Abstract
Catchment-scale transpiration is commonly determined by the use of sap-flow sensors, and its quantification, which is critical for water and forest management, relies crucially on the total catchment’s sapwood area (As). Species-specific allometric relationships between the trees As and the trees diameter at breast height (DBH) are widely used for determining stand or catchment As. However, substantial differences between studies challenge the robustness of these relationships between sites displaying various topographical and environmental characteristics. Our objectives for this study are to compare the parameters of these relationships between species of the Quercus genus from different sites across the globe and to test the role of topographical factors on the As-DBH relationship in Quercus petraea. Using 145 trees sampled within a 0.455 km2 catchment, we found that topography (slope, flow accumulation, aspect, curvature, and topographic wetness index) does not modulate the As-DBH relationship in Q. petraea, within our catchment. We compared our curve parameters with those from 16 studies on oak trees and found that the As-DBH relationship is not only species-specific, but depends on the site’s conditions. The use of species-specific parameters from other sites may lead to more than 100% difference in the calculation of As, and therefore in forest transpiration. In the light of these results, we recommend building site- and species-specific As-DBH relationships for determining stand or catchment transpiration, using a minimum of nine, randomly sampled trees, and different methods and azimuthal directions for determining sapwood depth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The estimation of catchment-scale transpiration remains critical for forest and water management. To date, a widely applied method is based on the use of sap-flow sensors (Hassler et al. 2018; Tsuruta et al. 2019; Mitra et al. 2020). Different types of sensors exist, but most rely on the measurement of sap-flux density in a subset of trees that is most commonly multiplied by the sapwood area of the trees to obtained catchment-scale transpiration (Granier et al. 1996; Flo et al. 2019). Sapwood area therefore plays a crucial role in estimating tree and catchment transpiration (Cermak and Nadezhdina 1998; Hölscher et al. 2005; Meinzer et al. 2005), which motivated numerous studies on sapwood allometric relations (e.g. Wang et al. 2010; Quiñonez-Piñón and Valeo 2017; Lubczynski et al. 2017; Mitra et al. 2020). Commonly, stand sapwood area is derived from empirical relationships with the DBH of the stand’s trees, because sampling all the trees in a catchment/stand is too resource intense (e.g. Hassler et al. 2018; Tsuruta et al. 2019). Given the importance of these empirical relationships for determining forest water use, they have been studied in a wide range of forested ecosystems and on various tree species like temperate broad-leaved species (Gebauer et al. 2008), boreal tree species (Quiñonez-Piñón and Valeo 2017), snow‑dependent tree species (Mitra et al. 2020), tropical rain forest species (Granier et al. 1996; Motzer et al. 2005; Horna et al. 2011; Aparecido et al. 2016; Kunert et al. 2015; Moore et al. 2018), Amazonian tree species (Parolin et al. 2008; Aparecido et al. 2019), desert tree species (Lubczynski et al. 2017), mountainous tree species (Vertessy et al. 1995; Tsuruta et al. 2019) or Australian tree species (Wang et al. 2016) among others. The relationships can take the form of different mathematical equations (e.g. linear, exponential, and hyperbolic). For most species from the Fagaceae family, including the Quercus genus, the relation commonly takes the form of a power-law function (Eq. 1),
where As is the sapwood area of the tree (cm2), DBH is the diameter at breast height (cm), and B0 and B1 are curve parameters.
Methods for determining these empirical relationships can be destructive, like the use of a wood disc taken at breast height (Miranda et al. 2009), or invasive, such as core analysis (Gebauer et al. 2008; Horna et al. 2011), dye-immersed (Parolin et al. 2008; Aparecido et al. 2019) or thermo-imaged fallen trees (Granier et al. 1994). Non-destructive methods also exist and are commonly based on electromagnetic imaging techniques (Čermák et al. 2004; Bieker and Rust 2010a, b; Wang et al. 2016; Benson et al. 2019; Salomón et al. 2020). The allometric relations of trees from the Quercus genus have been extensively studied (Table 1), due to its high natural abundance (Rüther and Walentowski 2008; Petritan et al. 2012) and a broad range of suitable growing conditions (Mette et al. 2013; Pretzsch et al. 2013). Additionally, the sapwood-heartwood limit from most Quercus trees can be distinguished straightforwardly by the darker colour of the heartwood (Mosedale et al. 1996; Taylor et al. 2002), which facilitates the quantification of As within this genus by direct inspection after coring.
Many eco-hydrological studies aiming at quantifying tree or stand water uses rely on so-called species-specific relationships (e.g. Chiu et al. 2016; Hassler et al. 2018; Schoppach et al. 2021). However, different studies on the same species have shown contrasting values for the B0 and B1 parameters (e.g. Aranda et al. 2005; Schmidt 2007; Grossiord et al. 2014), questioning the robustness of these relationships between sites (Horna et al. 2011) and raising concern on the level of difference in sapwood area, when measurements are up-scaled to the stand or catchment level using these parameter values. In the past decades, studies showed that the As-DBH allometric relationships of Japanese cedar and Japanese cypress may be affected by topographical factors as they vary with the slope position of the trees (Kumagai et al. 2007; Kume et al. 2016). These topographical effects may explain the inconsistencies of B0 and B1 between studies. However, topographical effects on As-DBH relationships have never been explored in the Quercus genus, despite the wide use of this relationship for quantifying stand-scale transpiration, especially across Europe. To date, most forest transpiration studies at the catchment scale rely on B0 and B1 parameters from the literature. There is a need for testing the robustness of these parameters between sites, specific species of a given genus, and topographical factors, to provide guidance for researchers and practitioners to build strong As-DBH allometric relationships.
