Abstract
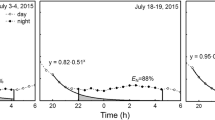
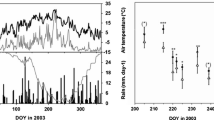
The water status of Fagus sylvatica L. and Quercus petraea (Matt) Liebl. was analysed during a cycle of progressive natural drought in southern Europe. Predawn (Ψpd) and midday water potential were measured in transpiring (Ψleaf) and non-transpiring leaves (Ψxyl). Furthermore, photosynthesis (A), stomatal conductance to water vapour (gs) and sap flow (Fd) were recorded on the same dates. Apparent leaf specific hydraulic conductance in the soil–plant–air continuum (Kh) and whole tree hydraulic conductance (Khsf) were calculated by using the simple analogy of the Ohm’s law. Kh was estimated at different points in the pathway as the ratio between transpiration (E) in the uppermost canopy leaves at midday and the gradient of water potential in the different compartments of the continuum soil–roots–stem–branches–leaves. There was a progressive decrease in water potential measured on non-transpiring leaves at the base of tree crown in both species (Ψlxyl) from the beginning of the growing season to the end of summer. A similar decrease was shown in shoot water potential (Ψuxyl) at the uppermost canopy. Predawn water potential (Ψpd) was high in both species until late July (28 July); afterwards, a significant decrease was registered in F. sylvatica and Q. petraea with minimum values of −0.81±0.03 and −0.75±0.06 MPa, respectively, by 15 September. In both species, leaf specific hydraulic conductance in the overall continuum soil–plant–air (Kh) decreased progressively as water stress increases. Minimum values of Kh and Khsf were recorded when Ψpd was lower. However, Q. petraea showed higher Kh than F. sylvatica for the same Ψpd. The decrease in Kh with water stress was mainly linked to its fall from the soil to the lowermost canopy (Ksrs). Nevertheless, a significant resistance in the petiole–leaf lamina (Kpl) was also recorded because significant differences in all dates were found on Ψ between transpiring and non-transpiring leaves from the same shoot. The decline in Kh was followed by an increase in stomatal control of daily water losses through the decrease of stomatal conductance to water vapour (gs) during the day. It promoted a seasonal increase in the stomatal limitation to carbon dioxide uptake for photosynthesis (A). These facts were more relevant in F. sylvatica, which had concurrently a higher decline in water use at the tree level than Q. petraea. The results showed a strong coupling in F. sylvatica and Q. petraea between processes at leaf and tree level. It may be hypothesised a role of specific hydraulic conductance not only in the regulation of water losses by transpiration but also of carbon uptake.
Similar content being viewed by others
References
N.N. Alder J.S. Sperry W.T. Pockman (1996) ArticleTitleRoot and stem xylem embolismstomatal conductance and leaf turgor in Acer grandidentatum populations along a soil moisture gradient Oecologia 105 293–301 Occurrence Handle10.1007/BF00328731
I. Aranda L. Gil J.A. Pardos (2000) ArticleTitleWater relations and gas exchange in Fagus sylvatica L. and Quercus petraea (Mattuschka) Liebl. in a mixed stand at their southern limit of distribution in Europe Trees, Struct. Funct. 14 344–452
F. Barataud C. Moyne N. Bréda A. Granier (1995) ArticleTitleSoil water dynamics in an oak stand. I. Soil moisturewater potentials and water uptake by roots Plant Soil 172 29–43 Occurrence Handle10.1007/BF00020857 Occurrence Handle1:CAS:528:DyaK2MXmsVagt7Y%3D
J.E. Begg N.C. Turner (1970) ArticleTitleWater potential gradients in field tobacco Plant Physiol. 46 343–346
N. Bréda H. Cochard E. Dreyer A. Granier (1993) ArticleTitleField comparison of transpiration, stomatal conductance and vulnerability to cavitation of Quercus petraeaQuercus robur under water stress Ann. Sci. Forest. 50 571–582
