagous spiders are assigned to hunting guilds (Blick unpubl.; see Online Resource Table S8), defined by Cardoso et al. (2011).
To reduce the noise caused by chance findings of species that are usually not detectable with pitfall traps, since they are not dwelling on the forest floor, only species that are to some extent associated with the forest floor are included in the analysis. This information is taken from the private databases of Frank Köhler (Coleoptera; Köhler unpubl.) and Theo Blick (Araneae; Blick unpubl.) and included in Tables. S7 and S8 in the Online Resource, respectively. In the case of beetles, these are eurytopic species, species actually dwelling on the forest floor (without any known preference for spatially delimited microhabitats), as well as species associated with microhabitats often found on the forest floor (decaying organic material, nesting sites (either without preference, or with preference for nesting sites of mammals or ants and other hymenopterans), and fungi) (Table 1; Köhler unpubl.). A similar definition of the ground dwelling beetle community was previously used by Köhler and Flechtner (2007) and Köhler (2010). For spiders, information on forest-floor-dwelling was directly available in the respective database (Blick unpubl.; see Online Resource Table S8). Species exclusively dwelling in open habitats are excluded from this forest habitat analyses, as they are most likely transient and represent chance findings (Dorow et al. 2019b; Schneider et al. 2021b).
Samples from severely damaged or destroyed traps were excluded from all analyses to minimize the effects of the trap conditions. For the descriptive comparison of the communities of individual sample years or surveys the respective individual samples (not the samples of the whole sample year) were excluded together with the respective samples from the other survey periods. Thus, in the case of one trap that was strongly damaged in May 2001 data from both May of 2001 and May 2002 were excluded from the interannual comparison of the first survey. In line with this approach we excluded samples from two, three and one trap(s) for May, June and July 2012 and 2013 data sets, respectively. Overall, we excluded May data of three traps, June data of three traps, and July data of one trap due to missing data for individual samples.
Since the biomass and the number of caught individuals are highly dependent upon the number of days sampled, and the number of sampling days differed between survey years, we calculated and analyzed biomass and number of individuals caught per sampling day. All statistical analyses are conducted in R (R Core Team 2021). Rarefaction and extrapolation of species numbers are conducted and depicted using the R-packages SpadeR (Chao et al. 2016) and iNEXT (Hsieh et al. 2020).
Temporal trends in the number of collected individuals per day, biomass collected per day, and functional diversity are analyzed by building models only including the year of the survey as a fixed effect in addition to a random trap-effect. Models with and without a fixed effect (year) or an additional random slope over time were compared, based on the AICc value and the model likelihood (package “AICcmodavg”; Mazerolle (2020)). Model fit was tested with the R-package DHARMa (Hartig 2021). If a trap was severely damaged during a sampling period the data collected with the respective trap during the respective year (not just the respective sample) are excluded from the analysis, to minimize the effects of the trap conditions. Data collected during other years with the respective traps were, in this case, not excluded. This procedure resulted in excluding data of 1 year in case of four traps and data of 2 years of one trap. To calculate species richness, the observed species numbers were rarefied to the respective smallest sample size that was caught during one of the 4 years with the respective trap, using the “rarefy”-function (package “vegan”; Oksanen et al. (2020)). As measure of the functional diversity Rao’s quadratic entropy (Botta-Dukát 2005) was calculated (package “SYNCSA”; Debastiani and Pillar (2012)). For beetles, food preference, body size, forest affinity, and biotope preference are used as functional species traits. For spiders, the hunting guild is used instead of the food preference. The mean body length was calculated from the minimal and maximal body length of a species, without separation of sexes. The dry biomass of the beetle species is calculated, based on its mean body length, as described by Rogers et al. (1976). The dry biomass of the spider species is calculated, based on the mean body length (separately for both sexes in this case), as described by Penell et al. (2018). Unreliable samples, e.g., when the respective trap was severely damaged or destroyed within the sampling period, were treated as missing data.
To test whether or not the observed long-term changes (differences between sampling periods) of the community measurements are greater than the observed short-term fluctuations, the absolute values of the differences between the sampling periods (based on mean values of the 2 years within each sampling period) and between the consecutive years within each sampling period were calculated separately for each trap. The same subset of data as for the previous analyses of temporal trends are used for this analysis. A one-way ANOVA for repeated measurements (package “rstatix” (Kassambara 2021)) was used to test for differences between the differences found between the 2 years within each of the two sampling periods (separate group for each sampling period) and between the sampling periods. When necessary data were transformed to match model assumptions (see Online Resource Table S5). If a significant difference was found pairwise t-tests (package “rstatix” (Kassambara 2021)) were conducted as post-hoc tests. The significance level was corrected for multiple testing, as described by Waite and Campbell (2006).
The high interannual fluctuations in the biomass or the number of individuals caught per day might be caused by climatic factors, independently of a long-term temporal trend. The tested climatic factors are given in Table S1 in the Online Resource. The temperature sum was calculated as the sum of the mean temperatures of each day (with a base temperature of 10 °C subtracted). Mean temperatures lower than 10 °C add nothing to the temperature sum (Kulhanek 2009). Calculations of precipitation and temperature sum are based on daily precipitation and mean temperature calculated for the center of HB by the Nordwestdeutsche Forstliche Versuchsanstalt (NW-FVA). As a phenological starting point, the first flowering day of hazel is taken from the phenological station Eversberg of the Deutsche Wetterdienst (DWD 2020b). The station Eversberg was chosen because it is the closest phenological station situated at a similar elevation level (360 m asl) (DWD 2020a) as the forest reserve (370–485 m asl) (BLE 2021b). These data are not meant to give the exact date of hazel flowering in HB but as a common phenological starting point of each sampling year. The values of all climatic variables are given in Table S1 in the Online Resource.
Since many of the climate factors were found to be correlated to each other (data not shown), a principle components analysis (PCA) was conducted to generate a reduced set of uncorrelated explanatory variables (principle components), based on the climatic factors. The prcomp-function was used to conduct the PCA in R (R Core Team 2021).
The first three principle components to which climate factors are aggregated were used as explanatory variables instead of the climate factors. The first principle component explains 42.0% of the total climatic variance, the second one 31.8%, and the third one 25.2%. The extent to which the individual climate factors are represented by individual principle components are given in Table S2 in the Online Resource.
The principle components were included as explanatory variables in mixed linear effects models [package lme4 (Bates et al. 2015)], to test for significant effects of climate on the animal biomass or abundance. Forward model selection was conducted to choose the best models. The same subset of data as for the previous analyses of temporal trends are used for this analysis.
We compare the overall similarities of the beetle and spider communities sampled in individual years using an NMDS, based on the relative abundances of the species separately for both species groups, with the “metaMDS”-function [package “vegan”; Oksanen et al. (2020)]. We used the Bray–Curtis-index as dissimilarity-measure.
To test whether or not species traits influence temporal changes in the occurrence probability, mixed effects models were built (package lme4 (Bates et al. 2015)), including interactions between species traits and the year of the survey, explaining presence-absence information for each species and year. A random species effect was also included in the models. This analysis was conducted separately for “all beetle species”, Carabidae (ground beetles), Staphylinidae (rove beetles) (the most abundant and species rich beetle families in the samples) and for spiders. Since individual traps were not analyzed separately in this case, all data of the five traps that were severely damaged during the sampling periods were excluded from these analyses. Therefore, these analyses are based on 11 of the 15 pitfall trap triplets. To prevent adding correlated traits in the same models, traits were tested for significant correlations (see Online Resource Table S3). Depending on the tested traits either Χ2-tests, ANOVAs, Kruskal–Wallace-tests, or Spearman-rank-correlations were conducted. The results were corrected for multiple testing, as described by Waite and Campbell (2006). In the case of all beetle species, backward model selection started with a model including the survey year, the forest affinity, the size of the species, and interactions between the year and one of the included species traits, each. In the case of Carabidae the starting model contained the survey year, the type of distribution, the forest affinity, the body size, the food preference, the Red List status, and the interactions between one of the species traits and the survey year, each. Since all detected ground-beetle-species (Carabidae) have the same biotope preference (gr; see Tables. 1, S7 (Online Resource)), the biotope preference is not included as an explanatory variable in this case. In the case of Staphylinidae, the starting model contained the survey year, the forest affinity and the body size of the species, in addition to the interactions between one of the species traits and survey year, each. For spiders the starting model contained the survey year, the forest affinity, the body size, the Red List status, and the biotope preference, in addition to the interactions between one of the species traits and the survey year, each.