In this study, we aim to fill these gaps by (i) comparing As-DBH allometric relationships between species of the Quercus genus growing in diverse site conditions from 17 field studies, (ii) investigating the role of topographical factors (i.e. slope, flow accumulation, aspect, curvature, and topographic wetness index) on As-DBH allometric relationships of Q. petraea, and (iii) determining the minimum number of samples required for a robust quantification of the As-DBH power-law function for Q. petraea in a temperate European forest.
Material and methods
Study site
The Weierbach is a forested catchment of 0.455 km2 located in north-western Luxembourg (lat 49.827, long 5.795). The forest is a mix of European beech (Fagus sylvatica L.) and pedunculate (Q. robur L.) and sessile oak (Q. petraea (Matt.) Liebl.) (Fabiani et al. 2021; Schoppach et al. 2021). Catchment’s elevation ranges between 458 and 514 m asl, and the geology is composed of Devonian slate and phyllites covered by 60 cm of silt and rock fragments (Juilleret et al. 2011). Distributions of the catchment topographical characteristics can be found in Schoppach et al. (2021).
Measurement of sapwood depth in oak trees and landscape characteristics analysis
During the 2019 and 2020 growing seasons, we measured sapwood depth of oak trees within 145 randomly selected trees in the catchment (Fig. 1b). Sapwood depth was determined by drilling one single core per tree directly coring horizontally, in a randomized azimuthal direction, at breast height. All sampled oak trees are mature trees in the co-dominant strata. Sapwood and heartwood were discriminated based on colour variation (Mosedale et al. 1996; Quiñonez-Piñón and Valeo 2018). Bark was removed from the cores before the sapwood depth was measured. Here, we assumed that the sapwood depth was constant on all azimuthal directions, and the tree trunks were considered as prefect cylinder for calculating As (Gebauer et al. 2008; Hassler et al. 2018).
Location of the study site within Luxembourg (a), location of the wood core samplings within the catchment (b), and 5 m contour lines (c). Panel B: Red circles represent the location of the trees sampled in 2019, and blue circles represent the location of the trees sampled in 2020. Light green line delineates the catchment boundaries. (Color figure online)
We used a high-resolution (1 m) digital elevation model (Luxembourgish air navigation administration 2017) and the Spatial Analysis Toolbox of ArcGIS Desktop 10.5 to determine the topographical characteristics at each sampling location.
We smoothed our raster maps by averaging topographical values of each one-square-metre grid plot with the values of the plots encompassed within a 10 m circle around it, to avoid the influence of small differences from the tree actual position to the analysis. The topographical characteristics within those 10 m circles were extracted for each sampled tree. Two relationships between sapwood depth and DBH were established based on a value criterion for each topographical characteristic. We selected these criteria to obtain roughly balanced (Table 2) and normally distributed groups in terms of DBH (Fig. 2), without significant differences in the means and variances (Table 2). Criteria, number of trees composing each group, and mean ± SE are displayed in Table 2. The slope values represent the steepness of the land surface. The flow accumulation represents the number of m2 of converging area flowing into each downslope area. The aspect is the orientation of the down-hill slope. The curvature indicates if the surface is upwardly convex or concave. The topographic wetness index (TWI) was calculated as follows (Eq. 2):
with flow ac. being the flow accumulation of a m2, a being the size (1 m2), and slope being the slope value in degrees.
Topographical characteristics associated with each group of trees. Left panels display boxplots of the topographical characteristics with horizontal lines representing the minimum, mean and maximum values of DBH. Right panels display the DBH distribution within each group of trees. Solid line filled with red and dashed line not filled delineate the distribution of the different groups. Threshold values defining each group are displayed on each pane. (Color figure online)
We tested the statistical differences between the As-DBH relationships derived from all pairs of datasets (two groups per topographical characteristic) using extra-sum-of-squares F tests and Akaike’s information criterions. For both approaches, we tested the probability that one model with identical B0 and B1 parameters fits the data as well as two models with different B0 and B1 values.
Comparison between A s-DBH allometric relationships from the literature
For determining the significance of the difference in As produced by the application of the various B1 and B0 parameters from the literature, we applied Eq. 1 to our dataset of 145 DBH and compared the resulting As. We used one-way analysis of variance (ANOVA) to determine the significance (p < 0.05) of the species and site effect on the produced As. Given the obvious differences between relationships of different species, we focused our analysis on Q. Petraea datasets, which present relatively similar curves, and ran a Tukey’s multiple comparison test in order to test the significance of the site effect alone.
Power analysis to determine the minimum number of samples required
We used a power analysis to estimate the minimum sample size required to reasonably detect an effect of a given size (Maxwell 2000). The effect size is a number measuring the strength of the relationship between two variables in a sampling panel. In our case, the relationship links As to DBH. Our effect size estimation is based on Lehr’s rule of thumb which states that the sample size n for a two-sided two-sample t test with power 80% (β = 0.2) and significance level (α = 0.05) should be (Lehr 1992; Van Belle 2011):
where Δ is the treatment difference to be detected in units of the standard deviation—the standardized difference. We used “pwr” R package (Champely et al. 2018) to calculate the number of samples needed to get at least the same level of coefficient of determination that we obtained with our 145 samples. As Eq. 1 is not linear, pwr was applied on the relation between As and pAs (the predicted As based on Eq. 1). The coefficient of determination is based on a linear assumption which is not technically appropriate for our nonlinear study, but we use them for a rough performance reference.