B. Bond K.L. Kavanagh (1999) ArticleTitleStomatal behaviour of four woody species in relation to leaf-specific hydraulic conductance and threshold water potential Tree Physiol. 19 503–510 Occurrence Handle12651540
J.S. Boyer (1985) ArticleTitleWater transport Annu. Rev. Plant Physiol. Plant Mol. Biol. 36 437–516 Occurrence Handle10.1146/annurev.arplant.36.1.473
N. Bréda A. Granier F. Barataud C. Moyne (1995) ArticleTitleSoil water dynamics in an oak stand. I. Soil moisturewater potentials and water uptake by roots Plant Soil 172 29–43 Occurrence Handle10.1007/BF00020857 Occurrence Handle1:CAS:528:DyaK2MXmsVagt7Y%3D
M.J. Clearwater F.C. Meinzer J.L. Andrade G. Goldstein N.M. Holbrook (1999) ArticleTitlePotential errors in measurements of non-uniform sap flow using heat dissipation probes Tree Physiol. 19 681–687 Occurrence Handle12651324
H. Cochard N. Bréda A. Granier G. Aussenac (1992) ArticleTitleVulnerability to air embolism of three European oak species (Quercus petraea (Matt) Liebl, Q. pubescens WilldQ. robur L) Ann. Sci. Forest. 49 225–233
H. Cochard N. Bréda A. Granier (1996) ArticleTitleWhole tree hydraulic conductance and water loss regulation in Quercus during drought: evidence for stomatal control of embolism? Ann. Sci. Forest. 53 197–206
H. Cochard D. Lemoine E. Dreyer (1999) ArticleTitleThe effects of acclimation to sunlight on the xylem vulnerability to embolism in Fagus sylvatica L Plant Cell Environ. 22 101–108 Occurrence Handle10.1046/j.1365-3040.1999.00367.x
J.P. Comstock (2000) ArticleTitleVariation in hydraulic architecture and gas-exchange in two desert sub-shrubs, Hymenoclea salsola (T. & G.) and Ambrosia dumosa (Payne) Oecologia 125 1–10
J.L. Croker W.T. Witte R.M. Augé (1998) ArticleTitleStomatal sensitivity of six temperatedeciduous tree species to non-hydraulic root-to-shoot signalling of partial soil drying J. Exp. Bot. 49 761–774 Occurrence Handle10.1093/jexbot/49.321.761 Occurrence Handle1:CAS:528:DyaK1cXivV2ntbs%3D
W.J. Davies J. Zhang (1991) ArticleTitleRoot signals and the regulation of growth and development of plants in drying soil Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 55–76 Occurrence Handle10.1146/annurev.pp.42.060191.000415 Occurrence Handle1:CAS:528:DyaK3MXltFSmsr8%3D
T.E. Dawson (1996) ArticleTitleDetermining water use by trees and forests from isotopic, energy balance and transpiration analyses: the roles of tree size and hydraulic lift Tree Physiol. 16 263–272 Occurrence Handle14871771
E.E. Fuchs N.J. Livingston (1996) ArticleTitleHydraulic control of stomatal conductance in Douglas fir [Pseudotsuga menziesii (Mirb.) Franco] and alder [Alnus rubra (Bong)] seedlings Plant Cell Environ. 19 1091–1098
M. Goulden (1996) ArticleTitleCarbon assimilation and water-use efficiency by neighboring Mediterranean-climate oaks that differ in water access Tree Physiol. 16 417–424 Occurrence Handle1:CAS:528:DyaK28XitlGqtbw%3D Occurrence Handle14871727
D.J.G. Gowing W.J. Davies H.G. Jones (1990) ArticleTitleA positive root-sourced signal as an indicator of soil drying in appleMalus x domestica Borkh J. Exp. Bot. 41 1535–1540
A. Granier (1985) ArticleTitleUne nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres Ann. Sci. Forest. 42 193–200
A. Granier (1987) ArticleTitleMesure du flux de sève dans le tronc du Douglas par une nouvelle méthode thermique Ann. Sci. Forest. 42 193–200
U. Hacke J.J. Sauter (1995) ArticleTitleVulnerability of xylem to embolism in relation to leaf water potential and stomatal conductance in Fagus sylvatica f. purpurea and Populus balsamifera J. Exp. Bot. 46 1177–1183 Occurrence Handle1:CAS:528:DyaK2MXovV2mt7w%3D
U. Hacke J.S. Sperry B.E. Ewers D.S. Ellsworth K.V. Schäfer R. Oren (2000) ArticleTitleInfluence of soil porosity on water use in Pinus taeda Oecologia 14 495–505
R.M. Hubbard B.J. Bond M.G. Ryan (1999) ArticleTitleEvidence that hydraulic conductance limits photosythesis in old Pinus ponderosa trees Tree Physiol. 19 165–172 Occurrence Handle12651579