Results and discussion
A total of 61,254 (first survey: 15,948; second survey: 45,306) adult beetles and 5568 (first survey: 1735; second survey: 3833) adult spiders were identified to species level comprising 506 (first survey: 318; second survey: 422) beetle species and 117 (first survey: 86; second survey: 107) spider species. Of these, 332 beetle species and 110 spider species are known to dwell on forest floors to some extent (Online Resource Tables. S7, S8; see Material and Methods section). A more detailed description of the results is given in the Online Resource.
Changes in community descriptors
Observed differences between surveys
14,381 ground dwelling beetles were caught during the first survey and 41,868 individuals were caught during the second survey, comprising 218 and 283 species, respectively. Similarly, 1728 of the spider individuals were caught during the first survey and 3816 individuals were caught during the second survey, comprising 84 and 101 species, respectively. This represents a marked, two to three-fold increase in the number of individuals caught between surveys, while the number of species increase by ~ 20–30%. These differences remain after exclusion of unreliable samples and correcting for these samples (see Material and Methods section) [ground dwelling beetles: first survey 12,538 individuals (211 species), second survey 35,819 (274) spiders: first survey 1511 (82), second survey 3184 (98)].
The number of detected species is known to depend on the sample size, i.e., the number of individuals analyzed (Gotelli and Colwell 2001; Martikainen and Kouki 2003; Burner et al. 2021). Thus, the higher number of detected species during the second survey might primarily be driven by greater number of individuals. In the cases of spiders this seems to be the case: The higher species numbers found during the second survey (Figs. 1b) are actually driven by the higher numbers of individuals caught. The extrapolated species numbers are similar between the surveys and the 95% confidence intervals (ACE-estimator; first survey: 111 (95–146), second survey: 112 (104–132), Fig. 1b). In the case of the ground dwelling beetles the expected (extrapolated) total number of species is also higher for the second survey than for the first survey (ACE-estimator; first survey: 261 (240–298), second survey: 348 (320–395), Fig. 1a) suggesting that this increase in species numbers is not solely a statistical effect of sampling more individuals. However, the difference in species richness between the two surveys is not significantly larger than the differences between the consecutive years within each of the two survey periods (Fig. 2b; Online Resource Table S5). Therefore, the higher number of ground dwelling beetle species caught during the second survey not necessarily indicates any long-term trend but might be driven by short-term-fluctuations in species richness.
Species accumulation curves. Species accumulation curves are calculated separately for both surveys for a Ground dwelling beetles, b Ground dwelling spiders. Solid lines represent interpolations, dashed lines represent extrapolations. The actual sample size is indicated by the mark depicted in the legend within the figure. The shades surrounding the inter- and extrapolation curves represent the 95% confidence intervals. HB1 first survey (2000–2001), HB2 second survey: 2012–2013
Changes of community descriptors of the ground dwelling beetle communities within and between surveys. The absolute differences in a individuals caught per day, b species richness, c functional diversity, d biomass (all species) caught per day, and e biomass without Anoplotrupes stercorosus caught per day of the ground dwelling beetle communities between surveys and between consecutive years within surveys are depicted as boxplots. The box extends from the 25% percentile to the 75% percentile, the horizontal bar in the box corresponds to the median and the whiskers include all values within the range of 1.5 times the interquartile range. Outliers are depicted as points. Abbreviations: 2000/2001: Difference between the individual sampling years of the first survey; 2012/2013: Difference between the individual sampling years of the second survey; surv1/surv2: Difference between surveys
The “number of individuals” and “biomass” caught per day also seem to increase and a significant positive trend over time was found in ground dwelling beetles and spiders (Table 2). However, the increase in the biomass of ground dwelling beetles caught per day is mostly driven by the high number of individuals of Anoplotrupes stercorosus during the second survey. When A. stercorosus was excluded, no significant increase over time of the biomass per day was found.
Short-term fluctuations
The short-term fluctuations of the “number of individuals” and “biomass” found in the ground dwelling beetle and spider communities of HB are high in comparison with any long-term-trend that might be present (Figs. 2, 3; Online Resource: Tables. S5, S6). In the case of the beetle biomass caught per day (with and without A. stercorosus), the difference between the consecutive years of at least one survey is even significantly higher than the difference between surveys. Therefore, it is not possible to distinguish between long-term trends and short-term or intermediate fluctuations based on the available data. Further data covering a longer timespan with a sufficient temporal resolution would have to be generated to detect long-term trends in the animal communities of HB. However, while we cannot exclude the possibility that the observed positive trends in the tested diversity measurements actually result from short-term fluctuations, we find no evidence for any severe decline in the ground dwelling beetle and spider communities in HB over the thirteen study years.
Changes of community descriptors of spider communities within and between surveys. The absolute differences of spider a individuals caught per day, b functional diversity, and c biomass caught per day between surveys and between consecutive years within surveys are depicted as boxplots. The boxplots are constructed in the same way as those in Fig. 2
Such a decline in the abundance, biomass, or species richness of the arthropod communities could have been expected following several recent reports of severe declines in insect biomass (e.g., Hallmann et al. 2017; Seibold et al. 2019; Roth et al. 2021), abundance (e.g., Conrad et al. 2004, 2006; Brooks et al. 2012; Roth et al. 2021), and species numbers (e.g., Franén and Johannesson 2007; Bojková et al. 2014; Seibold et al. 2019; Roth et al. 2021). Although, most of these studies focused on open habitats Homburg et al. (2019), Seibold et al. (2019), and Roth et al. (2021) also report declines of insect or arthropod populations in German forests. While these studies largely overlapped with the timeframe of our sampling (Homburg et al. (2019): 1994–2017, Seibold et al. (2019): 2008–2016, Roth et al. (2021): 1979–2018), they do include more recent years of data. Therefore, we are cautious in our comparisons and interpretations regarding overall trends in insect populations as the years after 2013 may have had disproportionate contributions to their observations. For example, Roth et al. (2021) found overall declines in species richness, abundance, and biomass of nocturnal moths in Bavarian forests across their entire data series through 2018, but this pattern was not observed when only the years prior to 2013 were considered (Fig. S7-2 in the Supporting information of Roth et al. (2021)). Seibold et al. (2019) found declines in biomass and species numbers, but not in abundance. These results also hold for their data for the timeframe of our study (Fig. 1 in Seibold et al. (2019)). According to Seibold et al. (2019) this indicates a long-term trend and is not an effect of individual survey years. Homburg et al. (2019) did not find a decline in biomass but in species richness and also in species numbers in the ground beetle community of a German forest reserve. In their depicted species-richness data (Fig. 1a in Homburg et al. 2019; species numbers not depicted) this decline over time seems to be also visible when only the years up to 2013 are considered. We found no evidence for a decline in the beetle and spider populations in HB, but our results are to be interpreted with caution because they are based only on two surveys of two consecutive years each and might be prone to interannual fluctuation (Fournier et al. 2019). More continuous data are needed to clarify trends in insect abundance in Hessian beech forest reserves.
High interannual fluctuations in arthropod communities between years, as were observed in HB, are common in arthropod populations (Liebhold and Kamata 2000; Roy et al. 2001; Günther and Assmann 2004). Also, individual beetle species becoming highly abundant in a single year was previously observed in forest surveys (Flechtner 2000; Günther and Assmann 2004) and are therefore probably not uncommon among beetles. In “Niddahänge östlich Rudingshain” (NI) one species of the family Leiodidae (Leiodes lucens) and three species of the family Staphylinidae (Aleochara sparsa, Atheta vaga (sub A. nigricornis), Placusa tachyporoides) became highly abundant in 1991 and were found in most traps, while in 1990 these species were found in much lower numbers and only in a few traps (Flechtner 2000). Similarly, Günther and Assmann (2004) report high interannual fluctuations in the population sizes of some ground beetle species (Carabidae) in a forest stand in northern Germany. The reasons behind such fluctuations are not fully understood, but thought to be either caused by climatic factors (Varley and Gradwell 1960; Courtney and Duggan 1983; Wallner 1987; Roy et al. 2001; Knape and de Valpine 2011) or by community driven effects, like predator prey interactions, parasitism, or diseases (Morris 1959; Wallner 1987; Liebhold and Kamata 2000).
Anoplotrupes stercorosus is the species that showed the most obvious fluctuation in the activity density in HB. This increase in individuals caught was also the main driver of changes in beetle biomass caught per day (see above). However, in no other Hessian strict forest reserve such an extremely high fluctuation in the activity density of A. stercorosus was observed to date. A. stercorosus was previously found to be among the most dominant beetle species in two of the four surveys that were conducted in other beech dominated Hessian strict forest reserves (Flechtner 2000, 2004; Köhler and Flechtner 2007; Köhler 2010). About 12,000 and 6000 individuals were caught during a 2 years survey (1994–1996) in the strict forest reserves “Goldbachs- und Ziebachsrück” (Köhler 2010) and “Hohestein” (Köhler and Flechtner 2007), respectively. In the remaining two Hessian strict forest reserves (Schönbuche and NI) that were both surveyed for 24-months-period within the years 1990–1992 A. stercorosus was not among the most abundant beetle species and less than 1000 individuals were caught per reserve (578 and 889 individuals, respectively) (Flechtner 2000, 2004). These findings are more similar to the findings of the first survey in HB. This indicates that although such high fluctuations in abundance of A. stercorosus might be common in beech forests, they are not regularly observed in the other Hessian strict forest reserves.