Results
Effect of topographical characteristics on A s-DBH relationships
According to the extra-sum-of-squares F test, among all tested topographical characteristics (i.e. slope, flow accumulation, aspect, curvature, and TWI), none produced a significant effect on the As-DBH relationship (Fig. 3). The strongest effect of topographical factor in our dataset was the aspect, but with only a p value of 0.1 between the two models. Results are similar using the Akaike’s information criterion. Except for the aspect, none of the topographical characteristics produced a likely effect.
Effect of the topographical characteristics on the As-DBH allometric relationship. Each circle represents one tree and the colours denote their group within each topographical characteristic. Black and red curves represent the As-DBH allometric relationship (Eq. 1) fitted to each group of data. (Color figure online)
Variations of the B 1 and B 0 coefficients between species of the Quercus genus and sites
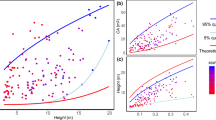
We identified 16 studies in the literature in which the allometric As-DBH relationship was determined for oak trees (Table 1). Within these studies and our additional study, 14 relationships have the shape of a power-law function (Fig. 4), while two others were linear and one exponential (Table 1). The As-DBH relationship was established for 13 different species from the Quercus genus (Fig. 4a, Table 1). We found a highly significant (p value < 0.0001) effect of the studies, which includes species and sites effects, on the different As datasets calculated using B1 and B0 coefficients from the literature and our DBH dataset. The mean As calculated varies by two orders of magnitude (between 56.7 and 6592 cm2) using the most extreme study-specific parameters. Beside our study, four other studies determined the As-DBH relationship on Q. petraea (Aranda et al. 2005; Schmidt 2007; Jonard et al. 2011; Grossiord et al. 2014). Our study has, on average, seven times more samples than the previous studies (145 vs. an average of 20). Our As-DBH relationship presents a R2 36% lower (0.65 vs an average of 0.88). The area covered by the sampling is almost a thousand time larger (904) than the mean area of all the other studies (Table 1). We found a highly significant site effect (p value < 0.0001—Fig. 5) on the calculated As of Q. Petraea. The mean As ranged from 368.5 ± 8.03 cm2 when applying Jonard’s B0 and B1 parameters to 1151 ± 35.0 cm2 when applying Grossiord’s parameters (Table 3).
As-DBH allometric curves determined on Quercus trees. Panel a displays the As-DBH relationships of all Quercus species from literature, while panel b displays the As-DBH relationship on Quercus petraea only. Different colours represent different studies and species (see legend). In panel b, each grey circle represents one of our core measurements. (Color figure online)
Sapwood areas (As) calculated via Eq. 1 using different B0 and B1 parameters from Q. petraea and our 145 DBH dataset. The box extends from the 25th to 75th percentiles. The line in the middle of the box is plotted at the median. Whiskers represent 5th and 95th percentiles. Dots represent data outside of the 5–95 percentiles. Letters denote significant differences between groups
Number of samples required for a reliable quantification of A s-DBH relationship
The power tests indicated that at least nine samples are needed to reach the same level of correlation, or even better, than what we obtained with our 145 samples (n = 8.7, r = 0.81).
Discussion
Topography does not modulate the allometric relation between DBH and sapwood area in Q. petraea at our study site
Topography does not influence the As-DBH allometric relationship in Q. petraea at the Weierbach study site. This contrasts with the results of Kumagai et al. (2007) and Kume et al. (2016) in Japanese cedar and Japanese cypress who found different relationships at different slope positions. Based on our analysis, we can argue that As-DBH relationship in Q. petraea is robust, independent of the topographical position of the sampled trees at the study site. This is a major finding for the robustness of future upscaling analysis relying on this allometric relation. Further research on different species and in different topographical settings is undoubtedly needed as a way forward.
Allometric curves vary between species of the Quercus genus
The allometric As-DBH relationships are commonly described as “species-specific”. In our study, we show that determining As using different Quercus’s B0 and B1 parameters from the literature produce highly significant differences. This site and species specificity of parameters led to a difference of two orders of magnitude in the calculated mean As. Tropical forests typically display a wide variety of species which commonly imposes the use of a single pair of parameters for determining As of a stand (e.g. Moore et al. 2018) with Eq. 1. For the species from the Quercus genus in temperate forests, we demonstrated that this method leads to under- or over-estimate As by up to hundred times. This massive difference confirms and reinforces the requirement of species-specific relationships when upscaling sap-flux density data from a subset of trees to stand or catchment scale.
Quercus petraea’s B 0 and B 1 parameters vary between sites
Our analysis showed that applying B0 and B1 parameters determined in different studies and at different sites on pedunculate oaks resulted in significantly different values of As (Table 3, Fig. 5). Using the parameters from other studies would induce a difference ranging from an overestimation of 107% (Grossiord et al. 2014) to an underestimation of 44% (Jonard et al. 2011) compared to the As determined with our own B0 and B1. These major differences highlight the need for, not only species-specific, but also site-specific allometric relations when estimating sap-flow at stand or catchment level. Similar investigations on larger catchments would help evaluating how far B0 and B1 remain valid.