A.J. Jarvis W.J. Davies (1998) ArticleTitleThe coupled response of stomatal conductance to photosynthesis and transpiration J. Exp. Bot. 49 399–406 Occurrence Handle10.1093/jexbot/49.suppl_1.399
H. Jones R.A. Sutherland (1991) ArticleTitleStomatal control of xylem embolism Plant Cell Environ. 14 607–612
K.J. Kolb J.S. Sperry (1999) ArticleTitleTransport constraints on water use by the Great Basin shrubArtemisia tridentata Plant Cell Environ. 22 925–935 Occurrence Handle10.1046/j.1365-3040.1999.00458.x
D. Lemoine H. Cochard A. Granier (2002) ArticleTitleWithin crown variation in hydraulic architecture in beech (Fagus sylvatica L): evidence for a stomatal control of xylem embolism Ann. For. Sci. 59 19–27 Occurrence Handle10.1051/forest:2001002
M.A. Lo Gullo S. Salleo (1988) ArticleTitleDifferent strategies of drought resistance in three Mediterranean sclerophyllous trees growing in the same environmental conditions New Phytol. 108 267–276
N.J. Loewenstein S.G. Pallardy (1998) ArticleTitleDrought tolerancexylem sap abscisic acid and stomatal conductance during soil drying: a comparison of canopy trees of three temperate deciduous angiosperms Tree Physiol. 18 431–439 Occurrence Handle1:CAS:528:DyaK1cXks1elt7w%3D Occurrence Handle12651354
F. Magnani M. Borghetti (1995) ArticleTitleInterpretation of seasonal changes of xylem embolism and plant hydraulic resistance in Fagus sylvatica Plant Cell Environ. 18 689–696
F.C. Meinzer (1993) ArticleTitleStomatal control of transpiration Trends Ecol. Evol. 8 289–293 Occurrence Handle10.1016/0169-5347(93)90257-P
F.C. Meinzer M.J. Clearwater G. Goldstein (2001) ArticleTitleWater transport in trees: current perspectives, new insights and some controversies Environ. Exp. Bot. 45 239–262 Occurrence Handle10.1016/S0098-8472(01)00074-0 Occurrence Handle11323032
A. Nardini M.A. Lo Gullo S. Salleo (1999) ArticleTitleCompetitive strategies for water availability in two Mediterranean Quercus species Plant Cell Environ. 22 109–116 Occurrence Handle10.1046/j.1365-3040.1999.00382.x
A. Nardini S. Salleo (2000) ArticleTitleLimitation of stomatal conductance by hydraulic traits: sensing or preventing xylem cavitation? Trees 15 14–24 Occurrence Handle10.1007/s004680000071
R. Oren D.E. Pataki (2001) ArticleTitleTranspiration in response to variation in microclimate and soil moisture in southeastern deciduous species Oecologia. 127 549–559 Occurrence Handle10.1007/s004420000622
D.E. Pataki R. Oren G. Katul J. Sigmon (1998) ArticleTitleCanopy conductance of Pinus taedaLiquidambar styracifluaQuercus phellos under varying atmospheric and soil water conditions Tree Physiol. 18 307–315 Occurrence Handle12651370
D.E. Pataki R. Oren W.K. Smith (2000) ArticleTitleTranspiration of co-occurring species in a western subalpine forest during seasonally developing drought Ecology 81 2557–2566
P.B. Reich T.M. Hinckley (1989) ArticleTitleInfluence of pre-dawn water potential and soil-to-leaf hydraulic conductance on maximum daily leaf diffusive conductance in two oak species Funct. Ecol. 3 719–726
M.G. Ryan B.J. Yoder (1997) ArticleTitleHydraulic limits to tree height and tree growth BioScience 47 235–242
N.Z. Saliendra F.C. Meinzer (1989) ArticleTitleRelationship between root/soil hydraulic properties and stomatal behavior in sugarcane Aust. J. Plant Physiol. 16 241–250
N.Z. Saliendra J.S. Sperry J.P. Comstock (1995) ArticleTitleInfluence of leaf water status on stomatal responses to hydraulic conductanceatmospheric droughtand soil drought in Betula occidentalis Planta 196 357–366 Occurrence Handle10.1007/BF00201396 Occurrence Handle1:CAS:528:DyaK2MXls1Wqs7s%3D
S. Salleo M.A. Lo Gullo (1993) Drought resistance strategies and vulnerability to cavitation of some Mediterranean sclerophyllous trees M. Borghetti J. Grace A. Raschi (Eds) Water Transport in Plants under Climatic Stress Cambridge University Press Cambridge 99–113
S. Salleo A. Nardini F. Pitt M.A. Lo Gullo (2000) ArticleTitleXylem cavitation and hydraulic control of stomatal conductance in Laurel (Laurus nobilis L.) Plant Cell and Environment 23 71–79 Occurrence Handle10.1046/j.1365-3040.2000.00516.x