Since the larvae of A. stercorosus feed on dung (Rößner 2012), the availability of dung probably has an influence on the abundance of A. stercorosus. However, the wildlife stock of the forest district in which HB is located was actively reduced by intensified hunting since 2009. Thus, the wildlife stock of HB and the surrounding area was probably lower during the second than during the first survey (Dienst pers. comm.). Therefore, the strong increase in the activity density of A. stercorosus from first to second survey is presumably not a function of dung availability.
In our study, the high fluctuations between survey years in the populations of ground dwelling beetles and spiders were found to be correlated with climatic conditions (Table 3). However, due to the correlation of many tested climatic factors it was not possible to separate the influence of individual climatic factors in our study. Regarding the ground dwelling beetles, usually all principle components, summarizing all tested climatic factors (see Online Resource Table S2 for an overview of the representation of individual climatic factors by the individual principle components), were found to significantly correlate with the number of caught individuals and the biomass. However, when excluding A. stercorosus from the analyses, the second principle component is not significantly correlated with the biomass. Concerning the ground dwelling spiders, the first and third principle components were found to show only a tendency to be significantly correlated with the biomass. The third principle component was not found to be significantly correlated with the number of caught spider individuals. Therefore, the fluctuations in beetle and spider populations are probably driven by climatic factors. However, since the principle components represent several climatic factors that are correlated to some extent, it is unclear which climatic factors actually drive the observed fluctuations.
Several studies found higher temperatures during the activity times of the previous generations (Courtney and Duggan 1983; Kingsolver 1989; Roy et al. 2001) or higher development temperatures (Kingsolver 1989; Roy et al. 2001) to have positive effects on the abundance of many species in the following year. However, since the temperature sum accumulated during the previous summer is nearly completely included in the third principle component (Online Resource Table S2), and this is not significantly correlated with the numbers of spider individuals and the spider biomass caught in our study, these effects are in this case probably not the main drivers or the temperature sums accumulated over other seasons of the previous year are of higher importance in this case.
Reduced drought stress during the development is also known to increase the numbers of individuals in the following year (Roy et al. 2001). In our study, the precipitation during the previous year, as well as that between the beginning of the hazel flowering and the start of the sampling period are mainly included in principle components significantly correlating with the numbers of individuals of ground dwelling beetles and spiders.
Not only the real population density, but also the activity of insects is highly influenced by the weather conditions and can account for fluctuations in abundances in faunistic surveys. Most insects are more active under dryer and warmer conditions (Greenslade 1964; Nunes et al. 2011). This is of high importance in the case of our study since the sampling was conducted with pitfall traps that are known to measure the activity density instead of real population densities (Greenslade 1964; Adis 1979; Siewers et al. 2014; Skvarla et al. 2014). Therefore, the per day temperature sum accumulated during the sampling period would be expected to positively influence the amount of caught beetles and spiders, while the per day precipitation would be expected to negatively influence the amount of caught beetles and spiders. On the other hand, ground dwelling beetles probably burry deeper in the ground, under dry conditions (Köhler 1996), which would lead to reduced effectivity of pitfall traps during especially hot and dry summers. Both, the temperature sum and precipitation during the sampling periods, are to some extend represented by principle components that were found to significantly correlate with the amounts of animals caught in the present study, although the temperature sum accumulated during the sampling period is nearly completely represented by the first principle component, that is not significantly correlated with the spider biomass. However, as mentioned above, it is not possible to separate the influence of individual climatic factors on the beetle or spider communities of HB.
Species composition
In both considered species groups (ground dwelling beetles, deadwood beetles, and ground dwelling spiders) also the species composition differed strongly between the two survey-periods and between individual years (Fig. 4a, b). Only a relatively small fraction of the species was found in every sampling year. In spiders, less than half of the species (45%) (Fig. 4b) and in ground dwelling beetles less than one third of the species (30%) (Fig. 4a). Species that were exclusively found in only 1 year or during only one survey usually were detected with only one or few individuals. Of the 147 species that were exclusively found in only 1 year (ground dwelling beetles: 115; spiders: 28) only one species (Dinaraea angustula (ground dwelling beetle; Staphylinidae) was found with more than 10 individuals. This indicates that especially the species found in only 1 year probably represent chance findings of species that either do not dwell at the trap locations but elsewhere in HB, or have very low population densities in HB, or are not effectively sampled with pitfall traps. Of the 46 species (ground dwelling beetles: 42; spiders: 4) that were found in both years of one survey but not during the respective other survey, 19 were found with more than 10 individuals (ground dwelling beetles: 18; spiders: 1). These species on the other hand might be indicators or sentinels of community change.
A high amount of species of which only few specimens are found is common in zoological surveys (McGill et al. 2007; Schneider et al. 2021a) and therefore probably a cause of random variance in most ecological studies based on survey data of invertebrates. The ground dwelling beetle communities of consecutive years differ nearly as much (Bray–Curtis-dissimilarity-index based on relative abundance; 2000/01: 0.433, 2012/13: 0.458), as between the two surveys (0.468) that are more than 10 years apart. For spiders this is similar, although the years 2012 and 2013 are more similar to each other (Bray–Curtis-dissimilarity-index based on relative abundance: 2000/01: 0.346, 2012/13: 0.257, first/second survey: 0.363). These patterns are also reflected in NMDS-ordinations comparing the communities of all individual sampling years (Online resource Figs. S1 and S2). However, since each survey includes 2 years, the differences caused by chance findings might have a smaller influence on the comparison of surveys.
Trait composition
The changes in species composition between survey periods (see above) might also represent changes in the functional compositions of the animal communities. However, although the species composition differs between individual years or surveys, no substantial changes in the functional community compositions (or rather trait composition) between surveys are obvious in any of the concerned species groups (ground dwelling beetles, ground dwelling spiders) on species level (Figs. 5a–i). One might expect a signal of climate change between the surveys. But we found no increase in the number of South-European-Mediterranean species or a reduced number of North-European-Siberian species, even though such species have been considered sentinels for climate-change induced community shifts (Konvicka et al. 2003; Hickling et al. 2006; Wilson et al. 2007; Feehan et al. 2009; Ott 2010; Köhler 2014; Kerr et al. 2015; Mason et al. 2015).
Community composition of ground dwelling beetles, ground dwelling spiders. For ground dwelling beetles a, c, e, g, i the community composition, regarding the species traits biotope preference (a), food preference (c), Red List status (e), forest affinity (g), and distribution patterns (i), based on species and individuals, are summarized as stacked bars, separately for both surveys. In the case of the ground dwelling spiders (b, d, f, h), the community compositions regarding the biotope preference (b), hunting type (d), Red list status (f), and forest affinity (h) are depicted. For an assignment of the abbreviations to the trait levels see Table 1
Changes in the forest structure could also influence the trait compositions of the arthropod communities of the forest reserve, since the overall forest structure in HB changed since the reserve was designated. The volume of living trees increased, indicating an increased canopy cover, and the amount deadwood also increased (Online Resource Table S9). There is also an increase in small gaps between the faunistic surveys reported. However, as mentioned above, the observed trait structures of the arthropod communities did not change substantially between surveys (Fig. 5a–i). This likely reflects the fact that we specifically chose sites where habitat structures remained comparable between the surveys to avoid confounding effects.
The amount of threatened species could also be expected to increase, as the forest reserve develops primeval-forest-like structures (Topp et al. 2006; Seibold et al. 2015). However, most primeval-forest-like structures are probably of low importance for the ground dwelling beetle or spider communities of forests. The effect of redeveloped primeval-forest-like structures should be strongest in deadwood-dependent species (Seibold et al. 2015). Also, a timespan of 10 years is a very short period for forest development and the biodiversity might just not have recovered detectably (Paillet et al. 2009). Most “primeval-forest”-like characteristics, like, e.g., a high density of overmature and dying trees including a wide variety of associated microhabitats, need much longer timespans to develop (Winter and Möller 2008; Larrieu et al. 2012; Meyer 2013; Nordén et al. 2014). However, other structures like gaps caused by windthrows develop much faster, and can harbor more red list species than the surrounding forests (Wermelinger et al. 2017). Being embedded in a landscape of managed forests might additionally reduce the recolonization potential of the reserve by threatened species, due to a reduced connectivity to forest stands already colonized by the respective species (Lindenmayer et al. 2000). Especially in the case of slowly dispersing species already colonized forest stands close by are a necessary precondition for a fast recolonization of forest stands that are redeveloping suitable habitat conditions (Bengtsson et al. 2000). It is unknown if there are any refuges of such species nearby that would allow a colonization of the forest reserve within this relatively short time span. A survey of more specialized dead wood traps to representatively sample saproxylic beetles, especially those threatened species that are dwelling in rare primeval-forest-like structures, would be a logical progression of the present study. A study of the fauna of naturally developed gaps as an important feature of natural forests is also highly recommended.