Interestingly, the use of B0 and B1 parameters from our study and Schmidt (2007) did not result in significant differences in As (Fig. 5). In Jonard et al. (2011), authors found a linear relationship, probably because of the too low DBH range of the sample trees (29–48 cm). Schmidt’s study was carried out in northern Bavaria, less than 300 km from Weierbach. Jonard’s study was carried out in the Belgian Ardennes, the northern site among the five, located within less than 100 km from Weierbach. Aranda’s study was carried out in Spain, and Grossiord’s study took place in Tuscany, both being much further south and in a clearly different climate than the two first cited. One explanation for the significantly different curve parameters within the same species could therefore be the growing conditions effect on the trees allometry. Indeed, higher water demand and lower water availability have been pointed out as a driver of defoliation, which subsequently affects the proportion of sapwood area (e.g. Bréda et al. 2006; Limousin et al. 2012). In Scots pine, summer vapour pressure deficit and maximum temperature have been shown to be negatively correlated with the leaf area/sapwood area ratio across Europe (Mencuccini and Bonosi 2001). Sapwood proportion is suspected to depend on the availability of light (Sellin 1994; Thurner et al. 2019) and the water demand (Gebauer et al. 2008; Horna et al. 2011). Trees exposed to higher level of light and potential evapotranspiration require to maximize sapwood area for sustaining the higher evaporative demand and thus preventing leaf overheating and dehydration (Aparecio et al. 2019). Importantly, the hydraulic conductivity of sapwood is also influenced by growth rate which depends on resources availability and demand (Medhurst and Beadle 2002). The total annual precipitation amount, the mean annual temperature, and the tree densities varies between the five above-mentioned studies on Q. petraea (Table 2). Total radiation received by each site and the associated potential evapotranspiration are not available in the original papers. However, Spanish and Tuscan sites are likely to receive higher level of annual radiation and are exposed to higher evaporative demands due to their southern locations compared with Luxembourgish, Belgian, and German sites (Cornes et al. 2018). Sites with lower tree densities present lower sapwood area for a given DBH, which contrasts with the findings of Benyon et al. (2015) in eucalypt forests, showing a decrease in sapwood thickness with an increase in forest density. Higher forest density is supposed to reduce the exposition to high radiation and VPD conditions, which influences the development of sapwood. Here, we cannot decipher the various effects of the growing conditions on the As-DBH relationship based on these annual data. Further research is certainly needed to unravel the role of temperature, radiation, water demand, water availability, and forest density on the As-DBH allometric relationship on Q. petraea.
Number of samples required for a reliable quantification of A s-DBH relationship
For upscaling purposes, a reliable quantification of the As-DBH allometric relationship remains crucial as it appeared that Eq. 1 parameters are constrained not only by species, but also by sites/stand characteristics. We thus recommend to experimentally determine Eq. 1 parameters for each site rather than using values from literature. According to our power test, at least nine samples are needed for building a robust As-DBH relationship. Importantly, the sampled trees should cover a range of DBH that is as wide as possible to avoid misinterpretation of the curve shape (e.g. Jonard et al. 2011). This number of samples is close to or lower than the number of samples most studies on Quercus genus used (Table 1). Therefore, Eq. 1 parameters extracted from these studies can be considered as robust for the species and sites concerned. However, the As-DBH allometric relation calculated in this study displays a substantially lower R2 compared to previous studies, which potentially pools down the required number of samples.
Confounding effects and methodological caveats
In this study, we were not able to highlight significant topographical effects on the As-DBH relationship of Q. petraea. Also, we determined an As-DBH relationship displaying a substantially lower R2 compared to literature. Confounding effects may be responsible for the lack of significance of the topographical effects and the lower R2. Confounding effects may include a much larger sampling area than other studies with higher unexplained variances and random errors, a higher number of sampled trees, a wider range of tree’s DBH, or methodological limitations.
A methodological limitation that may introduce some deviation of the measurement from the As-DBH curve is that As of each tree was inferred from a single core sampled at a random azimuth. We assumed the sapwood depth to be constant on all azimuths (Čermák et al. 2004; Tsuruta et al. 2010; Benson et al. 2019). Sapwood area was indeed inferred from a single core taken at random azimuth because we had no authorization from the forest owner to core each tree more than once during the experiment. However, the radial profile of sapwood thickness is suspected to be variable (e.g. Looker et al. 2016), especially in regard to slope (Pilate et al. 2004). Indeed, trees growing on a slope will produce tension wood to counteract the forces of gravity (Scurfield 1973; Pilate et al. 2004). This alters the production of wood, and presumably the sapwood thickness around the circumference of the tree, which could be a substantial confounding effect. The variability in radial sap-flux densities was found to be considerable in temperate broad-leaved species (Gebauer et al. 2008). For boreal trees, radial profile of sapwood thickness was shown to be dependent on the species; some species displaying constant sapwood depth, while others are growing thicker on the North-East side (Quiñonez-Piñón and Valeo 2017). For trembling aspen and white spruce, the influence of azimuthal direction on sapwood depth had a low impact (Merlin et al. 2020). In oak trees, the xylem vessel size was found to be larger on the northern side of Quercus suber, compared with the southern side (Barij et al. 2011), but, to our knowledge, the influence of azimuthal direction on sapwood depth itself remains unexplored in Q. petraea.
Topographical effect may also be confounded by the threshold values selected for comparing groups, which was imposed by catchment characteristics and done in such a way to force similar sample sizes, but was not necessarily tied to meaningful physical attributes. Further research on catchments displaying larger ranges of topographical characteristics could help untangling these effects.
Within studies on Q. petraea displaying power-law functions, Aranda et al. (2005), Schmidt (2007), and Grossiord et al. (2014) had a difference between their smaller and larger DBH of 14, 20, and 36 cm, respectively. Our dataset displayed a difference of 61 cm and our number of sampled trees was substantially higher. Also, our panel encompassed many trees with large DBH, while both Schmidt (2007) and Grossiord et al. (2014) had no trees larger than 50 cm. This value falls at 19 cm for Aranda et al. (2005). However, excluding the 85 trees larger than 50 cm in DBH from our dataset led to a substantially lower R2 (0.28) of the As-DBH relationship. Larger range of DBH within the sampled trees therefore did not explain the low R2 value displayed by our relationship.