K.V.R. Schäfer R. Oren J.D. Tenhunen (2000) ArticleTitleThe effect of tree height on crown level stomatal conductance Plant Cell Environ. 23 365–375 Occurrence Handle10.1046/j.1365-3040.2000.00553.x
H.R. Schultz M.A. Matthews (1993) ArticleTitleXylem development and hydraulic conductance in sun and shade shoots of grapevine (Vitis vinifera L.): evidence that low light uncouples water transport capacity from leaf area Planta 190 393–406 Occurrence Handle10.1007/BF00196969
J.S. Sperry W.T. Pockman (1993) ArticleTitleLimitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis Plant Cell Environ. 16 279–287
J.S. Sperry T. Ikeda (1997) ArticleTitleXylem cavitation in roots and stems of Douglas-fir and white fir Tree Physiol. 17 275–280 Occurrence Handle14759867
J.S. Sperry F.R. Adler G.S. Campbell J.P. Comstock (1998) ArticleTitleLimitation of plant water use by rhizosphere and xylem conductance: results from a model Plant Cell Environ. 21 347–359 Occurrence Handle10.1046/j.1365-3040.1998.00287.x
J.S. Sperry (2000) ArticleTitleHydraulic constraints on plant gas exchange Agric. For. Meteorol. 104 13–23 Occurrence Handle10.1016/S0168-1923(00)00144-1
P.C. Tausend F.C. Meinzer G. Golstein (2000) ArticleTitleControl of transpiration in three coffee cultivars: the role of hydraulic and crown architecture Trees Struct. Funct. 14 181–190
M. Tsuda M.T. Tyree (1997) ArticleTitleWhole-plant hydraulic resistance and vulnerability segmentation in Acer saccharinum Tree Physiol. 17 351–357 Occurrence Handle14759843
M.T. Tyree J.S. Sperry (1988) ArticleTitleDo woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Plant Physiol. 88 574–580
M.T. Tyree P. Yianoulis (1980) ArticleTitleThe site of water evaporation from sub-stomatal cavities, liquid path resistances and hydroactive stomatal closure Ann. Bot. 46 175–193
M.T. Tyree J.S. Sperry (1989) ArticleTitleVulnerability of xylem to cavitation and embolism Annu. Rev. Plant Physiol. Plant Mol. Biol. 40 19–38 Occurrence Handle10.1146/annurev.pp.40.060189.000315
M.T. Tyree H. Cochard P. Cruziat B. Sinclair T. Ameglio (1993) ArticleTitleDrought-induced leaf shedding in walnut: evidence for vulnerability segmentation Plant Cell Environ. 16 879–882
M.T. Tyree (2003) ArticleTitleHydraulic limits on tree performance: transpiration, carbon gain and growth of trees Trees Struct. Funct. 17 95–100
T.H. van den Honert (1948) ArticleTitleWater transport in plants as a catenary process Discussions Faraday Soc. 3 146–153 Occurrence Handle10.1039/df9480300146
D. Whitehead (1998) ArticleTitleRegulation of stomatal conductance and transpiration in forest canopies Tree Physiol. 18 633–644 Occurrence Handle12651352
G.B. West J.H. Brown B.J. Enquist (1999) ArticleTitleA general model for the structure and allometry of plant vascular systems Nature 400 664–667 Occurrence Handle10.1038/23251 Occurrence Handle1:CAS:528:DyaK1MXlsVCktLk%3D
S Yang M.T. Tyree (1993) ArticleTitleHydraulic resistance in Acer saccharum shoots and its influence of leaf water potential and transpiration Tree Physiol. 12 231–242 Occurrence Handle14969914
S. Yang M.T. Tyree (1994) ArticleTitleHydraulic architecture of Acer saccharumA. rubrum: comparison of branches to whole trees and the contribution of leaves to hydraulic resistance J. Exp. Bot. 45 179–186
C. Yao S. Moreshet B. Aloni (2001) ArticleTitleWater relations and hydraulic control of stomatal behaviour in bell pepper plant in partial soil drying Plant Cell Environ. 24 227–235
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aranda, I., Gil, L. & Pardos, J. Seasonal changes in apparent hydraulic conductance and their implications for water use of European beech (Fagus sylvatica L.) and sessile oak [Quercus petraea (Matt.) Liebl] in South Europe. Plant Ecol 179, 155–167 (2005). https://doi.org/10.1007/s11258-004-7007-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11258-004-7007-1