Regarding the ground dwelling spiders, the abundance-based trait structures of the communities are also similar between the surveys (Figs. 5b, d, f, h). In ground dwelling beetles, there are some changes in the abundance-based functional community structure: higher percentage of individuals belonging to species dwelling in decaying material or nests, higher percentage of individuals belonging to saprophagous species, and higher percentage of species predominantly dwelling in forests without any preference for light or dense forests, caught during the second survey (Figs. 5a, c, e, g, i). However, these differences in the abundance-based trait composition are mainly caused by Anoplotrupes stercorosus (first survey: 338 individuals; second survey: 12,593 individuals; see above), and to some extent also by Pella humeralis (first survey: 0 individuals; second survey: 360 individuals) which became highly abundant during the second survey. A. stercorosus is a saprophagous species, mainly dwelling in dung and other decaying materials (Köhler unpubl., Online Resource Table S7), that predominantly is found in forests without any preference for light or closed forests (Köhler et al. 2019). P. humeralis is a zoophagous species that is associated with ant nests (Köhler unpubl.), dwelling in forests as well as in open habitats (Köhler et al. 2019). Generally, more species and individuals of species associated with nests were caught during the second survey [first survey: 97 individuals (18 species); second survey: 811 individuals (26 species)]. This corresponds to an increase in ant species and abundance observed between the two surveys (Schmidt and Meyer 2022).
Influence of species traits on trends in population
Although the trait composition of the beetle and spider communities was found to be similar among surveys, there is evidence that changes in the occurrence probability of individual species over time might be influenced by species traits. Among all ground-dwelling beetle species analyzed, body size was found to show a strong tendency to predict changes in occurrence probability over time (Χ2 = 3.8385, Df = 1, p = 0.050088). The occurrence probability of the smallest species was found to increase the most and the occurrence probability of large species was even found to decrease (Fig. 6a). Since body size is correlated to biotope preference (e.g., species that predominantly dwell on the forest floor, without any preference for spatially delimited microhabitats, show the largest mean body length), food preference (e.g., mold feeding species have the smallest mean body length), and distribution patterns, these traits might also influence the changes in the occurrence probability of species over time. Carabid body size was found to significantly influence the change in occurrence probability over time (Χ2 = 4.4901, Df = 1, p = 0.03409; Fig. 6b), though no significant correlation between body size and any other species trait was found. When only Staphylinidae were analyzed, body size showed no influence on the change in the occurrence probability over time.
Changes in the occurrence probability over time. The interactions between sampling year and the body size of all ground dwelling beetle species (a) and Carabidae (b), as well as the interaction between the sampling year and the forest affinity of Carabidae (c) and spiders (d) are depicted. To illustrate the effect of body size on the changes of the occurrence probability of species over time the body size was transformed to a categorial variable (see Material and Methods section) and the models were recalculated with this new categorical variable. For an explanation of the abbreviations of the forest affinity levels see Table 1
The finding that the occurrence probability of large beetle species might have decreased over time while the occurrence probability of small species increased over time in HB goes in line with previous findings that larger species usually show a higher extinction risk than smaller species (McKinney 1997; Kotze and O’Hara 2003; Seibold et al. 2015; Hagge et al. 2021). Larger species are thought to be more likely threatened because larger body size usually correlates with life history traits, such as lower reproduction rates or smaller population size (Simberloff 1994). Homburg et al. (2019), on the contrary, found a stronger decrease of the occurrence probability of small ground beetle species than of medium-sized or large species. Our results might in this respect be driven by the strongly increasing numbers of individuals.
In Carabidae (Χ2 = 8.2445, Df = 4, p = 0.08302) and spider species (Χ2 = 9.4452, Df = 5, p = 0.0925723), the forest affinity shows a tendency to influence the occurrence probability. This might indicate changes in the forest structure. However, the patterns differ between Carabidae and spiders (Fig. 6 c, d). The decrease in the occurrence probability of spider species dwelling predominantly in light forests (fl) might be explained by an increase in canopy cover, due to the ceased harvesting. In Carabidae, the occurrence probability of species that predominantly dwell in light forests are, with exception of species that dwell in closed forests, the species group that increased the least strongly. On the other hand, the occurrence probability of species dwelling in open habitats as well as in forests (m, mm) increased and the occurrence probability of species predominantly dwelling in open habitats (mo) remained more or less unchanged. At least the occurrence probability of species predominantly dwelling in open habitats would also be expected to decrease with increasing canopy cover. In Carabidae, the occurrence probability of species that dwell in open habitats at least to the same extend as in forests (m, mm, mo) are even increasing the strongest.
Functional diversity
Although the trait compositions of the animal communities were found to be stable among the surveys, the functional diversity might have changed, as the species composition differs between years (see above) since the similar overall trait composition of the communities might be composed of species that differ more or less strongly in their individual trait compositions. In both considered species groups (ground dwelling beetles and spiders) the functional diversity either was found to significantly increase over time (beetles) or to show a tendency to do so (spiders) (Table 2). However, this increase in functional diversities is probably a result of the higher number of species caught during the second survey. Additionally, the difference in functional diversity of the beetle and spider communities between the two surveys is not significantly larger than the differences between the consecutive years (Figs. 2c, 3b).
Threatened species
Among the species caught in our survey the beetle species dwelling on the forest floor comprised three species that are near threatened, one species that is threatened to an unknown extend, and eight species that are not assessed to a threat status, due to insufficient data, and two species that were not assessed (see Online Resource “Detailed description of results”) (Schmidt et al. 2016; Esser 2021; Schaffrath 2021; Schmidl et al. 2021a, 2021b, 2021c). Of the spider species dwelling on the forest floor two species are near threatened and one species was not assessed to a thread status, due to insufficient data (see Online Resource “Detailed description of results”) (Blick et al. 2016).
Of most of the species that are threatened, near threatened, or which were not assigned to a thread status due to insufficient data only very few specimens were found. Martikainen and Kouki (2003) found that large sample sizes are necessary to reliably sample threatened beetle species in boreal forests. Therefore, it is not surprising that a higher number of these species was found during the second survey (13), during which a higher number of individuals were caught, than during the first survey (7). The higher number of red-list species might also be caused by changes in the forest structure. However, as mentioned above, a period of 10 years is a very short time span in terms of forest development (Winter and Möller 2008; Larrieu et al. 2012; Meyer 2013) and the red-listed beetle species of which more than ten individuals were caught and which increased in abundance from the first to the second survey (Pterostichus cristatus and Megaloscapa punctipennis) are either dwelling on the forest floor or eurytopic (Online Resource Table S7) and not associated with any primeval-forest-like structure. Additionally, the number of specimens that were caught of more abundant species strongly differ also between consecutive years [P. cristatus (2012: 2 individuals, 2013: 22), M. punctipennis (2012: 7 individuals, 2013: 16)]. This indicates that the high interannual fluctuations in abundance of individual species described above also seem to apply to threatened species. Generally, these results support the approach that red-list-oriented surveys should be conducted for more than 1 year, and preferably more than 2 years to generate reliable information on the occurrence of threatened species within an area.
Conclusion
Our results show that although ground dwelling beetle and spider populations undergo high short-term fluctuations in quantitative measurements (e.g., abundance or biomass), the functional compositions of the arthropod communities are relatively stable over time. The short-term fluctuations highlight the need to analyze time series of sufficient length and of sufficient temporal resolution to reliably identify long-term trends. In contrast to the findings of several other studies (Conrad et al. 2004, 2006; Franén and Johannesson 2007; Brooks et al. 2012; Bojková et al. 2014; Hallmann et al. 2017; Seibold et al. 2019; Roth et al. 2021) no evidence for a decline in the investigated communities is found, although our results are not directly comparable to these studies since they include more recent data and additionally the structure of our data does not allow completely ruling out an overall decline. Our results do show that climatic factors need to be considered in monitoring programs because the fluctuations in arthropod populations are also driven by these factors. This also means that the increasing number of long-term monitoring programs could also add a valuable contribution to the understanding of the effects of climate factors on insect populations if these factors are also monitored consequently.