Our dataset presents sapwood depth values varying between 10 and 86 mm with a mean of 42 mm. If we consider a deviation of 1 mm due to a recurrent measurement error, this one mm deviation would result in a deviation of 2.17% (14 cm2) of the As of a tree with average DBH (53 cm). For our smallest tree (DBH = 29.3 cm), the deviation increases to 9% (8.5 cm2). However, the potential effect of a systematic measurement mistake remains far too weak for explaining the observed spread of the points around the allometric curve (Fig. 3b). Dyeing experiments suggest that As is likely susceptible to be overestimated by the determination solely on heartwood coloration (Aparecido et al. 2019), despite other studies contradicting this suggestion (e.g. Githiomi and Dougal 2012). Experiments using dye are able to differentiate between observable sapwood and the functional conducting sapwood, the latter being typically lower. For this reason, we recommend the use of variable methods to validate the delimitation of sapwood.
The influence of the sampling area cannot be ignored. Most studies presented in Table 1 aimed at quantifying stand transpiration, and their main objectives was not to determine the As-DBH allometric relationship, which is commonly done only on few trees outside the field setup. In our experiment, we sampled within a catchment of 0.455 km2, which is by far wider than all previous studies on oak trees. Therefore, our As-DBH curve is likely to be influenced by non-accounted micro-climatic conditions, influencing the resources availability (i.e. water and radiation) and the evaporative demand.
Conclusion
Sapwood area plays a key role for determining sap-flow of a tree and upscaling sap-flow measurements from tree to stand or catchment level. A reliable quantification of sapwood area is therefore crucial for water and forest management. In this study, we confirmed that the As-DBH allometric relationship of the Quercus genus is not only species-specific, but depends on the site conditions. We showed that topography does not modulate the As-DBH relationship in Q. petraea, within our catchment. In the light of these results, we emphasize that each upscaling study should rely on its own site- and species-specific relationship and recommend a minimum of nine, randomly sampled trees, in order to build a robust As-DBH relationship. Due to the potentially high variability in radial profile of sapwood thickness and the caveats related to colour distinction, we recommend coring at different azimuthal locations in the trees and validating the delimitation of sapwood via different methods. Further research is certainly needed for evaluation on how far B0 and B1 parameters remain valid.
Data availability
The data used in this study are the property of the Luxembourg Institute of Science and Technology (LIST) and can be obtained upon request to the corresponding author, after approval by LIST.
Code availability
Not applicable
References
Aparecido LMT, Miller GR, Cahill AT, Moore GW (2016) Comparison of tree transpiration under wet and dry canopy conditions in a Costa Rican premontane tropical forest. Hydrol Process 30(26):5000–5011. https://doi.org/10.1002/hyp.10960
Aparecido LMT, Santos JD, Higuchi N, Kunert N (2019) Relevance of wood anatomy and size of Amazonian trees in the determination and allometry of sapwood area. Acta Amazon 49:1–10. https://doi.org/10.1590/1809-4392201800961
Aranda I, Gil L, Pardos JA (2005) Seasonal changes in apparent hydraulic conductance and their implications for water use of European beech (Fagus sylvatica L.) and sessile oak [Quercus petraea (Matt.) Liebl] in South Europe. Plant Ecol 179(2):155–167. https://doi.org/10.1007/s11258-004-7007-1
Barij N, Čermák J, Stokes A (2011) Azimuthal variations in xylem structure and water relations in cork oak (Quercus suber). IAWA J 32(1):25–40. https://doi.org/10.1163/22941932-90000040
Benson AR, Koeser AK, Morgenroth J (2019) Estimating conductive sapwood area in diffuse and ring porous trees with electronic resistance tomography. Tree Physiol 39(3):484–494. https://doi.org/10.1093/treephys/tpy092
Benyon RG, Lane PNJ, Jaskierniak D, Kuczera G, Haydon SR (2015) Use of a forest sapwood area index to explain long-term variability in mean annual evapotranspiration and streamflow in moist eucalypt forests. Water Resour Res 51(7):5318–5331. https://doi.org/10.1002/2015WR017321
Bieker D, Rust S (2010a) Non-destructive estimation of sapwood and heartwood width in Scots pine (Pinus sylvestris L.). Silva Fenn 44(2):267–273. https://doi.org/10.14214/sf.153
Bieker D, Rust S (2010b) Electric resistivity tomography shows radial variation of electrolytes in Quercus robur. Can J for Res 40(6):1189–1193. https://doi.org/10.1139/x10-076
Bovard BD, Curtis PS, Vogel CS, Su HB, Schmid HP (2005) Environmental controls on sap flow in a northern hardwood forest. Tree Physiol 25(1):31–38. https://doi.org/10.1093/treephys/25.1.31
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63(6):625–644. https://doi.org/10.1051/forest:2006042
Čermák J, Kučera J, Nadezhdina N (2004) Sap flow measurements with some thermodynamic methods, flow integration within trees and scaling up from sample trees to entire forest stands. Trees 18(5):529–546. https://doi.org/10.1007/s00468-004-0339-6
Cermak J, Nadezhdina N (1998) Sapwood as the scaling parameter-defining according to xylem water content or radial pattern of sap flow? In: Annales des sciences forestieres, vol 55, No. 5. EDP Sciences, pp 509–552. https://doi.org/10.1051/forest:19980501
Champely S, Ekstrom C, Dalgaard P, Gill J, Weibelzahl S, Anandkumar A, De Rosario MH (2018) Package ‘pwr’. R package version. 1(2)
Chiu CW, Komatsu H, Katayama A, Otsuki K (2016) Scaling-up from tree to stand transpiration for a warm-temperate multi-specific broadleaved forest with a wide variation in stem diameter. J For Res 21(4):161–169. https://doi.org/10.1007/s10310-016-0532-7
Cornes RC, van der Schrier G, van den Besselaar EJ, Jones PD (2018) An ensemble version of the E-OBS temperature and precipitation data sets. J Geophys Res Atmos 123(17):9391–9409. https://doi.org/10.1029/2017JD028200
Fabiani G, Schoppach R, Penna D, Klaus J (2021) Transpiration patterns and water use strategies of beech and oak trees along a hillslope. Ecohydrology 15(2):e2382. https://doi.org/10.1002/eco.2382
Flo V, Martinez-Vilalta J, Steppe K, Schuldt B, Poyatos R (2019) A synthesis of bias and uncertainty in sap flow methods. Agric For Meteorol 271:362–374. https://doi.org/10.1016/j.agrformet.2019.03.012
Gebauer T, Horna V, Leuschner C (2008) Variability in radial sap flux density patterns and sapwood area among seven co-occurring temperate broad-leaved tree species. Tree Physiol 28(12):1821–1830. https://doi.org/10.1093/treephys/28.12.1821
Githiomi JK, Dougal E (2012) Analysis of heartwood–sapwood demarcation methods and variation of sapwood and heartwood within and between 15 year old plantation grown Eucalyptus regnans. Int J Appl Sci Technol 2(8)
Granier A, Anfodillo T, Sabatti M, Cochard H, Dreyer E, Tomasi M, Bréda N (1994) Axial and radial water flow in the trunks of oak trees: a quantitative and qualitative analysis. Tree Physiol 14(12):1383–1396. https://doi.org/10.1093/treephys/14.12.1383
Granier A, Biron P, Breda N, Pontailler JY, Saugier B (1996) Transpiration of trees and forest stands: short and long-term monitoring using sapflow methods. Glob Change Biol 2(3):265–274. https://doi.org/10.1111/j.1365-2486.1996.tb00078.x
Grossiord C, Gessler A, Granier A, Pollastrini M, Bussotti F, Bonal D (2014) Interspecific competition influences the response of oak transpiration to increasing drought stress in a mixed Mediterranean forest. For Ecol Manage 318:54–61. https://doi.org/10.1016/j.foreco.2014.01.004
Hassler SK, Weiler M, Blume T (2018) Tree-, stand- and site-specific controls on landscape-scale patterns of transpiration. Hydrol Earth Syst Sci 22(1):13–30. https://doi.org/10.5194/hess-22-13-2018
Hölscher D, Koch O, Korn S, Leuschner C (2005) Sap flux of five co-occurring tree species in a temperate broad-leaved forest during seasonal soil drought. Trees 19(6):628–637. https://doi.org/10.1007/s00468-005-0426-3
Horna V, Schuldt B, Brix S, Leuschner C (2011) Environment and tree size controlling stem sap flux in a perhumid tropical forest of Central Sulawesi Indonesia. Ann for Sci 68(5):1027–1038. https://doi.org/10.1007/s13595-011-0110-2
Jonard F, André F, Ponette Q, Vincke C, Jonard M (2011) Sap flux density and stomatal conductance of European beech and common oak trees in pure and mixed stands during the summer drought of 2003. J Hydrol 409(1–2):371–381. https://doi.org/10.1016/j.jhydrol.2011.08.032
Juilleret J, Iffly JF, Pfister L, Hissler C (2011) Remarkable Pleistocene periglacial slope deposits in Luxembourg (Oesling): pedological implication and geosite potential. Bull Soc Nat Luxemb 112:125–130
Jung EY, Otieno D, Lee B, Lim JH, Kang SK, Schmidt MWT, Tenhunen J (2011) Up-scaling to stand transpiration of an Asian temperate mixed-deciduous forest from single tree sapflow measurements. Plant Ecol 212(3):383–395. https://doi.org/10.1007/s11258-010-9829-3
Kumagai TO, Aoki S, Shimizu T, Otsuki K (2007) Sap flow estimates of stand transpiration at two slope positions in a Japanese cedar forest watershed. Tree Physiol 27(2):161–168. https://doi.org/10.1093/treephys/27.2.161
Kume T, Tsuruta K, Komatsu H, Shinohara Y, Katayama A, Ide JI, Otsuki K (2016) Differences in sap flux-based stand transpiration between upper and lower slope positions in a Japanese cypress plantation watershed. Ecohydrology 9(6):1105–1116. https://doi.org/10.1002/eco.1709
Kunert N, Aparecido LMT, Higuchi N, dos Santos J, Trumbore S (2015) Higher tree transpiration due to road-associated edge effects in a tropical moist lowland forest. Agric For Meteorol 213:183–192. https://doi.org/10.1016/j.agrformet.2015.06.009
Lehr R (1992) Sixteen S-squared over D-squared: a relation for crude sample size estimates. Stat Med 11(8):1099–1102. https://doi.org/10.1002/sim.4780110811