Data availability
The data presented in this study are openly available at https://dataportal.senckenberg.de/dataset/4351ec2b-1365-486a-beff-8af8bc14b80f.
References
Adis J (1979) Problems of interpreting arthropod sampling with pitfall traps. Zool Anz 202:177–184
Althoff B, Hocke R, Willig J (1991) Naturwaldreservate in Hessen – Ein Überblick. Mitt Hess Landesforstverwalt 24:1–62
Assing V, Schülke M (2011) Freude-Harde-Lohse-Klausnitzer-Die Käfer Mitteleuropas Band 4: Staphylinidae 1. Spektrum-Verlag, Heidelberg
Assmann T (1999) The ground beetle fauna of ancient and recent woodlands in the lowlands of north-west Germany (Coleoptera, Carabidae). Biodivers Conserv 8:1499–1517. https://doi.org/10.1023/A:1008974413376
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bengtsson J, Nilsson SG, Franc A, Menozzi P (2000) Biodiversity, disturbances, ecosystem function and management of European forests. For Ecol Manage 132:39–50. https://doi.org/10.1016/S0378-1127(00)00378-9
Bense U, Bussler H, Möller G, Schmidl J (2021) Rote Liste und Gesamtartenliste der Bockkäfer (Coleoptera: Cerambycidae) Deutschlands—3. Fassung, Stand September 2011. Naturschutz und Biologische Vielfalt 70(5):269–290
BLE (2021a) Ergebnisdatenbank–Dritte Bundeswaldinventur 2012. https://bwi.info/?lang=en. Accessed 1 Nov 2021a
BLE (2021b) Naturwaldreservate. online database. https://fgrdeu.genres.de/naturwaldreservate/details/. Accessed 1 Nov 2021b
Blick T, Dorow WHO, Kopelke J-P (2012) Kinzigaue–Zoologische Untersuchungen 1999–2001, Teil 1. Naturwaldreservate in Hessen 12:1–348
Blick T, Dorow WHO, Köhler G (2014) Kinzigaue–Zoologische Untersuchungen 1999–2001, Teil 2. Naturwaldreservate in Hessen 13:1–238
Blick T, Finch O-D, Harms KH, Kiechle J, Kielhorn K-H, Kreuels M, Malten A, Martin D, Muster C, Nährig D, Platen R, Rödel I, Scheidler M, Staudt A, Stumpf H, Tolke D (2016) Rote Liste und Gesamtartenliste der Spinnen (Arachnida: Araneae) Deutschlands—3. Fassung, Stand: April 2008, einzelne Änderungen und Nachträge bis August 2015. Naturschutz und Biologische Vielfalt 70(4):383–510
Blick T, Buchholz S, Kielhorn K-H, Muster C (2019) Die Waldbindung der Spinnen (Araneae) Deutschlands. BfN-Skripten 544:24–56. https://doi.org/10.19217/skr544
Boggs AD, Moorman CE, Hazel DW, Greenberg CH, Sorger DM, Sorenson CE (2020) Ground-dwelling invertebrate abundance positively related to volume of logging residues in the southern appalachians, USA. Forests 11(1149):1–17. https://doi.org/10.3390/f11111149
Bojková J, Rádková V, Soldán T, Zahrádková S (2014) Trends in species diversity of lotic stoneflies (Plecoptera) in the Czech Republic over five decades. Insect Conserv Div 7:252–262. https://doi.org/10.1111/icad.12050
Brooks DR, Bater JE, Clark SJ, Monteith DT, Andrews C, Corbett SJ, Beaumont DA, Chapman JW (2012) Large carabid beetle declines in a United Kingdommonitoring network increases evidence for a widespread loss in insect biodiversity. J Appl Ecol 49:1009–1019. https://doi.org/10.1111/j.1365-2664.2012.02194.x
Burner RC, Birkemoe T, Åström J, Sverdrup-Thygeson A (2021) Flattening the curve: approaching complete sampling for diverse beetle communities. Insect Conserv Div 15:157–167. https://doi.org/10.1111/icad.12540
Buse J (2012) ‘“Ghosts of the past”’: flightless saproxylic weevils (Coleoptera: Curculionidae) are relict species in ancient woodlands. J Insect Conserv 16:93–102. https://doi.org/10.1007/s10841-011-9396-5
Bussler H, Bense U (2021) Rote Liste und Gesamtartenliste der Borkenkäfer, Kernkäfer und Breitrüssler (Coleoptera: Scolytidae, Platypodidae, Anthribidae) Deutschlands—3 Fassung, Stand September 2011. Naturschutz und Biologische Vielfalt 70(5):415–432
Cardoso P, Pekár S, Jocqué R, Coddington JA (2011) Global patterns of guild composition and functional diversity of spiders. PLoS ONE 6:1–10. https://doi.org/10.1371/journal.pone.0021710
Chao A, Ma KH, Hsieh TC, Chiu C-H (2016) SpadeR: Species-richness prediction and diversity estimation with R, R package version (1). https://CRAN.R-project.org/package=SpadeR
Conrad KF, Woiwod IP, Parsons M, Fox R, Warren MS (2004) Long-term population trends in widespread British moths. J Insect Conserv 8:119–136. https://doi.org/10.1023/B:JICO.0000045810.36433.c6
Conrad KF, Warren MS, Fox R, Parsons M, Woiwod IP (2006) Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol Cons 132:279–291. https://doi.org/10.1016/j.biocon.2006.04.020
Courtney SP, Duggan AE (1983) The population biology of the Orange Tip butterfly Anthocharis cardamines in Britain. Ecol Entomol 8:271–281. https://doi.org/10.1111/j.1365-2311.1983.tb00508.x
de Warnaffe GDB, Lebrun P (2004) Effects of forest management on carabid beetles in Belgium: implications for biodiversity conservation. Biol Cons 118:219–234. https://doi.org/10.1016/j.biocon.2003.08.015
Debastiani VJ, Pillar VD (2012) SYNCSA—R tool for analysis of metacommunities based on functional traits and phylogeny of the community components. Bioinformatics 28:2067–2068. https://doi.org/10.1093/bioinformatics/bts325
Dorow WHO, Kopelke J-P (2007) Hohestein–Zoologische Untersuchung 1994–1996, Teil 2. Mitt Hess Landesforstverwalt 42:1–341
Dorow WHO, Flechtner G, Kopelke J-P (1992) Naturwaldreservate in Hessen No. 3. Zoologische Untersuchungen—Konzept. Mitt Hess Landesforstverwalt 26:1–159
Dorow WHO, Flechtner G, Kopelke J-P (2001) Schönbuche zoologische untersuchungen 1990–1992. Hess for FIV Ergeb Forsch 28(1):1–306
Dorow WHO, Flechtner G, Kopelke J-P (2004) Schönbuche zoologische untersuchungen 1990–1992, Teil 2. Hess for FIV Ergeb Forsch 28(2):1–352
Dorow WHO, Blick T, Kopelke J-P (2009) Goldbachs-und Ziebachsrück–Zoologische Untersuchungen 1994–1996, Teil 1. Mitt Hess Landesforstverwalt 45:1–326
Dorow WHO, Blick T, Kopelke J-P (2010) Goldbachs—und Ziebachsrück–Zoologische Untersuchungen 1994–1996, Teil 2. Mitt Hess Landesforstverwalt 46:1–271
Dorow WHO, Blick T, Pauls SU, Schneider A (2019a) Waldbindung ausgewählter Tiergruppen Deutschlands. BfN-Skripten 544:1–388. https://doi.org/10.19217/skr544
Dorow WHO, Blick T, Pauls SU, Schneider A (2019b) Zusammenfassende Auswertung der Waldbindung ausgewählter Tiergruppen Deutschlands. BfN-Skripten 544:382–387. https://doi.org/10.19217/skr544
DWD (2020a) Deutscher Wetterdienst - Climate data center - Index of/climate_environment/CDC/help/. https://opendata.dwd.de/climate_environment/CDC/help/PH_Beschreibung_Phaenologie_Stationen_Jahresmelder.txt. Accessed 20 March 2020a
DWD (2020b) Deutscher Wetterdienst—Climate data center—Index of/climate_environment/CDC/observations_germany/phenology/. https://opendata.dwd.de/climate_environment/CDC/observations_germany/phenology/. Accessed 20 March 2020b
Eckelt A, Müller J, Bense U, Brustel H, Bußler H, Chittaro Y, Cizek L, Frei A, Holzer E, Kadej M, Kahlen M, Köhler F, Möller G, Mühle H, Sanchez A, Schaffrath U, Schmidl J, Smolis A, Szallies A, Németh T, Wurst C, Thorn S, Christensen RHB, Seibold S (2017) “Primeval forest relict beetles” of Central Europe: a set of 168 umbrella species for the protection of primeval forest remnants. J Insect Conserv 22:15–28. https://doi.org/10.1007/s10841-017-0028-6
Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen. Ulmer, Stuttgart. https://doi.org/10.36198/9783825281045
Esser J (2021) Rote Liste und Gesamtartenliste der „Clavicornia“ (Coleoptera: Cucujoidea) Deutschlands—3 Fassung, Stand September 2011. Naturschutz und Biologische Vielfalt 70(5):127–161
Feehan J, Harley M, Minnen J (2009) Climate change in Europe. 1 Impact on terrestrial ecosystems and biodiversity. Rev Agron Sustain Dev 29:409–421. https://doi.org/10.1051/agro:2008066