Limousin JM, Rambal S, Ourcival JM, Rocheteau A, Joffre R, Rodriguez-Cortina R (2009) Long-term transpiration change with rainfall decline in a Mediterranean Quercus ilex forest. Glob Change Biol 15(9):2163–2175. https://doi.org/10.1111/j.1365-2486.2009.01852.x
Limousin JM, Rambal S, Ourcival JM, Rodriguez-Calcerrada J, Perez-Ramos I, Rodriguez-Cortina R et al (2012) Morphological and phenological shoot plasticity in a Mediterranean evergreen oak facing long-term increased drought. Oecologia 169(2):565–577. https://doi.org/10.1007/s00442-011-2221-8
Looker N, Martin J, Jencso K, Hu J (2016) Contribution of sapwood traits to uncertainty in conifer sap flow as estimated with the heat-ratio method. Agric For Meteorol 223:60–71. https://doi.org/10.1016/j.agrformet.2016.03.014
Lubczynski MW, Chavarro-Rincon DC, Rossiter DG (2017) Conductive sapwood area prediction from stem and canopy areas—allometric equations of Kalahari trees Botswana. Ecohydrology 10(6):e1856. https://doi.org/10.1002/eco.1856
Luxembourgish air navigation administration (2017) https://data.public.lu/fr/datasets/digital-surface-model-high-dem-resolution/Downloaded. Accessed 15 May 2020
Martin JG, Kloeppel BD, Schaefer TL, Kimbler DL, McNulty SG (1998) Aboveground biomass and nitrogen allocation of ten deciduous southern Appalachian tree species. Can J For Res 28(11):1648–1659. https://doi.org/10.1139/x98-146
Maxwell SE (2000) Sample size and multiple regression analysis. Psychol Methods 5(4):434. https://doi.org/10.1037/1082-989X.5.4.434
Medhurst JL, Beadle CL (2002) Sapwood hydraulic conductivity and leaf area—sapwood area relationships following thinning of a Eucalyptus nitens plantation. Plant Cell Environ 25:1011–1019. https://doi.org/10.1046/j.1365-3040.2002.00880.x
Meinzer FC, Bond BJ, Warren JM, Woodruff DR (2005) Does water transport scale universally with tree size? Funct Ecol 19(4):558–565. https://doi.org/10.1111/j.1365-2435.2005.01017.x
Mencuccini M, Bonosi L (2001) Leaf/sapwood area ratios in Scots pine show acclimation across Europe. Can J For Res 31:442–456. https://doi.org/10.1139/x00-173
Merlin M, Solarik KA, Landhäusser SM (2020) Quantification of uncertainties introduced by data-processing procedures of sap flow measurements using the cut-tree method on a large mature tree. Agric For Meteorol 287:107926. https://doi.org/10.1016/j.agrformet.2020.107926
Mette T, Dolos K, Meinardus C, Bräuning A, Reineking B, Blaschke M, Wellstein C (2013) Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 4(12):1–19. https://doi.org/10.1890/ES13-00115.1
Miranda I, Gominho J, Pereira H (2009) Variation of heartwood and sapwood in 18-year-old Eucalyptus globulus trees grown with different spacings. Trees 23(2):367–372. https://doi.org/10.1007/s00468-008-0285-9
Mitra B, Papuga SA, Alexander MR, Swetnam TL, Abramson N (2020) Allometric relationships between primary size measures and sapwood area for six common tree species in snow-dependent ecosystems in the Southwest United States. J for Res 31(6):2171–2180. https://doi.org/10.1007/s11676-019-01048-y
Moore GW, Orozco G, Aparecido LM, Miller GR (2018) Upscaling transpiration in diverse forests: insights from a tropical premontane site. Ecohydrology 11(3):e1920. https://doi.org/10.1002/eco.1920
Mosedale JR, Charrier B, Janin G (1996) Genetic control of wood colour, density and heartwood ellagitannin concentration in European oak (Quercus petraea and Q. robur). For an Int J for Res 69(2):111–124. https://doi.org/10.1093/forestry/69.2.111
Motzer T, Munz N, Küppers M, Schmitt D, Anhuf D (2005) Stomatal conductance, transpiration and sap flow of tropical montane rain forest trees in the southern Ecuadorian Andes. Tree Physiol 25(10):1283–1293. https://doi.org/10.1093/treephys/25.10.1283
Parolin P, Müller E, Junk WJ (2008) Sapwood area in seven common tree species of Central Amazon floodplains. Area Pesqui Bot 59:277–286
Petritan AM, Biris IA, Merce O, Turcu DO, Petritan IC (2012) Structure and diversity of a natural temperate sessile oak (Quercus petraea L.)—European Beech (Fagus sylvatica L.) forest. For Ecol Manage 280:140–149. https://doi.org/10.1016/j.foreco.2012.06.007
Pilate G, Chabbert B, Cathala B, Yoshinaga A, Leplé JC, Laurans F, Ruel K (2004) Lignification and tension wood. CR Biol 327(9–10):889–901. https://doi.org/10.1016/j.crvi.2004.07.006
Poyatos R, Čermák J, Llorens P (2007) Variation in the radial patterns of sap flux density in pubescent oak (Quercus pubescens) and its implications for tree and stand transpiration measurements. Tree Physiol 27(4):537–548. https://doi.org/10.1093/treephys/27.4.537
Pretzsch H, Schütze G, Uhl E (2013) Resistance of European tree species to drought stress in mixed versus pure forests: evidence of stress release by inter-specific facilitation. Plant Biol 15(3):483–495. https://doi.org/10.1111/j.1438-8677.2012.00670.x
Quiñonez-Piñón MR, Valeo C (2017) Allometry of sapwood depth in five boreal trees. Forests 8(11):457. https://doi.org/10.3390/f8110457
Quiñonez-Piñón MR, Valeo C (2018) Assessing the translucence and color-change methods for estimating sapwood depth in three boreal species. Forests 9(11):686. https://doi.org/10.3390/f9110686