Flechtner G (2000) Coleoptera (Käfer). In: Flechtner G, Dorow WHO, Kopelke J-P (eds) Niddahänge östlich Rudingshain—Zoologische Untersuchungen II 1990–1992, vol 32. Mitteilungen der Hessischen Landesforstverwaltung, Wiesbaden, pp 5–349
Flechtner G, Dorow WHO, Kopelke J-P (1999) Niddahänge östlich Rudingshain—Zoologische Untersuchungen 1990–1992. Mitt der Hess Landesforstverwalt 32(1):1–746
Flechtner G, Dorow WHO, Kopelke J-P (2000) Niddahänge östlich Rudingshain—Zoologische Untersuchungen II 1990–1992. Mitt der Hess Landesforstverwalt 32(2):1–550
Flechtner G, Dorow WHO, Kopelke J-P (2006) Hohestein—Zoologische Untersuchung 1994–1996, teil 1. Mitt der Hess Landesforstverwalt 41(1):1–247
Flechtner G (2004) Coleoptera (Käfer). In: Dorow WHO, Flechtner G, Kopelke J-P (eds) Schönbuche Zoologische Untersuchungen 1990–1992, Teil 2. Hessen-Forst—FIV Ergebnis—und Forschungsbericht 28/2, Wiesbaden, pp 5–126
Flensted KK, Bruun HH, Ejrnæs R, Eskildsen A, Thomsen PF, Heilmann-Clausen J (2016) Red-listed species and forest continuity—a multi-taxon approach to conservation in temperate forests. For Ecol Manage 376:144–159. https://doi.org/10.1016/j.foreco.2016.07.029
Fournier AMV, White ER, Heard SB (2019) Site-selection bias and apparent population declines in long-term studies. Conserv Biol 33:1370–1379. https://doi.org/10.1111/cobi.13371
Franén M, Johannesson M (2007) Predicting extinction risk of butterflies and moths (Macrolepidoptera) from distribution patterns and species characteristics. J Insect Conserv 11:367–390. https://doi.org/10.1007/s10841-006-9053-6
Freude H, Harde KW, Lohse GA (1967) Die Käfer Mitteleuropas Band 7: Clavicornia. Goecke & Evers, Krefeld
Freude H, Harde KW, Lohse GA (1969) Die Käfer Mitteleuropas Band 8: Teredilia, Heteromera. Clavicornia. Goecke & Evers, Krefeld
Freude H, Harde KW, Lohse GA (1974) Die Käfer Mitteleuropas Band 5: Staphylinidae II (Hypocyphtinae und Aleocharinae). Pselaphidae. Goecke & Evers, Krefeld
Freude H, Harde KW, Lohse GA (1976) Die Käfer Mitteleuropas Band 2: Adephaga 1. Goecke & Evers, Krefeld
Freude H, Harde KW, Lohse GA (1979) Die Käfer Mitteleuropas Band 6: Diversicornia. Goecke & Evers, Krefeld
Glaser FF, Hauke U (2004) Historisch alte Waldstandorte und Hudewälder in Deutschland. Angewandte Landschaftsökologie 61:1–193
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Greenslade PJM (1964) Pitfall trapping as a method for studying populations of Carabidae (Coleoptera). J Anim Ecol 33:301–310. https://doi.org/10.2307/2632
Grodsky SM, Moorman CE, Fritts SR, Campbell JW, Sorenson CE, Bertone M, Castleberry SB, Wigley TB (2018) Invertebrate community response to coarse woody debris removal for bioenergy production from intensively managed forests. Ecol Appl 28:135–148. https://doi.org/10.1002/eap.1634
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annu Rev Ecol Syst 33:1–23. https://doi.org/10.1146/annurev.ecolsys.33.010802.150507
Günther J, Assmann T (2004) Fluctuations of carabid populations inhabiting an ancient woodland (Coleoptera, Carabidae). Pedobiologia 48:159–164. https://doi.org/10.1016/j.pedobi.2003.11.002
Hagge J, Müller J, Birkemoe T, Buse J, Christensen RHB, Gossner MM, Gruppe A, Heibl C, Jarzabek-Müller A, Seibold S, Siitonen J, Soutinho JG, Sverdrup-Thygeson A, Thorn S, Drag L (2021) What does a threatened saproxylic beetle look like? Modelling extinction risk using a new morphological trait database. J Anim Ecol 90:1934–1947. https://doi.org/10.1111/1365-2656.13512
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. https://doi.org/10.1371/journal.pone.0185809
Hampicke U (2018) Kulturlandschaft—Äcker, Wiesen, Wälder und ihre Produkte. Springer, Berlin. https://doi.org/10.1007/978-3-662-57753-0
Hartig F (2021) DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models, R package version 0.4.1. https://CRAN.R-project.org/package=DHARMa
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Change Biol 12:450–455. https://doi.org/10.1111/j.1365-2486.2006.01116.x
Homburg K, Drees C, Boutaud E, Nolte D, Schuett W, Zumstein P, von Ruschkowski E, Assmann T (2019) Where have all the beetles gone? Long-term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Cons Div 12:268–277. https://doi.org/10.1111/icad.12348
Horion A (1941) Faunistik der deutschen Käfer Band 1: Adephaga—Caraboidea. Goecke, Krefeld
Horion A (1949) Faunistik der Mitteleuropäischen Käfer Band 2. Klostermann, Frankfurt am Main
Horion A (1953) Faunistik der Mitteleuropäischen Käfer Band 3 Malacodermata, Sternoxia (Elateridae—Throscidae). Eigenverlag, München
Horion A (1955) Faunistik der Mitteleuropäischen Käfer Band 4 Sternoxia (Buprestidae), Fossipedes, Macrodactylia, Brachymera. Eigenverlag, München
Horion A (1956) Faunistik der Mitteleuropäischen Käfer Band 5 Heteromera. Eigenverlag, München
Horion A (1958) Faunistik der Mitteleuropäischen Käfer Band 6 Lamellicornia. Eigenverlag, Überlingen
Horion A (1960) Faunistik der Mitteleuropäischen Käfer Band 7 Clavivornia, 1. Teil, (Sphaeritidae bis Phalacridae). Eigenverlag, Überlingen
Horion A (1961) Faunistik der Mitteleuropäischen Käfer Band 8 Clavicornia 2. Teil (Thorictidae bis Cisidae), Teredilia. Eigenverlag, Überlingen, Coccinellidae
Horion A (1963) Faunistik der Mitteleuropäischen Käfer Band 9 Staphylinidae, 1 Teil Micropeplinae bis Euaesthetinae. Eigenverlag, Überlingen
Horion A (1965) Faunistik der Mitteleuropäischen Käfer Band 10 Staphylinidae 2. Teil Paederinae bis Staphylininae. Eigenverlag, Überlingen
Horion A (1967) Faunistik der Mitteleuropäischen Käfer Band 11 Staphylinidae 2. Teil Habrocerinae bis Aleocharinae (ohne Subtribus Athetae). Eigenverlag, Überlingen
Horion A (1974) Faunistik der Mitteleuropäischen Käfer Band 12 Cerambycidae. Eigenverlag, Überlingen
Hsieh TC, Ma KH, Chao A (2020) iNEXT: iNterpolation and EXTrapolation for species diversity, R package version 2, 20 http://chao.stat.nthu.edu.tw/wordpress/software_download/inext-online/
Kassambara A (2021) rstatix: Pipe-friendly framework for basic statistical tests, R package version 0.7.0. https://CRAN.R-project.org/package=rstatix
Kerr JT, Pindar A, Galpern P, Packer L, Potts SG, Roberts SM, Rasmont P, Schweiger O, Colla SR, Richardson LL, Wagner DL, Gall LF, Sikes DS, Pantoja A (2015) Climate change impacts on bumblebees converge across continents. Science 349:177–180. https://doi.org/10.1126/science.aaa7031
Kingsolver JG (1989) Weather and the population dynamics of insects: Integrating physiological and population ecology. Physiol Zool 62:314–334. https://doi.org/10.1086/physzool.62.2.30156173
Knape J, de Valpine P (2011) Effects of weather and climate on the dynamics of animal population time series. Proc R Soc B Biol Sci 278:985–992. https://doi.org/10.1098/rspb.2010.1333
Knapp HD (2007) Buchenwälder als spezifisches Naturerbe Europas. Bfn-Skripten 222:13–39
Köhler F (1996) Käferfauna in Naturwaldzellen und Wirtschaftswald—Vergleichsuntersuchungen im Waldreservat Kermeter in der Nordeifel. Landwirtschaftsverlag, Münster
Köhler F (2010) Die Käfer (Coleoptera) des Naturwaldreservats Goldbachs- und Ziebachsrück (Hessen). Untersuchungszeitraum 1994–1996. Mitteilungen der Hessischen Landesforstverwaltung 46:7–98
Köhler F, Bense U, Fritze M-A, Gürlich S, Köhler J, Schneider A (2019) Waldbindung der Käfer (Coleoptera) Deutschlands. BfN-Skripten 544:115–217. https://doi.org/10.19217/skr544
Köhler F, Flechtner G (2007) Coleoptera (Käfer). In: Dorow WHO, Kopelke J-P (eds) Hohestein - Zoologische Untersuchung 1994–1996, Teil 2. Mitteilungen der Hessischen Landesforstverwaltung 42:103–192.
Köhler F (2014) Die klimabedingte Veränderung der Totholzkäferfauna (Coleoptera) des nördlichen Rheinlandes - Analysen zur Gesamtfauna und am Beispiel von Wiederholungsuntersuchungen in ausgewählten Naturwaldzellen. Wald und Holz NRW, Münster
Konvicka M, Maradova M, Benes J, Fric Z, Kepka P (2003) Uphill shifts in distribution of butterflies in the Czech Republic: effects of changing climate detected on a regional scale. Glob Ecol Biogeogr 12:403–410. https://doi.org/10.1046/j.1466-822X.2003.00053.x
Kotze DJ, O’Hara RB (2003) Species decline—but why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 135:138–148. https://doi.org/10.1007/s00442-002-1174-3
Kulhanek AL (2009) User-friendly methods for timing integrated pest management strategies: an analysis of degree-day models and biological calendars. Master Thesis, The Ohio State University
Lange M, Turke M, Pasalic E, Boch S, Hessenmoller D, Muller J, Prati D, Socher SA, Fischer M, Weisser WW, Gossner MM (2014) Effects of forest management on ground-dwelling beetles (Coleoptera; Carabidae, Staphylinidae) in Central Europe are mainly mediated by changes in forest structure. For Ecol Manage 329:166–176. https://doi.org/10.1016/j.foreco.2014.06.012
Larrieu L, Cabanettes A, Delarue A (2012) Impact of silviculture on dead wood and on the distribution and frequency of tree microhabitats in montane beech-fir forests of the Pyrenees. Eur J Forest Res 131:773–786. https://doi.org/10.1007/s10342-011-0551-z
Larsson T-B (2001) Biodiversity evaluation tools for European forests. Ecol Bull 50:75–81
Leuschner C, Immenroth J (1994) Landschaftsveränderungen in der Lüneburger Heide 1775–1985: Dokumentation und Bilanzierung auf der Grundlage historischer Karten. Archiv Für Naturschutz Und Landschaftsforschung 23:85–139
Liebhold A, Kamata N (2000) Introduction: Are population cycles and spatial synchrony a universal characteristic of forest insect populations? Popul Ecol 42:205–209
Lindenmayer DB, Margules CR, Botkin DB (2000) Indicators of biodiversity for ecologically sustainable forest management. Conserv Biol 14:941–950. https://doi.org/10.1046/j.1523-1739.2000.98533.x
Ludwig G, Haupt H, Gruttke H, Binot-Hafke M (2009) Methodik der Gefärdungsanalyse für Rote Listen. Naturschutz und Biologische Vielfalt 70(1):23–71
Martikainen P, Kouki J (2003) Sampling the rarest: threatened beetles in boreal forest biodiversity inventories. Biodivers Conserv 12:1815–1831. https://doi.org/10.1023/A:1024132829581
Mason SC, Palmer G, Fox R, Gillings S, Hill JH, Thomas CD, Oliver TH (2015) Geographical range margins of many taxonomic groups continue to shift polewards. Biol J Lin Soc 115:586–597. https://doi.org/10.1111/bij.12574
Mazerolle MJ (2020) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c), R package version 2.3–1. https://cran.r-project.org/package=AICcmodavg
McCain CM, Garfinkel CF (2021) Climate change and elevational range shifts in insects. Curr Opin Insect Sci 47:111–118. https://doi.org/10.1016/j.cois.2021.06.003
McGill BJ, Etienne RS, Gray JS, Alonso D, Anderson MJ, Benecha HK, Dornelas M, Enquist BJ, Green JL, He F, Hurlbert AH, Magurran AE, Marquet PA, Maurer BA, Ostling A, Soykan CU, Ugland KI, White EP (2007) Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol Lett 10:995–1015. https://doi.org/10.1111/j.1461-0248.2007.01094.x
McKinney ML (1997) Extinction vulnerability and selectivity: Combining ecological and paleontological views. Annu Rev Ecol Syst 28:495–516. https://doi.org/10.1146/annurev.ecolsys.28.1.495
Meyer P (2013) Wie schnell werden Wirtschaftswälder zu Naturwäldern? AFZ-Der Wald 2013(24):11–13
Meyer P, Schmidt M, Feldmann E, Willig J, Larkin R (2021) Long-term development of species richness in a central European beech (Fagus sylvatica) forest affected by windthrow—Support for the intermediate disturbance hypothesis? Ecol Evol 11:12801–12815. https://doi.org/10.1002/ece3.8028
Morris RF (1959) Single-factor analysis in population dynamics. Ecology 40:580–588. https://doi.org/10.2307/1929811
Müller-Motzfeld G (2006) Freude-Harde-Lohse-Klausnitzer-Die Käfer Mitteleuropas Band 2: Adephaga 1 (Carabidae (Laufkäfer)). Spektrum-Verlag, Berlin
Neckermann C, Neckermann-Achterholt B (2014) Biotop- und Strukturkartierung im Naturwaldreservat Hasenblick (Landkreis Waldeck-Frankenberg). Neckermann-Achterholt Ökologische Gutachten, Göttingen
Nentwig W, Blick T, Bosmans R, Gloor D, Hänggi A, Kropf C (2021) Spiders of Europe. Version 10.2021. https://www.araneae.nmbe.ch. Accessed 27 Oct 2021
Nordén B, Dahlberg A, Brandrud TE, Fritz Ö, Ejrnæs R, Ovaskainen O (2014) Effects of ecological continuity on species richness and composition in forests and woodlands: a review. Ecoscience 21:34–45. https://doi.org/10.2980/21-1-3667
Nunes FA, Segundo GBM, Vasconcelos YB, Azevedo R, Quinet Y (2011) Ground-foraging ants (Hymenoptera: Formicidae) and rainfall effect on pitfall trapping in a deciduous thorn woodland (Caatinga), Northeastern Brazil. Rev Biol Trop 59:1637–1650. https://doi.org/10.15517/rbt.v59i4.3426
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: Community ecology package, R package version 2.5–7. https://CRAN.R-project.org/package=vegan
Ott J (2010) Dragonflies and climatic changes—recent trends in Germany and Europe. BioRisk 5:253–286. https://doi.org/10.3897/biorisk.5.857
Paillet Y, Bergés L, Hjältén J, Ódor P, Avon C, Bernhardt-Römermann M, Bijlsma R-J, De Bruyn L, Fuhr M, Grandin U, Kanka R, Lundin L, Luque S, Magura T, Matesanz S, Mészéros I, Sebastià M-T, Schmidt W, Standovár T, Tóthmérész B, Uotila A, Valladares F, Vellak K, Virtanen R (2009) Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv Biol 24:101–112. https://doi.org/10.1111/j.1523-1739.2009.01399.x
Penell A, Raub F, Höfer H (2018) Estimating biomass from body size of European spiders based on regression models. J Arachnol 46:413–419. https://doi.org/10.1636/JoA-S-17-044.1
Rogers LE, Hinds WT, Buschbom RL (1976) A general weight vs. length relationship for insects. Ann Entomol Soc Am 69:387–389. https://doi.org/10.1093/aesa/69.2.387
Rößner E (2012) Die Hirschkäfer und Blatthornkäfer Ostdeutschlands (Coleoptera: Scarabaeoidea). Verein der Freunde & Förderer des Naturkundemuseums Erfurt e. V., Erfurt
Roth N, Hacker HH, Heidrich L, Friess N, García-Barros E, Habel JC, Thorn S, Müller J (2021) Host specificity and species colouration mediate the regional decline of nocturnal moths in central European forests. Ecography 44:941–952. https://doi.org/10.1111/ecog.05522
Roy DB, Rothery P, Moss D, Pollard E, Thomas JA (2001) Butterfly numbers and weather: predicting historical trends in abundance and the future effects of climate change. J Anim Ecol 70:201–217. https://doi.org/10.1111/j.1365-2656.2001.00480.x
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Cons 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Schaefer M (1999) The diversity of the fauna of two beech forests: some thoughts about possible mechanisms causing the observed patterns. In: Kratochwil A (ed) Biodiversity in Ecosystems. Kluwer, Dodrecht, pp 39–57. https://doi.org/10.1007/978-94-011-4677-7_3