Rüther C, Walentowski H (2008) Tree species composition and historic changes of the Central European oak/beech region. In: Canopy arthropod research in Europe, pp 61–88
Salomón RL, Steppe K, Ourcival JM, Villers S, Rodríguez-Calcerrada J, Schapman R, Limousin JM (2020) Hydraulic acclimation in a Mediterranean oak subjected to permanent throughfall exclusion results in increased stem hydraulic capacitance. Plant Cell Environ 43(6):1528–1544. https://doi.org/10.1111/pce.13751
Schmidt M (2007) Canopy transpiration of beech forests in Northern Bavaria–Structure and function in pure and mixed stands with oak at colline and montane sites. Doctoral dissertation
Schoppach R, Chun KP, He Q, Fabiani G, Klaus J (2021) Species-specific control of DBH and landscape characteristics on tree-to-tree variability of sap velocity. Agric For Meteorol 307:108533. https://doi.org/10.1016/j.agrformet.2021.108533
Scurfield G (1973) Reaction Wood: its Structure and Function: lignification may generate the force active in restoring the trunks of leaning trees to the vertical. Science 179(4074):647–655. https://doi.org/10.1126/science.179.4074.647
Sellin A (1994) Sapwood–heartwood proportion related to tree diameter, age, and growth rate in Picea abies. Can J For Res 24(5):1022–1028. https://doi.org/10.1139/x94-133
Taylor AM, Gartner BL, Morrell JJ (2002) Heartwood formation and natural durability—a review. Wood Fiber Sci 34:587–611
Thurner M, Beer C, Crowther T, Falster D, Manzoni S, Prokushkin A, Schulze ED (2019) Sapwood biomass carbon in northern boreal and temperate forests. Glob Ecol Biogeogr 28(5):640–660. https://doi.org/10.1111/geb.12883
Tsuruta K, Kume T, Komatsu H, Higashi N, Umebayashi T, Kumagai TO, Otsuki K (2010) Azimuthal variations of sap flux density within Japanese cypress xylem trunks and their effects on tree transpiration estimates. J For Res 15(6):398–403. https://doi.org/10.1007/s10310-010-0202-0
Tsuruta K, Komatsu H, Kume T, Otsuki K, Kosugi Y, Kosugi KI (2019) Relationship between stem diameter and transpiration for Japanese cypress trees: implications for estimating canopy transpiration. Ecohydrology 12(5):e2097. https://doi.org/10.1002/eco.2097
Van Belle G (2011) Statistical rules of thumb, vol 699. Wiley
Vertessy RA, Benyon RG, O’sullivan SK, Gribben PR (1995) Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Physiol 15(9):559–567. https://doi.org/10.1093/treephys/15.9.559
Wang X, Wang C, Zhang Q, Quan X (2010) Heartwood and sapwood allometry of seven Chinese temperate tree species. Ann For Sci 67(4):410. https://doi.org/10.1051/forest/2009131
Wang H, Guan H, Guyot A, Simmons CT, Lockington DA (2016) Quantifying sapwood width for three Australian native species using electrical resistivity tomography. Ecohydrology 9(1):83–92. https://doi.org/10.1002/eco.1612
Wullschleger SD, Hanson PJ (2006) Sensitivity of canopy transpiration to altered precipitation in an upland oak forest: evidence from a long-term field manipulation study. Glob Change Biol 12(1):97–109. https://doi.org/10.1111/j.1365-2486.2005.001082.x
Wullschleger SD, Hanson PJ, Todd DE (2001) Transpiration from a multi-species deciduous forest as estimated by xylem sap flow techniques. For Ecol Manag 143(1–3):205–213. https://doi.org/10.1016/S0378-1127(00)00518-1
Zhu L, Hu Y, Zhao X, Zeng X, Zhao P, Zhang Z, Ju Y (2017) The impact of drought on sap flow of cooccurring Liquidambar formosana Hance and Quercus variabilis Blume in a temperate forest. Cent China Ecohydrol 10(4):e1828. https://doi.org/10.1002/eco.1828
Acknowledgements
This work was supported by the Luxembourg National Research Fund (FNR/CORE/C17/SR/11702136/EFFECT). KP Chun is supported by the Accelerator Programme (AP) 2022-24 and the Starter Scheme by the University of West of England, Bristol. We thank the members of the Catchment and Eco-hydrology group at LIST, and especially Jean-François Iffly and Jérome Juilleret for their support in field work. We thank the Luxembourgish air navigation administration for providing the Digital Elevation Model.
Funding
This work was supported by the Luxembourg National Research Fund (FNR/CORE/C17/SR/11702136/EFFECT).
Author information
Authors and Affiliations
Contributions
RS contributed to conceptualization, investigation, data analysis, data visualization, and writing original draft and editing. KPC performed data analysis and reviewing and editing. JK contributed to funding acquisition, supervision, reviewing and editing, and project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
Consent for publication
Not applicable
Ethical approval
Not applicable
Consent to participate
Not applicable
Additional information
Communicated by Ruediger Grote.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schoppach, R., Chun, K.P. & Klaus, J. Allometric relations between DBH and sapwood area for predicting stand transpiration: lessons learned from the Quercus genus. Eur J Forest Res 142, 797–809 (2023). https://doi.org/10.1007/s10342-023-01558-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-023-01558-9