Schaffrath U (2021) Rote Liste und Gesamtartenliste der Blatthornkäfer (Coleoptera: Scarabaeoidea) Deutschlands—3. Fassung, Stand: 10. August 2020. Naturschutz und Biologische Vielfalt 70(5):189–266
Schmidl J, Bense U, Bussler H, Fuchs H, Lange F, Möller G (2021a) Rote Liste und Gesamtartenliste der „Teredilia“ und Heteromera (Coleoptera: Bostrichoidea: lyctidae, Bostrichidae, Anobiidae, Ptinidae; Tenebrionidea) Deutschlands—Stand September 2011. Naturschutz und Biol Vielfalt 70(5):165–186
Schmidl J, Bussler H, Hofmann G, Esser J, Schülke M (2021b) Rote Liste und Gesamtartenliste der Kurzflüglerartigen, Stutzkäferartigen, landbewohnenden Kolbenwasserkäfer und Ufer-Kugelkäfer (Coleoptera: Polyphaga: Staphylinoidea, Histeroidea, Hydrophiloidea partim; Myxophaga: Sphaeriusidae) Deutschlands - Stand September 2020; Nachtrag bis September 2021. Naturschutz und Biologische Vielfalt 70(5):31–95
Schmidl J, Wurst C, Bussler H (2021c) Rote Liste und Gesamtartenliste der „Diversicornia“ (Coleoptera) Deutschlands - Stand September 2011. Naturschutz und Biologische Vielfalt 70(5):99–124
Schmidt J, Trautner J, Müller-Motzfeld G (2016) Rote Liste und Gesamtartenliste der Laufkäfer (Coleoptera: Carabidae) Deutschlands. Naturschutz und Biologische Vielfalt 70(4):139–204
Schmidt M, Meyer P (2022) Hasenblick. Hessische Naturwaldreservate im Portrait. Nordwestdeutsche forstliche Versuchsanstalt (NW-FVA) and Landesbetrieb HessenForst, Göttingen, Kassel
Schneider A, Blick T, Köhler F, Pauls SU, Römbke J, Zub P, Dorow WHO (2021a) Animal diversity in beech forests – An analysis of 30 years of intense faunistic research in Hessian strict forest reserves. For Ecol Manage 499(119564):1–13. https://doi.org/10.1016/j.foreco.2021.119564
Schneider A, Blick T, Pauls SU, Dorow WHO (2021b) The list of forest affinities for animals in Central Europe—a valuable resource for ecological analysis and monitoring in forest animal communities? For Ecol Manag 479(118542):1–8. https://doi.org/10.1016/j.foreco.2020.118542
Seibold S, Brandl R, Buse J, Hothorn T, Schmidl J, Thorn S, Müller J (2015) Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conserv Biol 29:382–390. https://doi.org/10.1111/cobi.12427
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze E-D, Vogt J, Wöllauer S, Weisser WW (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674. https://doi.org/10.1038/s41586-019-1684-3
Siewers J, Schirmel J, Buchholz S (2014) The efficiency of pitfall traps as a method of sampling epigeal arthropods in litter rich forest habitats. Eur J Entomol 111:69–74. https://doi.org/10.14411/eje.2014.008
Simberloff D (1994) The ecology of extinction. Acta Palaeontol Pol 38:159–174
Skvarla MJ, Larson JL, Dowling APG (2014) Pitfalls and preservatives: a review. J the Entomol Soc Ontario 145:15–43
Sprick P, Behne L, Maus C (2021) Rote Liste und Gesamtartenliste der Rüsselkäfer (i. e. S.) Deutschlands (Überfamilie Curculionoidea, exklusive Anthribidae, Scolytidae, Platypodidae)—3. Fassung, Einstufung Stand Mai 2011, Nomenklatur Stand August 2013; vereinzelte inhaltliche Beiträge bis August 2019. Naturschutz und Biologische Vielfalt 70(5):335–412
Topp W, Kappes H, Kulfan J, Zach P (2006) Litter-dwelling beetles in primeval forests of Central Europe: does deadwood matter? J Insect Conserv 10:229–239. https://doi.org/10.1007/s10841-005-3814-5
Varley GC, Gradwell GR (1960) Key factors in population studies. J Anim Ecol 29:399–401. https://doi.org/10.2307/2213
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the anthropocene: death by a thousand cuts. Proc Natl Acad Sci USA 118:1–10. https://doi.org/10.1073/pnas.2023989118
Waite TA, Campbell LG (2006) Controlling the false discovery rate and increasing statistical power in ecological studies. Ecoscience 13:439–442. https://doi.org/10.2980/1195-6860(2006)13[439:CTFDRA]2.0.CO;2
Wallner DE (1987) Factors affecting insect population dynamics: differences between outbreak and non-outbreak species. Annu Rev Entomol 32:317–340. https://doi.org/10.1146/annurev.en.32.010187.001533
Wermelinger B, Moretti M, Duelli P, Lachat T, Pezzatti GB, Obrist MK (2017) Impact of windthrow and salvage-logging on taxonomic and functional diversity of forest arthropods. For Ecol Manage 391:9–18. https://doi.org/10.1016/j.foreco.2017.01.033
Willig J (2002) Natürliche Entwicklung von Wäldern nach Sturmwurf—10 Jahre Forschung im Naturwaldreservat Weiherskopf. Naturwaldreservate in Hessen 8. Mitteilungen Der Hessischen Landesforstverwaltung 38:1–186
Wilson RJ, Gutierrez D, Gutierrez J, Monserrat VJ (2007) An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob Change Biol 13:1873–1887. https://doi.org/10.1111/j.1365-2486.2007.01418.x
Winter S, Möller GC (2008) Microhabitats in lowland beech forests as monitoring tool for nature conservation. For Ecol Manage 255:1251–1261. https://doi.org/10.1016/j.foreco.2007.10.029
Winter S, Begehold H, Herrmann M, Lüderitz M, Möller G, Rzanny M, Flade M (2016) Praxishandbuch—Naturschutz im Buchenwald. Ministerium für Ländliche Entwicklung. Umwelt und Landwirtschaft Brandenburg, Schorfheide-Chorin
Acknowledgements
We wish to thank Michael-Andreas Fritze, Eckersdorf, for the identification of the Carabidae caught during the first survey, the employees of the ÖKOTEAM-Institute, Graz, Austria, for the identification of most of the beetles caught during the second survey, Jens Esser, Berlin, for the identification of some Atomaria-specimens, Jürgen Vogel, Görlitz, for the identification of some Aleocharinae-specimens, and the many people in our team that were involved in field work, sorting trap catches, and identifying the Geotrupidae caught during the second survey. We also wish to thank Viktor Hartung, LWL-Museum für Naturkunde – Westfälisches Museum mit Planetarium, Münster, for his valuable comments on the manuscript and Joachim Dienst, Landesbetrieb HessenForst, Forstamt Frankenberg-Vöhl, for the information on the wildlife stock of the forest district Osterfeld. Additionally, we wish to thank the “Landesbetrieb HessenForst” for the cooperation and financial support. We thank two anonymous reviewers for their constructive comments that helped improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Research was conducted in cooperation with and financially supported by “Landesbetrieb HessenForst”.
Author information
Authors and Affiliations
Contributions
AS: Conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing. TB: Investigation, data curation, writing—review and editing. WD: Conceptualization, methodology, investigation, data curation, writing—review and editing. FK: Investigation, writing—review and editing. PM: Conceptualization, writing—review and editing. SP.: Conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Consent to participate
All authors have agreed to be named as authors.
Consent for publication
All authors have agreed to the version of the manuscript.
Additional information
Communicated by Claus Bässler.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schneider, A., Blick, T., Dorow, W.H.O. et al. Temporal changes in the beetle and spider communities in a Hessian (German) strict forest reserve. Eur J Forest Res 143, 45–64 (2024). https://doi.org/10.1007/s10342-023-01607-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-023-01607-3